Hypothesizing Nutrigenomic-Based Precision Anti-Obesity Treatment and Prophylaxis: Should We Be Targeting Sarcopenia Induced Brain Dysfunction?
Abstract
:1. Introduction
- Central Nervous System Stimulants (CNSS) that artificially stimulate the rate of calorie burning (Basal Metabolic Rate [BMR]).
- Appetite Suppressants
- Fat Blockers
- Starch Blockers
- Diuretics (Water Pills)
- Low Calorie Diets
- Low Food Diets
- Meal Replacement Programs (Diet Shakes, bars, etc.)
- High Protein Diets
- High Carbohydrate Diets
- Low/No Carbohydrate Diets
- Low-Fat Diets
- Pre-Meal Fiber/Water “Fill-You-Up” Programs
- Fruit and Fruit juice “Rapid “weight loss”” Programs
- Overnight “weight loss” Programs
- Vegetable Soup Diet Programs
- Liposuction
- Radical Digestive Tract Surgeries
- Acupuncture
- Laxatives
- Hypnosis
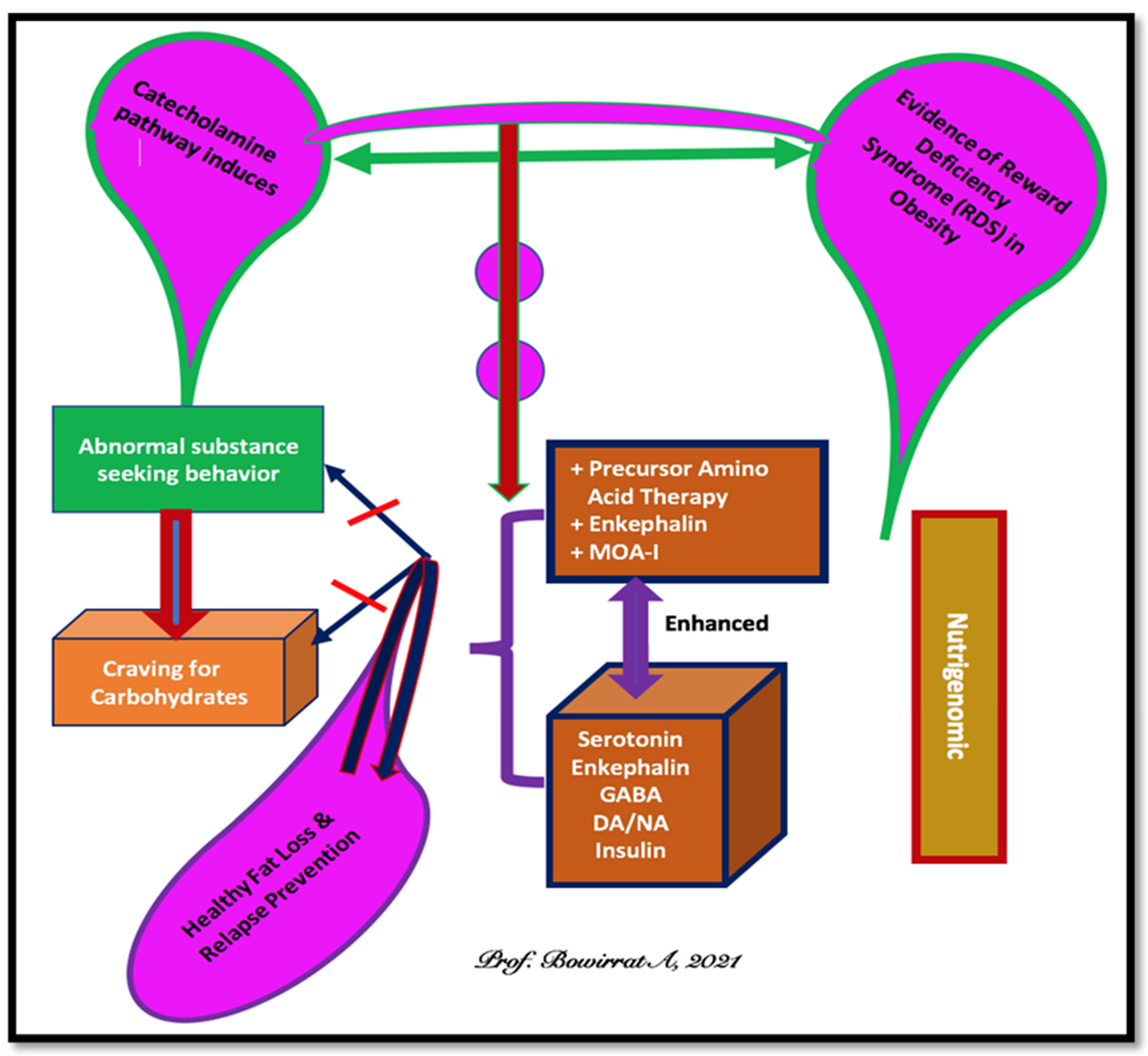
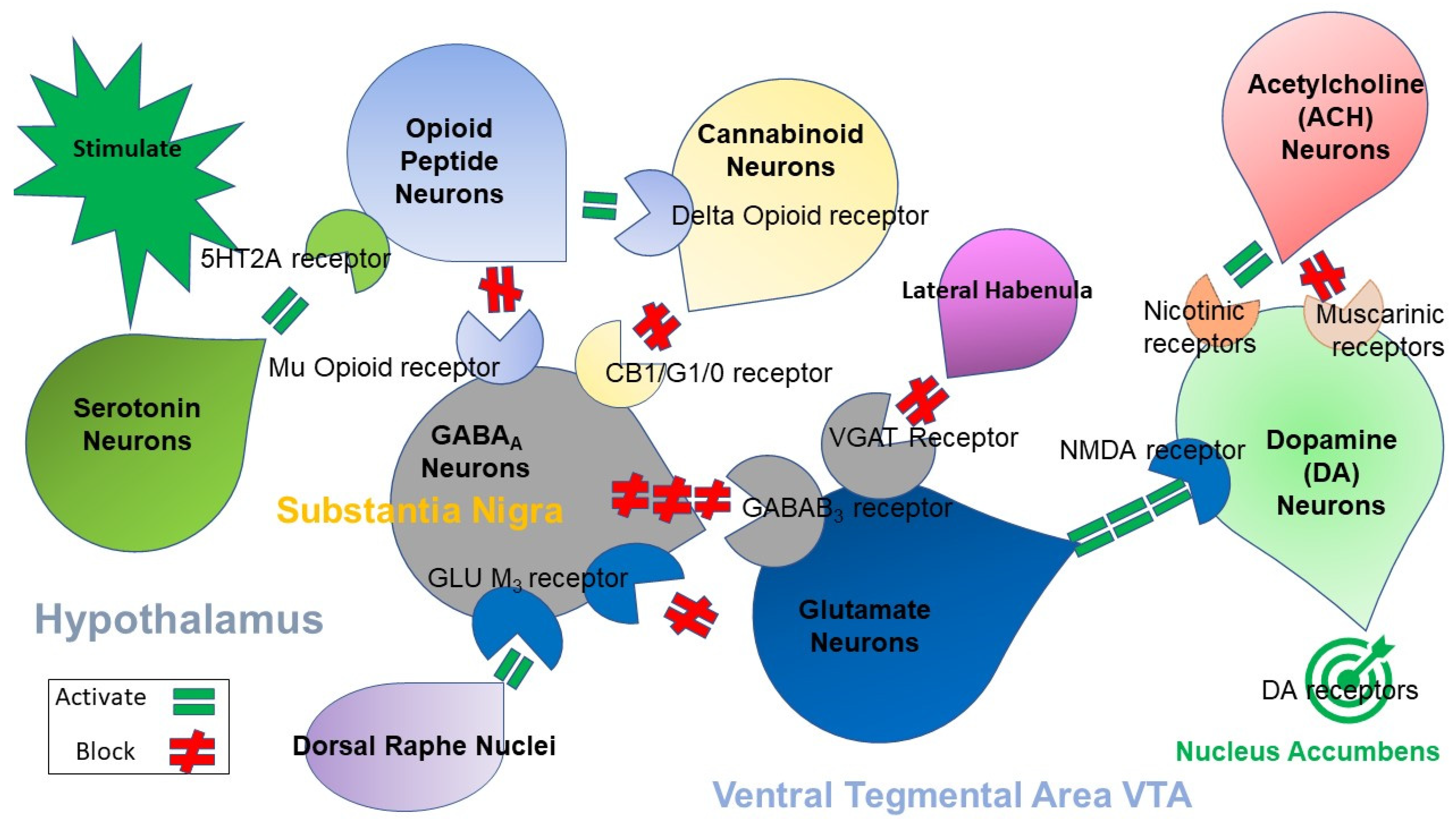
2. Brain Reward Mechanisms and Nutrigenomic Solutions
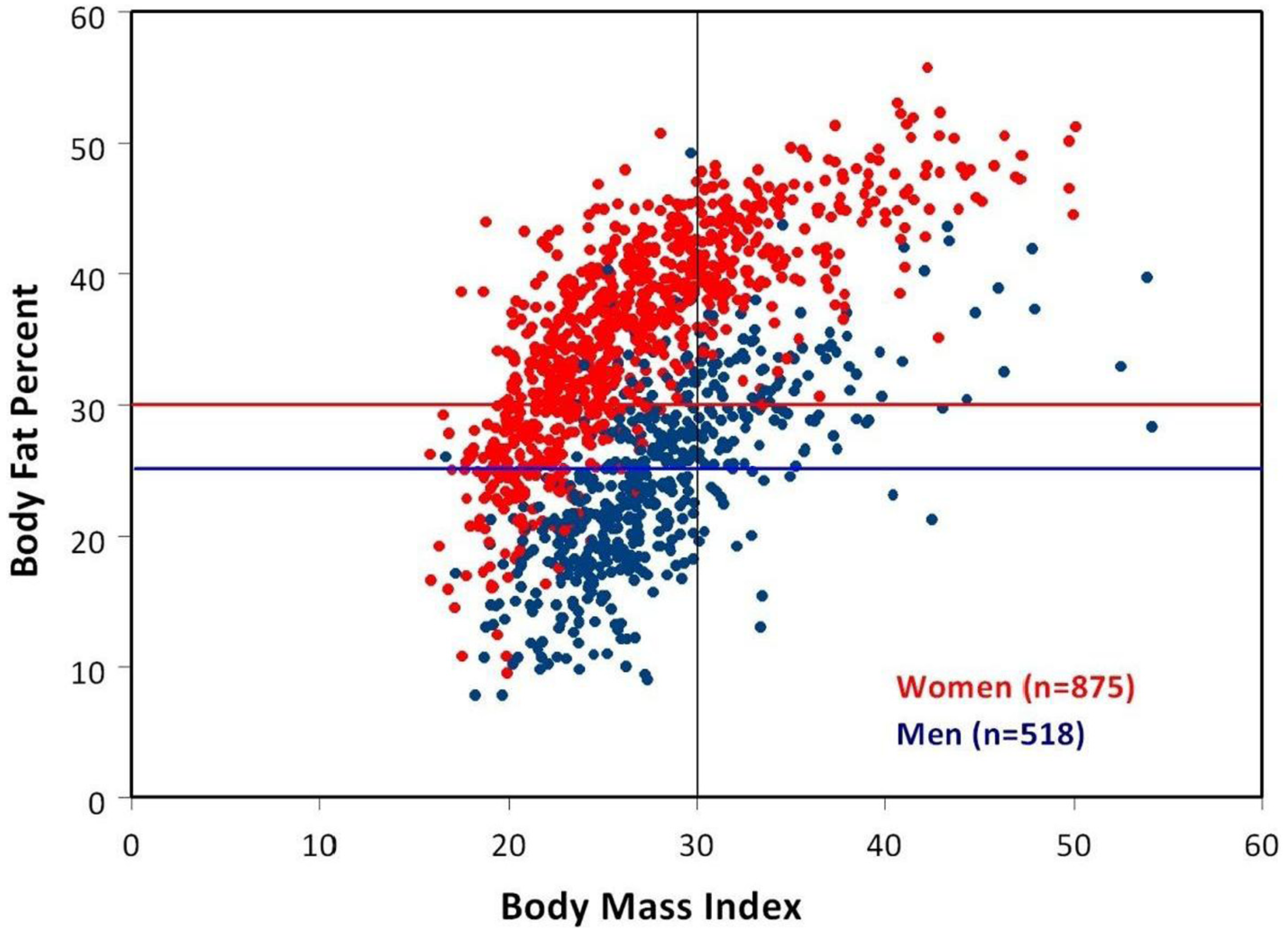
3. Body Mass Index (BMI) vs. Percent Body Fat
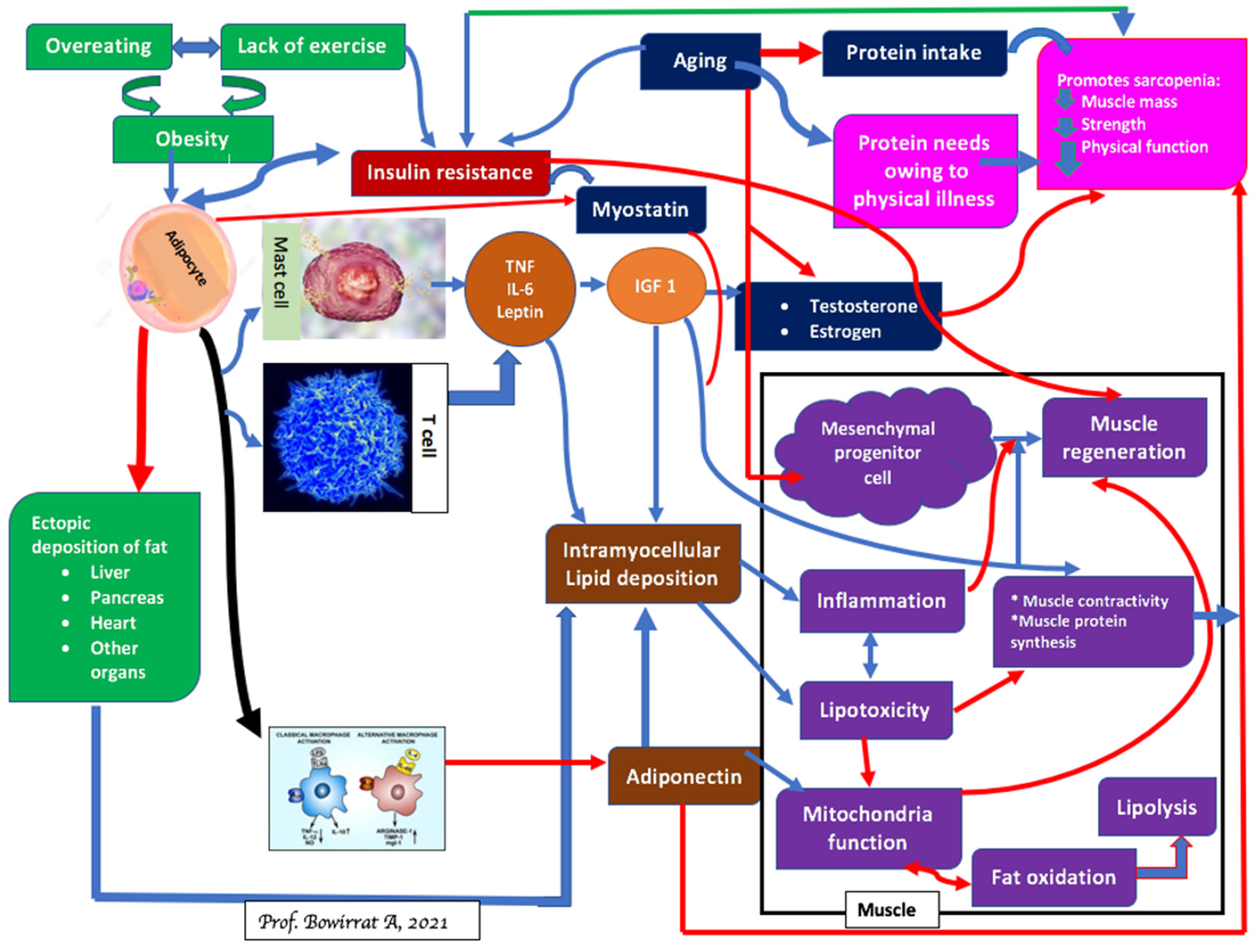
4. Sarcopenia and Brain Function
5. Sarcopenia and Obesity: Can We Treat?
6. Summary
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goodarzi, M.O. Genetics of obesity: What genetic association studies have taught us about the biology of obesity and its complications. Lancet Diabetes Endocrinol. 2018, 3, 223–236. [Google Scholar] [CrossRef]
- Schulte, E.M.; Avena, N.M.; Gearhardt, A.N. Which foods may be addictive? The roles of processing, fat content, and glycemic load. PLoS ONE 2015, 10, e0117959. [Google Scholar] [CrossRef]
- Prior, S.J.; Joseph, L.J.; Brandauer, J.; Katzel, L.I.; Hagberg, J.M.; Ryan, A.S. Reduction in midthigh low-density muscle with aerobic exercise training and weight loss impacts glucose tolerance in older men. J. Clin. Endocrinol. Metab. 2007, 92, 880–886. [Google Scholar] [CrossRef] [Green Version]
- Hall, K.D.; Bemis, T.; Brychta, R.; Chen, K.Y.; Courville, A.; Crayner, E.J.; Goodwin, S.; Guo, J.; Howard, L.; Knuth, N.D.; et al. Calorie for calorie, dietary fat restriction results in more body fat loss than carbohydrate restriction in people with obesity. Cell Metab. 2015, 22, 427–436. [Google Scholar] [CrossRef] [Green Version]
- Carden, A.; Blum, K.; Arbaugh, C.J.; Trickey, A.; Eisenberg, D. Low socioeconomic status is associated with lower weight-loss outcomes 10-years after Roux-en-Y gastric bypass. Surg. Endosc. 2019, 33, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Tataranni, P.A.; Baier, L.; Jenkinson, C.; Harper, I.; Del Parigi, A.; Bogardus, C. A Ser311Cys mutation in the human dopamine receptor D2 gene is associated with reduced energy expenditure. Diabetes 2001, 50, 901–904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jenkinson, C.P.; Hanson, R.; Cray, K.; Wiedrich, C.; Knowler, W.C.; Bogardus, C.; Baier, L. Association of dopamine D2 receptor polymorphisms Ser311Cys and TaqIA with obesity or type 2 diabetes mellitus in Pima Indians. Int. J. Obes. Relat. Metab. Disord. 2000, 24, 1233–1238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hales, C.N.; Barker, D.J. The thrifty phenotype hypothesis. Br. Med. Bull. 2001, 60, 5–20. [Google Scholar] [CrossRef] [Green Version]
- Izquierdo, A.G.; Crujeiras, A.B.; Casanueva, F.F.; Carreira, M.C. Leptin, obesity, and leptin resistance: Where are we 25 years later? Nutrients 2019, 11, 2704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blum, K.; Chen, A.L.; Chen, T.J.; Rhoades, P.; Prihoda, T.J.; Downs, B.W.; Waite, R.L.; Williams, L.; Braverman, E.R.; Braverman, D.; et al. LG839: Anti-obesity effects and polymorphic gene correlates of reward deficiency syndrome. Adv. Ther. 2008, 25, 894–913. [Google Scholar] [CrossRef]
- Downs, B.W.; Chen, A.L.; Chen, T.J.; Waite, R.L.; Braverman, E.R.; Kerner, M.; Braverman, D.; Rhoades, P.; Prihoda, T.J.; Palomo, T.; et al. Nutrigenomic targeting of carbohydrate craving behavior: Can we manage obesity and aberrant craving behaviors with neurochemical pathway manipulation by immunological compatible substances (nutrients) using a genetic positioning system (GPS) map? Med. Hypotheses 2009, 73, 427–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blum, K.; Chen, T.J.; Meshkin, B.; Downs, B.W.; Gordon, C.A.; Blum, S.; Mangucci, J.F.; Braverman, E.R.; Arcuri, V.; Deutsch, R.; et al. Genotrim, a DNA-customized nutrigenomic product, targets genetic factors of obesity: Hypothesizing a dopamine-glucose correlation demonstrating reward deficiency syndrome (RDS). Med. Hypotheses 2007, 68, 844–852. [Google Scholar] [CrossRef]
- Blum, K.; Simpatico, T.; Badgaiyan, R.D.; Demetrovics, Z.; Fratantonio, J.; Agan, G.; Febo, M.; Gold, M.S. Coupling neurogenetics (GARS™) and a nutrigenomic based dopaminergic agonist to treat Reward Deficiency Syndrome (RDS): Targeting polymorphic reward genes for carbohydrate addiction algorithms. J. Reward Defic. Syndr. 2015, 1, 75–80. [Google Scholar] [CrossRef] [Green Version]
- Blum, K.; Modestino, E.J.; Gondré-Lewis, M.C.; Neary, J.; Siwicki, D.; Hauser, M.; Barh, D.; Steinberg, B.; Badgaiyan, R.D. Global opioid epidemic: Doomed to fail without genetically based precision addiction medicine (Pam™): Lessons learned from America. Precis. Med. 2017, 2, 17–22. [Google Scholar]
- Park, S.; Ahn, I.S.; Kim, D.S. Central infusion of leptin improves insulin resistance and suppresses beta-cell function, but not beta-cell mass, primarily through the sympathetic nervous system in a type 2 diabetic rat model. Life Sci. 2010, 86, 854–862. [Google Scholar] [CrossRef]
- Duarte, L.C.; Speakman, J.R. Low resting metabolic rate is associated with greater lifespan because of a confounding effect of body fatness. Age 2014, 36, 9731. [Google Scholar] [CrossRef] [Green Version]
- Hoebel, B.G.; Avena, N.M.; Bocarsly, M.E.; Rada, P. Natural addiction: A behavioral and circuit model based on sugar addiction in rats. J. Addict. Med. 2009, 3, 33–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Zwaan, M.; Aslam, Z.; Mitchell, J.E. Research on energy expenditure in individuals with eating disorders: A review. Int. J. Eat. Disord. 2002, 32, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Krueger, D.W. Eating disorders. In Substance Abuse: A Comprehensive Textbook, 2nd ed.; Lowenson, J.H., Ruiz, P., Milman, R.B., Langrod, J.G., Eds.; Williams and Wilkins: Baltimore, MD, USA, 1992; pp. 371–379. [Google Scholar]
- Blum, K.; Thanos, P.K.; Gold, M.S. Dopamine and glucose, obesity, and reward deficiency syndrome. Front. Psychol. 2014, 5, 919. [Google Scholar] [CrossRef] [Green Version]
- Blum, K.; Oscar-Berman, M.; Demetrovics, Z.; Barh, D.; Gold, M.S. Genetic Addiction Risk Score (GARS): Molecular neurogenetic evidence for predisposition to Reward Deficiency Syndrome (RDS). Mol. Neurobiol. 2014, 50, 765–796. [Google Scholar] [CrossRef] [Green Version]
- Blum, K.; Baron, D.; McLaughlin, T.; Gold, M.S. Molecular neurological correlates of endorphinergic/dopaminergic mechanisms in reward circuitry linked to Endorphinergic Deficiency Syndrome (EDS). J. Neurol. Sci. 2020, 411, 116733. [Google Scholar] [CrossRef] [Green Version]
- Noble, E.P.; Blum, K.; Ritchie, T.; Montgomery, A.; Sheridan, P.J. Allelic association of the D2 dopamine receptor gene with receptor-binding characteristics in alcoholism. Arch. Gen. Psychiatry 1991, 48, 648–654. [Google Scholar] [CrossRef]
- Gluskin, B.S.; Mickey, B.J. Genetic variation and dopamine D2 receptor availability: A systematic review and meta-analysis of human in vivo molecular imaging studies. Transl. Psychiatry 2016, 6, e747. [Google Scholar] [CrossRef] [Green Version]
- Eisenstein, S.A.; Bogdan, R.; Love-Gregory, L.; Corral-Frías, N.S.; Koller, J.M.; Black, K.J.; Moerlein, S.M.; Perlmutter, J.S.; Barch, D.M.; Hershey, T. Prediction of striatal D2 receptor binding by DRD2/ANKK1 TaqIA allele status. Synapse 2016, 70, 418–431. [Google Scholar] [CrossRef] [Green Version]
- Noble, E.P.; Blum, K.; Khalsa, M.E.; Ritchie, T.; Montgomery, A.; Wood, R.C.; Fitch, R.J.; Ozkaragoz, T.; Sheridan, P.J.; Anglin, M.D.; et al. Allelic association of the D2 dopamine receptor gene with cocaine dependence. Drug Alcohol Depend. 1993, 33, 271–285. [Google Scholar] [CrossRef]
- Blanco-Gandía, M.C.; Rodríguez-Arias, M. Bingeing on fat increases cocaine reward. Oncotarget 2017, 8, 16105–16106. [Google Scholar] [CrossRef] [PubMed]
- Noble, E.P. The DRD2 gene in psychiatric and neurological disorders and its phenotypes. Pharmacogenomics 2000, 1, 309–333. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Kaufman, T. Childhood obesity. Panminerva Med. 2018, 60, 200–212. [Google Scholar] [CrossRef] [PubMed]
- Thanos, P.K.; Hamilton, J.; O’Rourke, J.R.; Napoli, A.; Febo, M.; Volkow, N.D.; Blum, K.; Gold, M. Dopamine D2 gene expression interacts with environmental enrichment to impact lifespan and behavior. Oncotarget 2016, 7, 19111–19123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lecca, D.; Piras, G.; Driscoll, P.; Giorgi, O.; Corda, M.G. A differential activation of dopamine output in the shell and core of the nucleus accumbens is associated with the motor responses to addictive drugs: A brain dialysis study in Roman high- and low-avoidance rats. Neuropharmacology 2004, 46, 688–699. [Google Scholar] [CrossRef] [PubMed]
- Blum, K.; Chen, T.J.; Meshkin, B.; Waite, R.L.; Downs, B.W.; Blum, S.H.; Mengucci, J.F.; Arcuri, V.; Braverman, E.R.; Palomo, T. Manipulation of catechol-O-methyl-transferase (COMT) activity to influence the attenuation of substance seeking behavior, a subtype of Reward Deficiency Syndrome (RDS), is dependent upon gene polymorphisms: A hypothesis. Med. Hypotheses 2007, 69, 1054–1060. [Google Scholar] [CrossRef]
- Blum, K.; Chen, T.J.; Downs, B.W.; Bowirrat, A.; Waite, R.L.; Braverman, E.R.; Madigan, M.; Oscar-Berman, M.; DiNubile, N.; Stice, E.; et al. Neurogenetics of dopaminergic receptor supersensitivity in activation of brain reward circuitry and relapse: Proposing “deprivation-amplification relapse therapy” (DART). Postgrad. Med. 2009, 121, 176–196. [Google Scholar] [CrossRef] [Green Version]
- Chen, A.L.; Blum, K.; Chen, T.J.; Giordano, J.; Downs, B.W.; Han, D.; Barh, D.; Braverman, E.R. Correlation of the Taq1 dopamine D2 receptor gene and percent body fat in obese and screened control subjects: A preliminary report. Food Funct. 2012, 3, 40–48. [Google Scholar] [CrossRef]
- Shah, N.R.; Braverman, E.R. Measuring adiposity in patients: The utility of body mass index (BMI), percent body fat, and leptin. PLoS ONE 2012, 7, e33308. [Google Scholar] [CrossRef] [Green Version]
- Sun, Q.; Townsend, M.K.; Okereke, O.I.; Franco, O.H.; Hu, F.B.; Grodstein, F. Adiposity and weight change in mid-life in relation to healthy survival after age 70 in women: Prospective cohort study. BMJ 2009, 339, b3796. [Google Scholar] [CrossRef] [Green Version]
- Flegal, K.M.; Carroll, M.D.; Ogden, C.L.; Curtin, L.R. Prevalence and trends in obesity among US adults, 1999–2008. JAMA 2010, 303, 235–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Q.; Van Dam, R.M.; Spiegelman, D.; Heymsfield, S.B.; Willett, W.C.; Hu, F.B. Comparison of dual-energy X-Ray absorptiometric and anthropometric measures of adiposity in relation to adiposity-related biologic factors. Am. J. Epidemiol. 2010, 172, 1442–1454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Monaco, M.; Vallero, F.; Di Monaco, R.; Tappero, R. Prevalence of sarcopenia and its association with osteoporosis in 313 older women following a hip fracture. Arch. Gerontol. Geriatr. 2011, 52, 71–74. [Google Scholar] [CrossRef]
- Ruiz, J.R.; Sui, X.; Lobelo, F.; Morrow, J.R.; Jackson, A.W.; Sjöström, M.; Blair, S.N. Association between muscular strength and mortality in men: Prospective cohort study. BMJ 2008, 337, a439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenberg, I. Summary comments: Epidemiological and methodological problems in determining nutritional status of older persons. Am. J. Clin. Nutr. 1989, 50, 1231–1233. [Google Scholar] [CrossRef]
- Rosenberg, I.H. Sarcopenia: Origins and clinical relevance. J. Nutr. 1997, 127, 990S–991S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morley, J.E.; Baumgartner, R.N.; Roubenoff, R.; Mayer, J.; Nair, K.S. Sarcopenia. J. Lab. Clin. Med. 2001, 137, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Paddon-Jones, D.; Short, K.R.; Campbell, W.W.; Volpi, E.; Wolfe, R.R. Role of dietary protein in the sarcopenia of aging. Am. J. Clin. Nutr. 2008, 87, 1562S–1566S. [Google Scholar] [CrossRef] [Green Version]
- Sayer, A.A.; Syddall, H.; Martin, H.; Patel, H.; Baylis, D.; Cooper, C. The developmental origins of sarcopenia. J. Nutr. Health Aging 2008, 12, 427–432. [Google Scholar] [CrossRef] [Green Version]
- Thompson, D.D. Aging and sarcopenia. J. Musculoskelet Neuronal. Interact. 2007, 7, 344–345. [Google Scholar]
- Rolland, Y.; Czerwinski, S.; Van Kan, G.A.; Morley, J.E.; Cesari, M.; Onder, G.; Woo, J.; Baumgartner, R.; Pillard, F.; Boirie, Y.; et al. Sarcopenia: Its assessment, etiology, pathogenesis, consequences and future perspectives. J. Nutr. Health Aging 2008, 12, 433–450. [Google Scholar] [CrossRef] [Green Version]
- Rivard, A.; Fabre, J.E.; Silver, M.; Chen, D.; Murohara, T.; Kearney, M.; Magner, M.; Asahara, T.; Isner, J.M. Age-dependent impairment of angiogenesis. Circulation 1999, 99, 111–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez-Lopez, C.; LeRoith, D.; Torres-Aleman, I. Insulin-like growth factor I is required for vessel remodeling in the adult brain. Proc. Natl. Acad. Sci. USA 2004, 101, 9833–9838. [Google Scholar] [CrossRef] [Green Version]
- Loddick, S.A.; Liu, X.J.; Lu, Z.X.; Liu, C.; Behan, D.P.; Chalmers, D.C.; Foster, A.C.; Vale, W.W.; Ling, N.; De Souza, E.B. Displacement of insulin-like growth factors from their binding proteins as a potential treatment for stroke. Proc. Natl. Acad. Sci. USA 1998, 95, 1894–1898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guan, J.; Bennet, L.; George, S.; Wu, D.; Waldvogel, H.J.; Gluckman, P.D.; Faull, R.L.; Crosier, P.S.; Gunn, A.J. Insulin-like growth factor-1 reduces postischemic white matter injury in fetal sheep. J. Cereb. Blood Flow Metab. 2001, 21, 493–502. [Google Scholar] [CrossRef] [Green Version]
- Guan, J.; Bennet, L.; George, S.; Waldvogel, H.J.; Faull, R.L.M.; Gluckman, P.D.; Keunen, H.; Gunn, A.J. Selective neuroprotective effects with insulin-like growth factor-1 in phenotypic striatal neurons following ischemic brain injury in fetal sheep. Neuroscience 2000, 95, 831–839. [Google Scholar] [CrossRef]
- Liu, J.P.; Baker, J.; Perkins, A.S.; Robertson, E.J.; Efstratiadis, A. Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r). Cell 1993, 75, 59–72. [Google Scholar] [CrossRef]
- Liu, P.; Smith, P.F.; Darlington, C.L. Glutamate receptor subunits expression in memory-associated brain structures: Regional variations and effects of aging. Synapse 2008, 62, 834–841. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.F.; Fawcett, J.R.; Thorne, R.G.; DeFor, T.A.; Frey, W.H., II. Intranasal administration of insulin-like growth factor-I bypasses the blood–brain barrier and protects against focal cerebral ischemic damage. J. Neurol. Sci. 2001, 187, 91–97. [Google Scholar] [CrossRef]
- Schäbitz, W.R.; Hoffmann, T.T.; Heiland, S.; Kollmar, R.; Bardutzky, J.; Sommer, C.; Schwab, S. Delayed neuroprotective effect of insulin-like growth factor-I after experimental transient focal cerebral ischemia monitored with MRI. Stroke 2001, 32, 1226–1233. [Google Scholar] [CrossRef] [Green Version]
- Mackay, K.B.; Loddick, S.A.; Naeve, G.S.; Vana, A.M.; Verge, G.M.; Foster, A.C. Neuroprotective effects of insulin-like growth factor-binding protein ligand inhibitors in vitro and in vivo. J. Cereb. Blood Flow Metab. 2003, 23, 1160–1167. [Google Scholar] [CrossRef] [Green Version]
- Leinninger, G.M.; Feldman, E.L. Insulin-like growth factors in the treatment of neurological disease. Endocr. Dev. 2005, 9, 135–159. [Google Scholar] [CrossRef]
- Zhu, G.; Song, M.; Wang, H.; Zhao, G.; Yu, Z.; Yin, Y.; Zhao, X.; Huang, L. Young environment reverses the declined activity of aged rat-derived endothelial progenitor cells: Involvement of the phosphatidylinositol 3-kinase/Akt signaling pathway. Ann. Vasc. Surg. 2009, 23, 519–534. [Google Scholar] [CrossRef]
- Zhu, W.; Fan, Y.; Hao, Q.; Shen, F.; Hashimoto, T.; Yang, G.Y.; Gasmi, M.; Bartus, R.T.; Young, W.L.; Chen, Y. Postischemic IGF-1 gene transfer promotes neurovascular regeneration after experimental stroke. J. Cereb. Blood Flow Metab. 2009, 29, 1528–1537. [Google Scholar] [CrossRef]
- Zhu, W.; Fan, Y.; Frenzel, T.; Gasmi, M.; Bartus, R.T.; Young, W.L.; Yang, G.Y.; Chen, Y. Insulin growth factor-1 gene transfer enhances neurovascular remodeling and improves long-term stroke outcome in mice. Stroke 2008, 39, 1254–1261. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.L.; Callahan, M.F.; Blum, K.; Dinubile, N.A.; Chen, T.J.; Waite, R.L. H-Wave® effects on blood flow and angiogenesis in longitudinal studies in rats. J. Surg Orthop. Adv. 2011, 20, 255–259. [Google Scholar] [PubMed]
- Pizzimenti, M.; Meyer, A.; Charles, A.L.; Giannini, M.; Chakfé, N.; Lejay, A.; Geny, B. Sarcopenia and peripheral arterial disease: A systematic review. J. Cachexia Sarcopenia Muscle 2020, 11, 866–886. [Google Scholar] [CrossRef] [PubMed]
- Hendrickse, P.; Degens, H. The role of the microcirculation in muscle function and plasticity. J. Muscle Res. Cell Motil. 2019, 40, 127–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalle, C.; Ostermann, A.I.; Konrad, T.; Coudy-Gandilhon, C.; Decourt, A.; Barthélémy, J.C.; Roche, F.; Féasson, L.; Mazur, A.; Béchet, D.; et al. Muscle loss associated changes of oxylipin signatures during biological aging: An exploratory study from the PROOF cohort. J. Gerontol. A Biol. Sci. Med. Sci. 2019, 74, 608–615. [Google Scholar] [CrossRef]
- Choi, K.M. Sarcopenia and sarcopenic obesity. Korean J. Intern. Med. 2016, 31, 1054–1060. [Google Scholar] [CrossRef] [Green Version]
- Batsis, J.A.; Villareal, D.T. Sarcopenic obesity in older adults: Aetiology, epidemiology and treatment strategies. Nat. Rev. Endocrinol. 2018, 14, 513–537. [Google Scholar] [CrossRef]
- Milne, A.C.; Potter, J.; Vivanti, A.; Avenell, A. Protein and energy supplementation in elderly people at risk from malnutrition. Cochrane Database Syst. Rev. 2009, 2, CD003288. [Google Scholar] [CrossRef]
- Sammarco, R.; Marra, M.; Di Guglielmo, M.L.; Naccarato, M.; Contaldo, F.; Poggiogalle, E.; Donini, L.M.; Pasanisi, F. Evaluation of hypocaloric diet with protein supplementation in middle-aged sarcopenic obese women: A pilot study. Obes. Facts 2017, 10, 160–167. [Google Scholar] [CrossRef] [Green Version]
- Bouchonville, M.; Armamento-Villareal, R.; Shah, K.; Napoli, N.; Sinacore, D.R.; Qualls, C.; Villareal, D.T. Weight loss, exercise or both and cardiometabolic risk factors in obese older adults: Results of a randomized controlled trial. Int. J. Obes. 2014, 38, 423–431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbenhardt, C.; McTiernan, A.; Alfano, C.M.; Wener, M.H.; Campbell, K.L.; Duggan, C.; Foster-Schubert, K.E.; Kong, A.; Toriola, A.T.; Potter, J.D.; et al. Effects of individual and combined dietary weight loss and exercise interventions in postmenopausal women on adiponectin and leptin levels. J. Intern. Med. 2013, 274, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Beavers, K.M.; Hsu, F.C.; Isom, S.; Kritchevsky, S.B.; Church, T.; Goodpaster, B.; Pahor, M.; Nicklas, B.J. Long-term physical activity and inflammatory biomarkers in older adults. Med. Sci. Sports Exerc. 2010, 42, 2189–2196. [Google Scholar] [CrossRef]
- Cohen, P.G. Obesity in men: The hypogonadal estrogen receptor relationship and its effect on glucose homeostasis. Med. Hypotheses 2008, 70, 358–360. [Google Scholar] [CrossRef]
- Bergen, H.R., III; Farr, J.N.; Vanderboom, P.M.; Atkinson, E.J.; White, T.A.; Singh, R.J.; Khosla, S.; LeBrasseur, N.K. Myostatin as a mediator of sarcopenia versus homeostatic regulator of muscle mass: Insights using a new mass spectrometry-based assay. Skelet. Muscle 2015, 5, 21. [Google Scholar] [CrossRef] [Green Version]
- Koshinaka, K.; Toshinai, K.; Mohammad, A.; Noma, K.; Oshikawa, M.; Ueno, H.; Yamaguchi, H.; Nakazato, M. Therapeutic potential of ghrelin treatment for unloading-induced muscle atrophy in mice. Biochem. Biophys. Res. Commun. 2011, 412, 296–301. [Google Scholar] [CrossRef]
- Shah, K.; Gleason, L.; Villareal, D.T. Vitamin K and bone health in older adults. J. Nutr. Gerontol. Geriatr. 2014, 33, 10–22. [Google Scholar] [CrossRef]
- Sacco, A.; Doyonnas, R.; Kraft, P.; Vitorovic, S.; Blau, H.M. Self-renewal and expansion of single transplanted muscle stem cells. Nature 2008, 456, 502–506. [Google Scholar] [CrossRef] [Green Version]
- Yarnell, S.; Oscar-Berman, M.; Avena, N.; Blum, K.; Gold, M. Pharmacotherapies for overeating and obesity. J. Genet. Syndr. Gene Ther. 2013, 4, 131. [Google Scholar] [CrossRef] [PubMed]
- Fried, L.; Modestino, E.J.; Siwicki, D.; Lott, L.; Thanos, P.K.; Baron, D.; Badgaiyan, R.D.; Ponce, J.V.; Giordano, J.; Downs, W.B.; et al. Hypodopaminergia and “Precision Behavioral Management” (PBM): It is a generational family affair. Curr. Pharm. Biotechnol. 2020, 21, 528–541. [Google Scholar] [CrossRef]
- Blum, K.; Gondré-Lewis, M.C.; Baron, D.; Thanos, P.K.; Braverman, E.R.; Neary, J.; Elman, I.; Badgaiyan, R.D. Introducing precision addiction management of reward deficiency syndrome, the construct that underpins all addictive behaviors. Front. Psychiatry 2018, 9, 548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swenson, S.; Blum, K.; McLaughlin, T.; Gold, M.S.; Thanos, P.K. The therapeutic potential of exercise for neuropsychiatric diseases: A review. J. Neurol Sci. 2020, 412, 116763. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ji, G.; Hu, Y.; Li, G.; Ding, Y.; Hu, C.; Liu, L.; Zhang, W.; von Deneen, K.M.; Han, Y.; et al. Laparoscopic sleeve gastrectomy induces sustained changes in gray and white matter brain volumes and resting functional connectivity in obese patients. Surg. Obes. Relat. Dis. 2020, 16, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Febo, M.; Blum, K.; Badgaiyan, R.D.; Perez, P.D.; Colon-Perez, L.M.; Thanos, P.K.; Ferris, C.F.; Kulkarni, P.; Giordano, J.; Baron, D.; et al. Enhanced functional connectivity and volume between cognitive and reward centers of naïve rodent brain produced by pro-dopaminergic agent KB220Z. PLoS ONE 2017, 12, e0174774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blum, K.; Liu, Y.; Wang, W.; Wang, Y.; Zhang, Y.; Oscar-Berman, M.; Smolen, A.; Febo, M.; Han, D.; Simpatico, T.; et al. rsfMRI effects of KB220Z™ on neural pathways in reward circuitry of abstinent genotyped heroin addicts. Postgrad Med. 2015, 127, 232–241. [Google Scholar] [CrossRef]
- Cerdó, T.; García-Santos, J.A.; GBermúdez, M.; Campoy, C. The Role of Probiotics and Prebiotics in the Prevention and Treatment of Obesity. Nutrients 2019, 11, 635, PMCID:PMC6470608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gul, K.; Singh, A.K.; Jabeen, R. Nutraceuticals and Functional Foods: The Foods for the Future World. Crit. Rev. Food Sci. Nutr. 2016, 56, 2617–2627. [Google Scholar] [CrossRef] [PubMed]
- Conroy, K.P.; Davidson, I.M.; Warnock, M. Pathogenic obesity and nutraceuticals. Proc. Nutr. Soc. 2011, 70, 426–438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anton, S.D.; Hida, A.; Mankowski, R.; Layne, A.; Solberg, L.M.; Mainous, A.G.; Buford, T. Nutrition and Exercise in Sarcopenia. Curr. Protein. Pept. Sci. 2018, 19, 649–667. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Li, G.; Wang, J.; von Deneen, K.M.; Wu, K.; Yang, Y.; She, J.; Ji, G.; Nie, Y.; Cui, G.; et al. Comparing the Impact of Laparoscopic Sleeve Gastrectomy and Gastric Cancer Surgery on Resting-State Brain Activity and Functional Connectivity. Front. Neurosci. 2020, 14, 614092, PMCID:PMC7726325. [Google Scholar] [CrossRef] [PubMed]

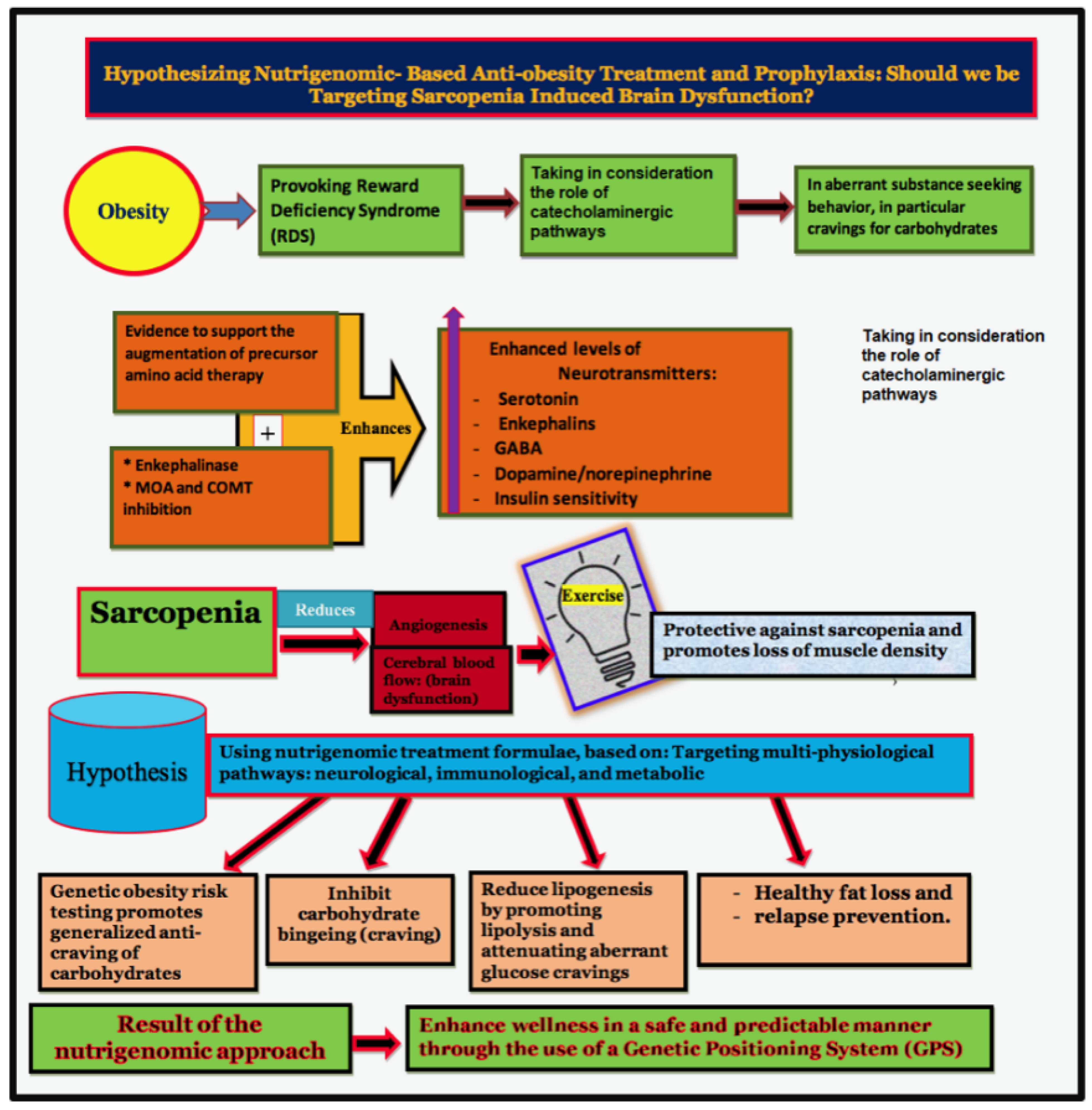
|
|
|
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blum, K.; Gold, M.S.; Llanos-Gomez, L.; Jalali, R.; Thanos, P.K.; Bowirrat, A.; Downs, W.B.; Bagchi, D.; Braverman, E.R.; Baron, D.; et al. Hypothesizing Nutrigenomic-Based Precision Anti-Obesity Treatment and Prophylaxis: Should We Be Targeting Sarcopenia Induced Brain Dysfunction? Int. J. Environ. Res. Public Health 2021, 18, 9774. https://doi.org/10.3390/ijerph18189774
Blum K, Gold MS, Llanos-Gomez L, Jalali R, Thanos PK, Bowirrat A, Downs WB, Bagchi D, Braverman ER, Baron D, et al. Hypothesizing Nutrigenomic-Based Precision Anti-Obesity Treatment and Prophylaxis: Should We Be Targeting Sarcopenia Induced Brain Dysfunction? International Journal of Environmental Research and Public Health. 2021; 18(18):9774. https://doi.org/10.3390/ijerph18189774
Chicago/Turabian StyleBlum, Kenneth, Mark S. Gold, Luis Llanos-Gomez, Rehan Jalali, Panayotis K. Thanos, Abdalla Bowirrat, William B. Downs, Debasis Bagchi, Eric R. Braverman, David Baron, and et al. 2021. "Hypothesizing Nutrigenomic-Based Precision Anti-Obesity Treatment and Prophylaxis: Should We Be Targeting Sarcopenia Induced Brain Dysfunction?" International Journal of Environmental Research and Public Health 18, no. 18: 9774. https://doi.org/10.3390/ijerph18189774






