Cognitive Training Improves Disconnected Limbs’ Mental Representation and Peripersonal Space after Spinal Cord Injury
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
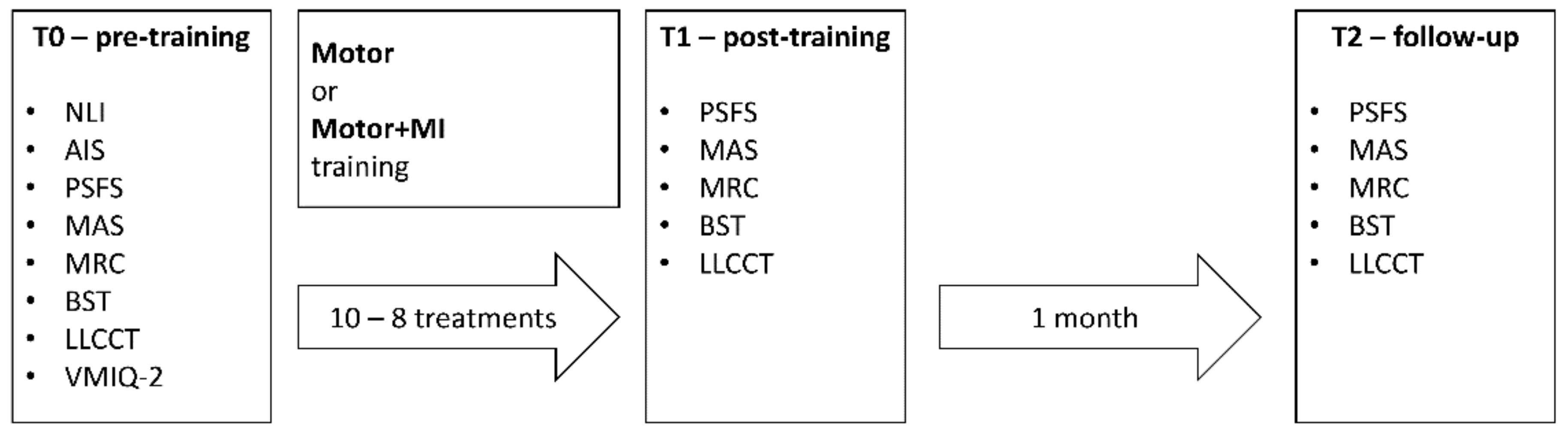
2.2. Overall Design
2.3. Materials
2.3.1. Questionnaires and Clinical Scales
- The Neurological Level of Injury (NLI), that coincides with the most caudal part of the spinal cord with completely spared sensorimotor functions [13].
- The ASIA Impairment Scale (AIS), that is a 5-point scale concerning the completeness of the lesion [48].
- The Vividness of Motor Imagery Questionnaire-2 (VMIQ—Second Version) [55,56] is a measure of an individual’s capacity to imagine actions. In the present study, it was administered in the version adapted for spinal cord injured people [29] only at T0 with the aim of identifying potential correlations between the patient’s imagery capacity and any effects of the interventions carried out.It assesses three components of motor imagery: (I) visual imagery from a first person perspective (i.e., subjects are asked to visualise their body performing the action as if they were inside their body watching it with their own eyes; (II) visual imagery from a third person perspective (i.e., subjects are asked to visualise their body performing the action as if they were watching themselves from an external position such as in a mirror) or (III) Kinesthetic imagery, KIN (i.e., subjects are asked to simulate the musculo-skeletal sensations generated by executing the actions). These activate partially different processes [57,58,59], with KIN probably being the most sensitive measure of Motor Imagery.
- The Penn Spasms Frequency Scale (PSFS) [49] is used to estimate the intensity and frequency of spasms as reported by the patient.
- The Ashworth Scale-Modified (MAS) [50] is used by clinicians to assess the presence and degree of spasticity on a 5 point scale.
- The Medical Research Council (MRC) scale [51] is used to assess the muscular strength of the right and left legs in movements involving: the flexion, extension and abduction of the hips; the extension of the knee and the dorsal and plantar flexion of the ankle.
2.3.2. Lower Limbs Crossmodal Congruency Task (LLCCT)
2.3.3. Body Sidedness Task (BST)
2.4. Procedure
2.5. Data Handling and Statistical Analysis
2.5.1. VMIQ-2, PSFS and Clinical Data Analysis
2.5.2. LLCCT Analyses
2.5.3. BST Analyses
3. Results
3.1. General Imagery Ability—VMIQ-2, PSFS and Clinical Data Results
3.2. LLCCT Results
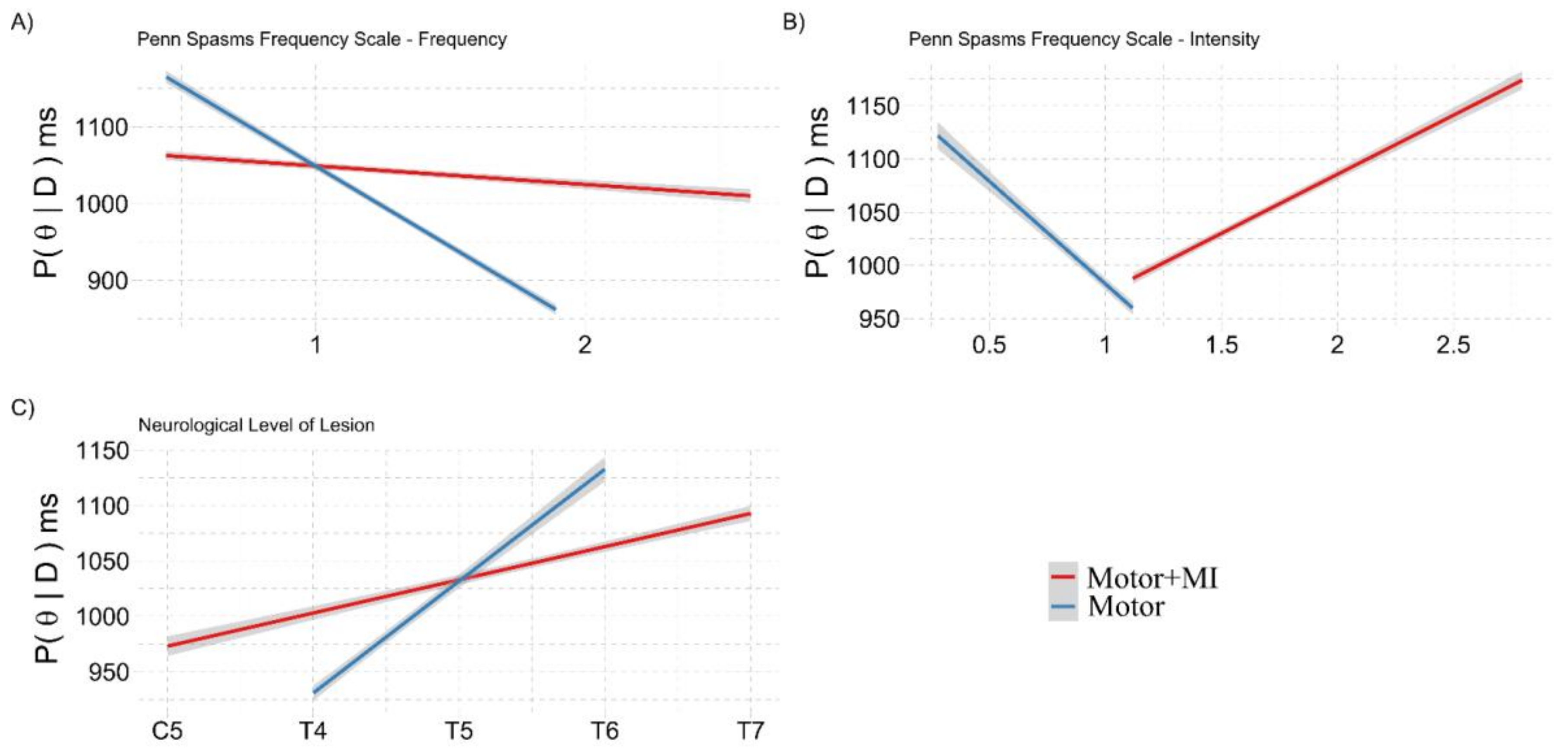
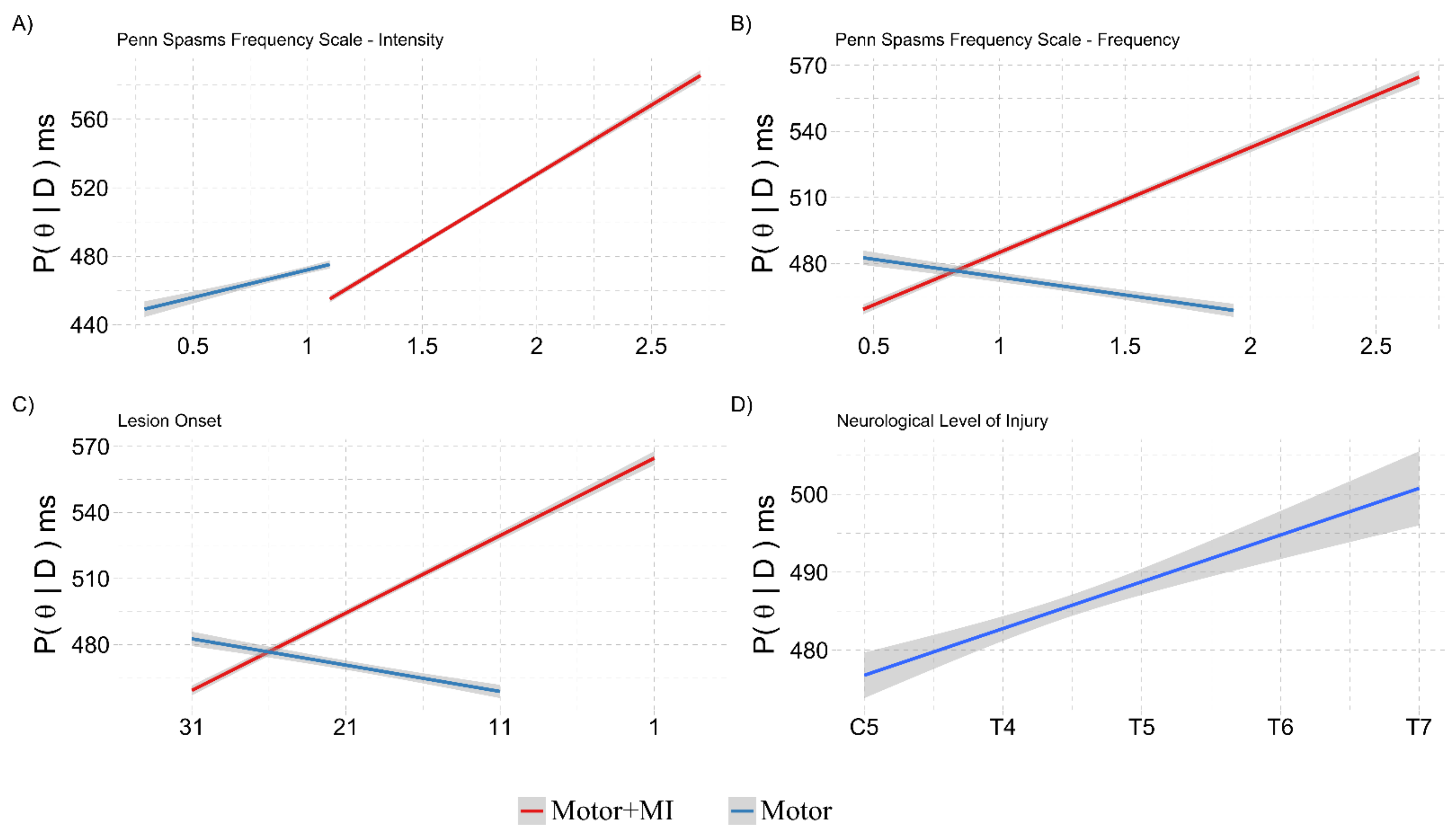
3.2.1. Covariation with NLI, Lesion Onset, PSFS and VMIQ-2
3.2.2. Ad-Interim Discussion
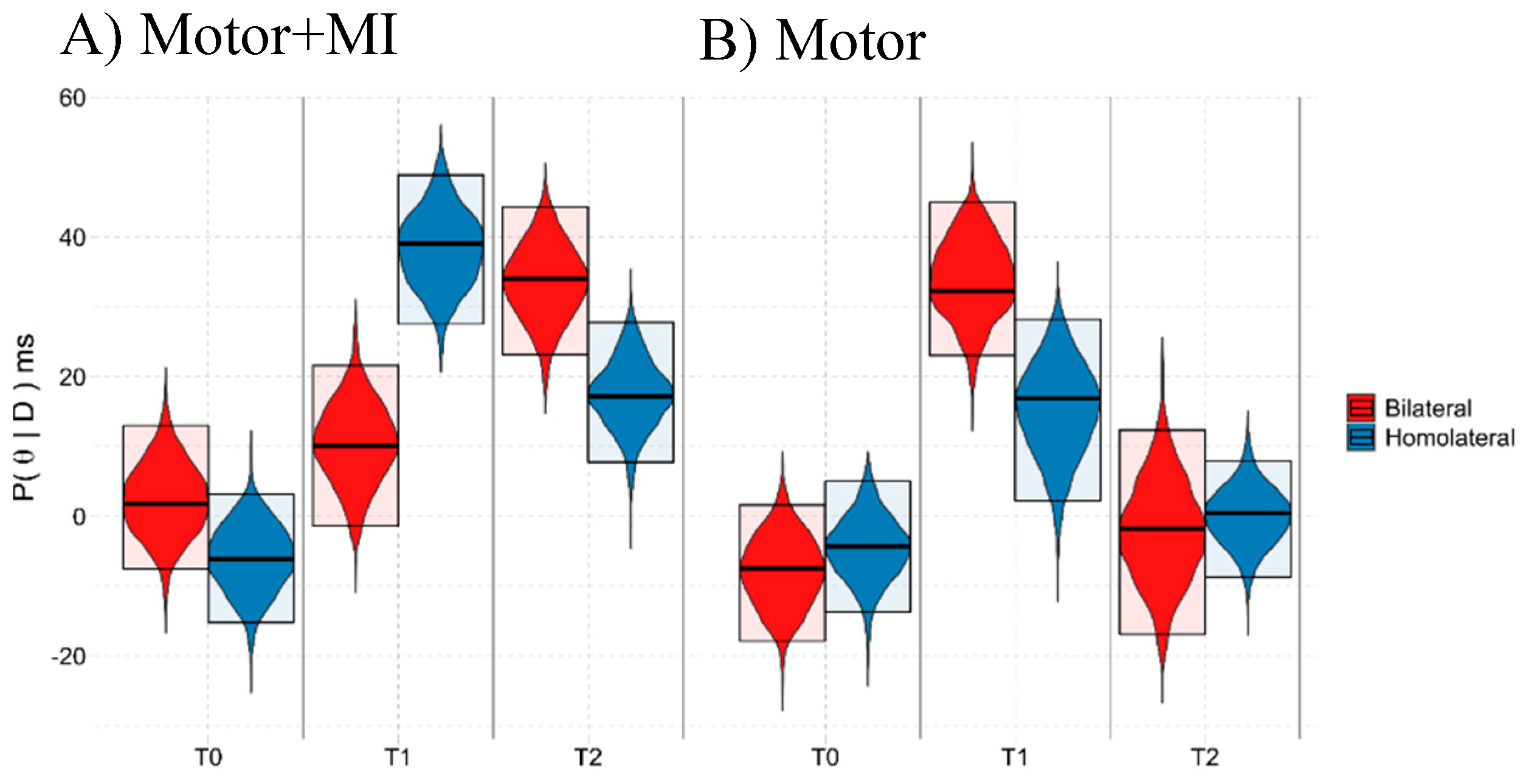
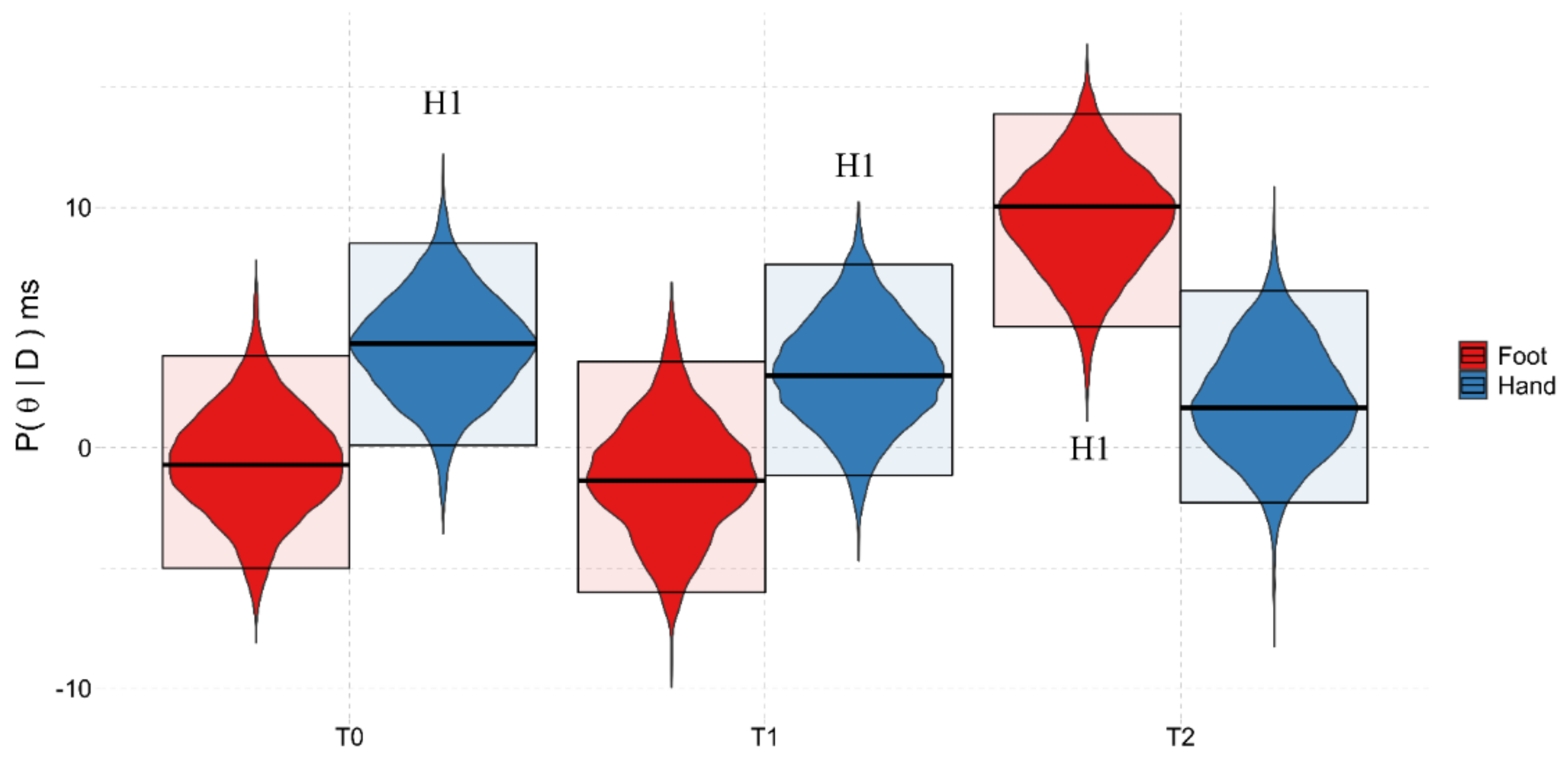
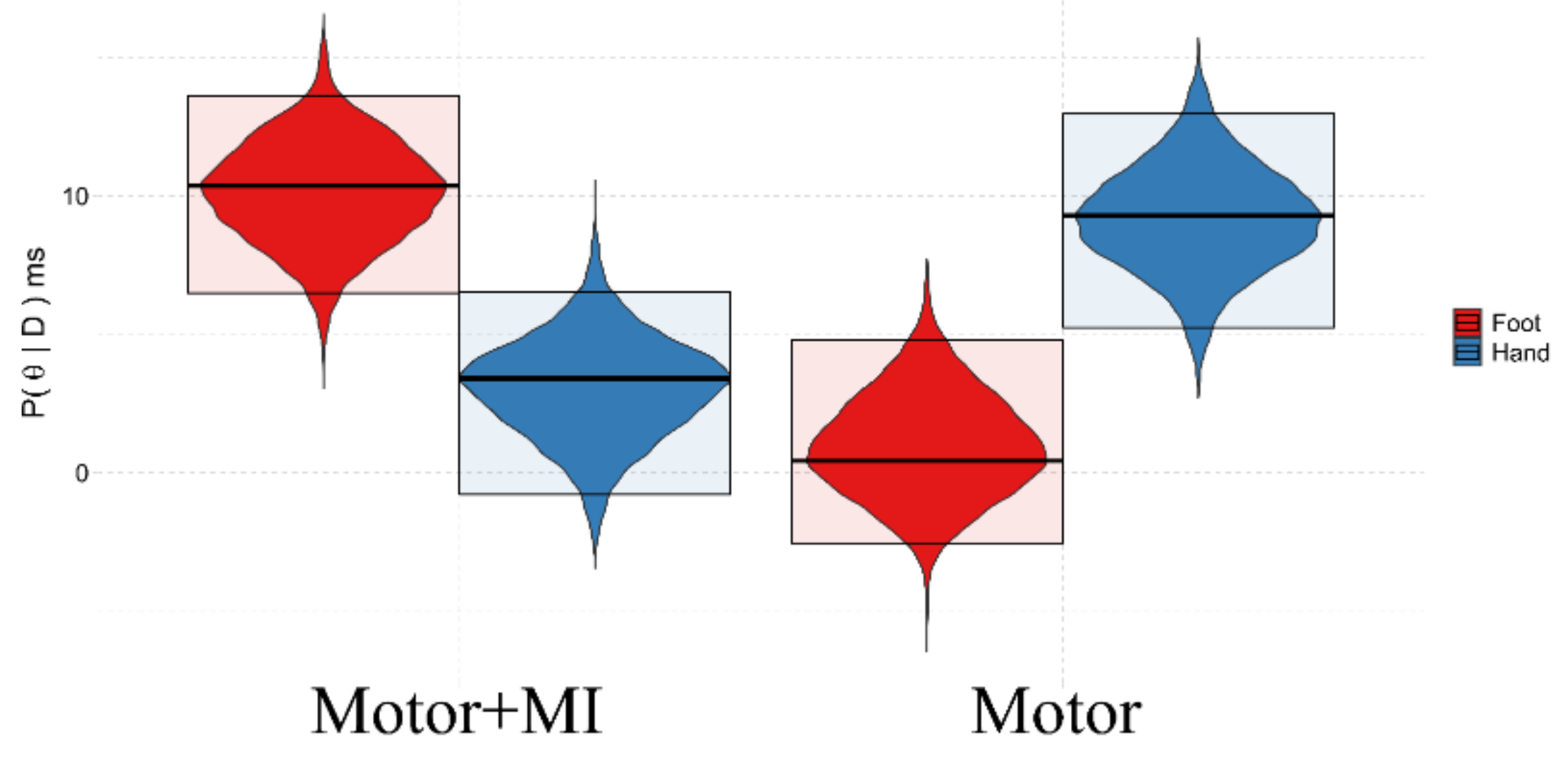
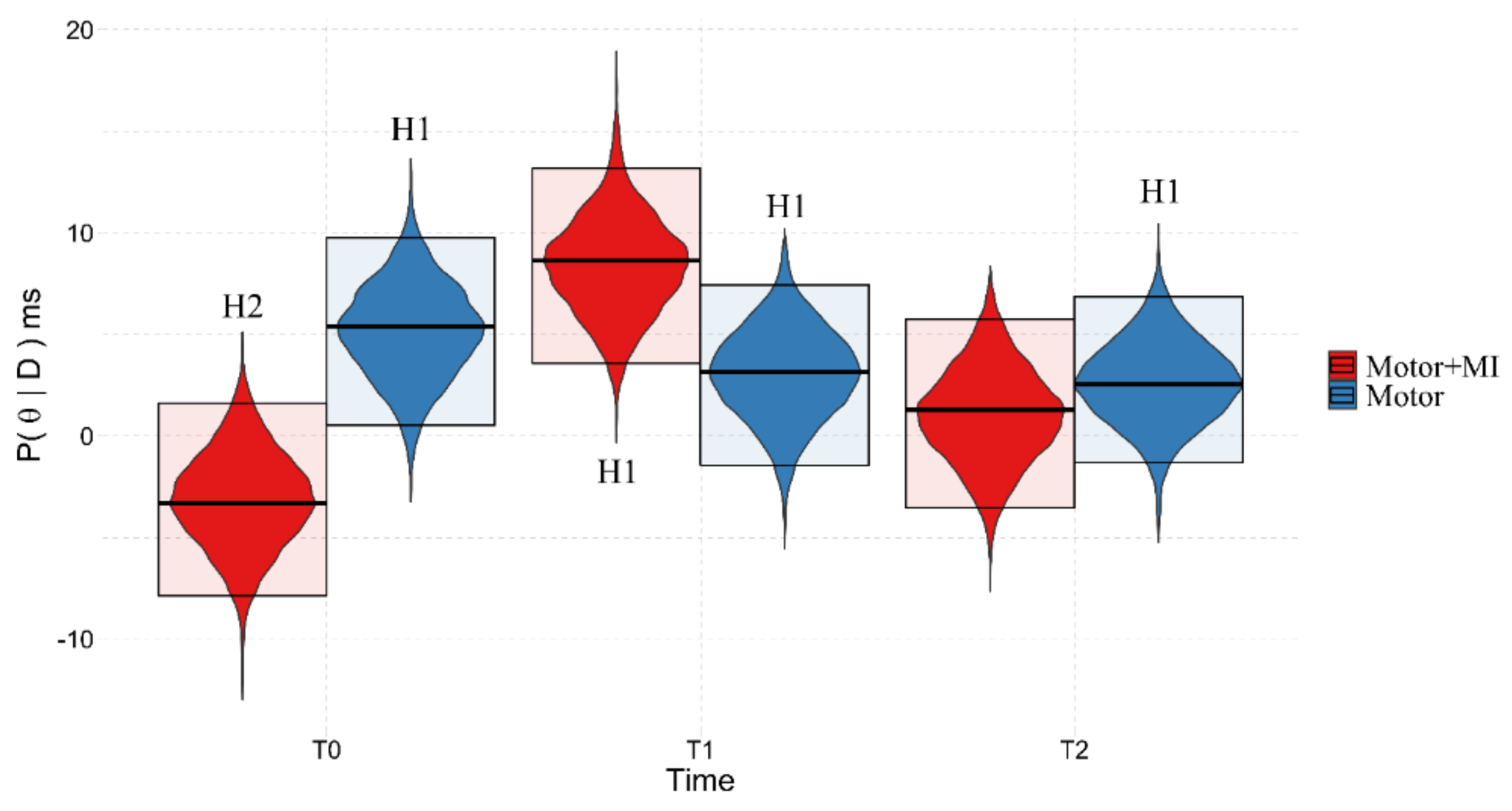
3.3. BST Results
3.3.1. Background:Time2
3.3.2. Background:Group
3.3.3. Group:Time2
3.3.4. Covariations with NLI, Lesion Onset, PSFS and VMIQ-2
3.3.5. Ad-Interim Discussion
4. Discussion
4.1. The Effects of Training on PPS
4.2. The Effects of Training on Body Representations
4.3. Pathological Below-Lesion Sensations and Better Clinical Scores Facilitate Body and Peripersonal Space Recovery in MI Training
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix B

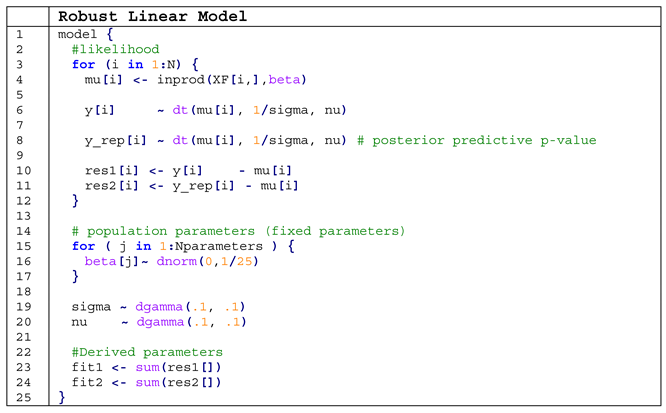
Appendix C

- computing the average of reaction times we are implicitly assuming that they are normally distributed, when they are not [83];
- also when computing the differences between the averages, we do not consider the whole data set, with the consequence that these averages are more prone to outliers and the power of the analysis is thus weaker (1 − β).
If Congruent trials:
μ = Xβ + Zξ with β being the population- and ξ the group-level effects,
If Incongruent trails:
μ = X(βCongruency Effect + βCongruent Trials)+ Zξ
λ ~ Uniform (0.01, 100),
ξ ~ MultiGaussian (ξμ, Ω),
ξμ ~ Gaussian(0, 1000),
Appendix D
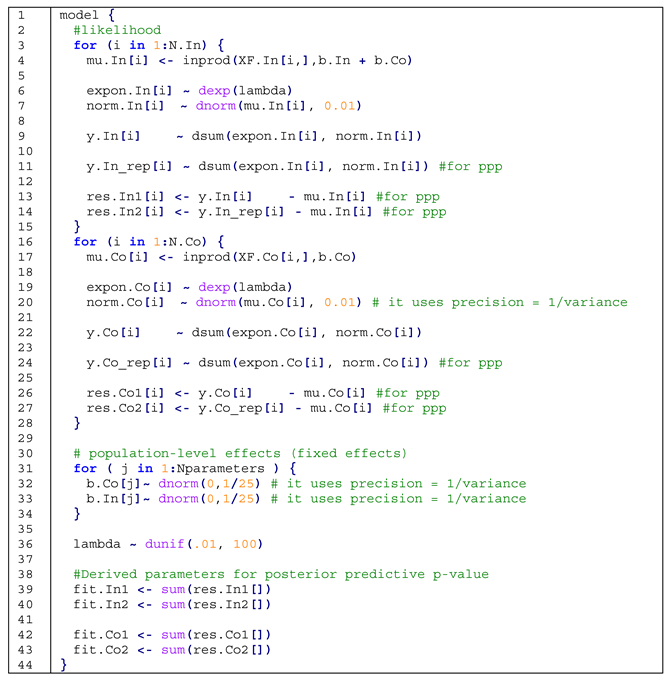
Appendix E
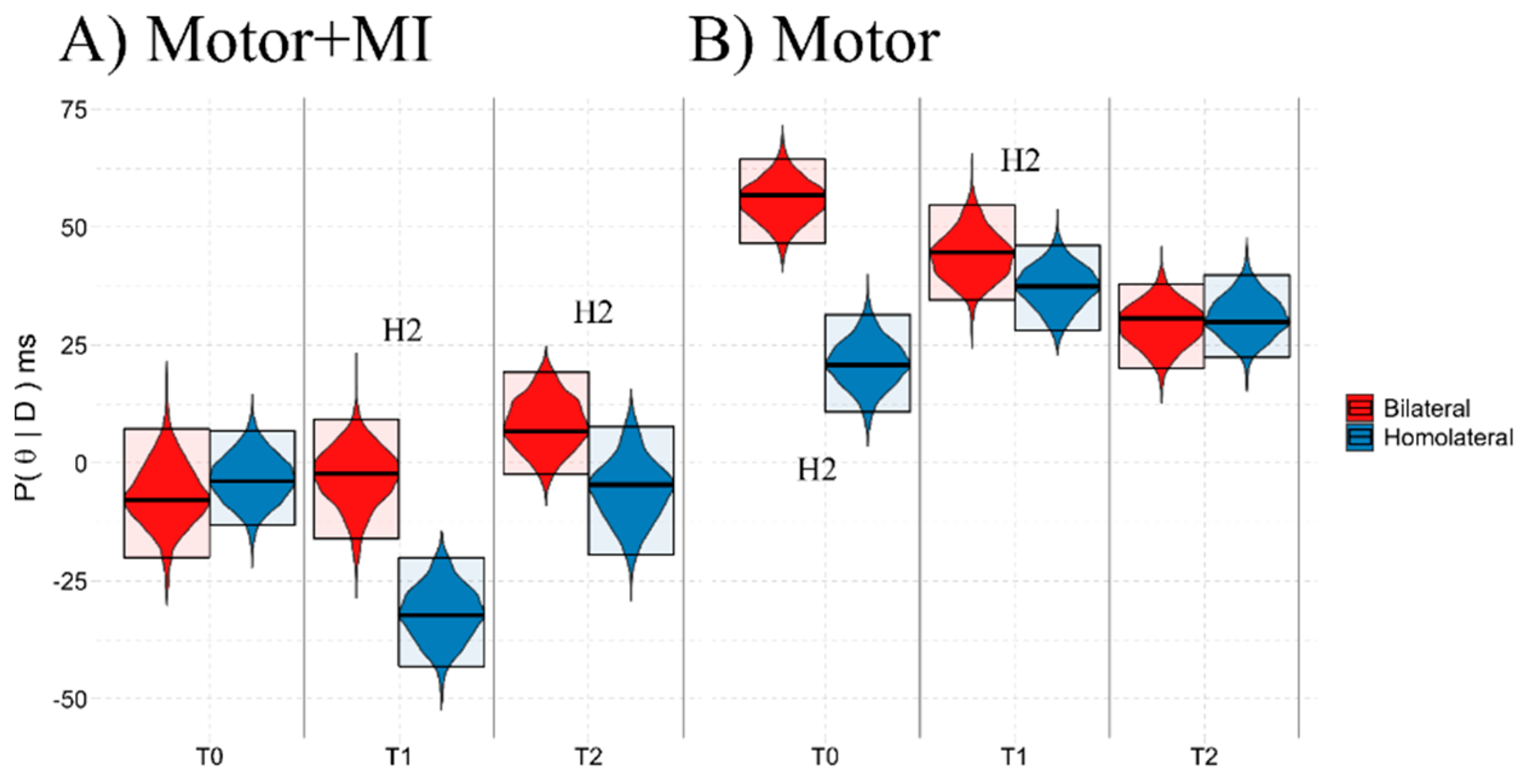
References
- Jackson, P.; Lafleur, M.F.; Malouin, F.; Richards, C.L.; Doyon, J. Potential role of mental practice using motor imagery in neurologic rehabilitation. Arch. Phys. Med. Rehabil. 2001, 82, 1133–1141. [Google Scholar] [CrossRef]
- Bonda, E.; Petrides, M.; Frey, S.; Evans, A. Neural correlates of mental transformations of the body-in-space. Proc. Natl. Acad. Sci. USA 1995, 92, 11180–11184. [Google Scholar] [CrossRef] [Green Version]
- Corradi-Dell’Acqua, C.; Tomasino, B.; Fink, G.R. What Is the Position of an Arm Relative to the Body? Neural Correlates of Body Schema and Body Structural Description. J. Neurosci. 2009, 29, 4162–4171. [Google Scholar] [CrossRef] [PubMed]
- Decety, J. Neural Representations for Action. Rev. Neurosci. 1996, 7, 285–297. [Google Scholar] [CrossRef]
- Gerardin, E.; Sirigu, A.; Lehéricy, S.; Poline, J.-B.; Gaymard, B.; Marsault, C.; Agid, Y.; Le Bihan, D. Partially Overlapping Neural Networks for Real and Imagined Hand Movements. Cereb. Cortex 2000, 10, 1093–1104. [Google Scholar] [CrossRef]
- Perruchoud, D.; Michels, L.; Piccirelli, M.; Gassert, R.; Ionta, S. Differential neural encoding of sensorimotor and visual body representations. Sci. Rep. 2016, 6, 37259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersen, R.A.; Hwang, E.J.; Mulliken, G.H. Cognitive Neural Prosthetics. Annu. Rev. Psychol. 2010, 61, 169–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conson, M.; Sacco, S.; Sarà, M.; Pistoia, F.; Grossi, D.; Trojano, L. Selective motor imagery defect in patients with locked-in syndrome. Neuropsychologia 2008, 46, 2622–2628. [Google Scholar] [CrossRef]
- Fiori, F.; Sedda, A.; Ferrè, E.R.; Toraldo, A.; Querzola, M.; Pasotti, F.; Ovadia, D.; Piroddi, C.; Dell’Aquila, R.; Lunetta, C.; et al. Exploring motor and visual imagery in Amyotrophic Lateral Sclerosis. Exp. Brain Res. 2013, 226, 537–547. [Google Scholar] [CrossRef]
- Fiorio, M.; Aglioti, S.M.; Tinazzi, M. Selective impairment of hand mental rotation in patients with focal hand dystonia. Brain 2006, 129, 47–54. [Google Scholar] [CrossRef] [Green Version]
- Coslett, H.B.; Medina, J.; Kliot, D.; Burkey, A.R. Mental motor imagery indexes pain: The hand laterality task. Eur. J. Pain 2010, 14, 1007–1013. [Google Scholar] [CrossRef]
- Schwoebel, J.; Friedman, R.; Duda, N.; Coslett, H.B. Pain and the body schema: Evidence for peripheral effects on mental representations of movement. Brain 2001, 124, 2098–2104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirshblum, S.C.; Burns, S.P.; Biering-Sørensen, F.; Donovan, W.; Graves, D.; Jha, A.; Johansen, M.; Jones, L.; Krassioukov, A.; Mulcahey, M.; et al. International standards for neurological classification of spinal cord injury (Revised 2011). J. Spinal Cord Med. 2011, 34, 535–546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferro, S.; Cecconi, L.; Bonavita, J.; Pagliacci, M.C.; Biggeri, A.; Franceschini, M. Incidence of traumatic spinal cord injury in Italy during 2013–2014: A population-based study. Spinal Cord 2017, 55, 1103–1107. [Google Scholar] [CrossRef] [PubMed]
- Dahlberg, L.S.; Becerra, L.; Borsook, D.; Linnman, C. Brain changes after spinal cord injury, a quantitative meta-analysis and review. Neurosci. Biobehav. Rev. 2018, 90, 272–293. [Google Scholar] [CrossRef] [PubMed]
- Nardone, R.; Höller, Y.; Brigo, F.; Seidl, M.; Christova, M.; Bergmann, J.; Golaszewski, S.; Trinka, E. Functional brain reorganization after spinal cord injury: Systematic review of animal and human studies. Brain Res. 2013, 1504, 58–73. [Google Scholar] [CrossRef]
- Kokotilo, K.J.; Eng, J.J.; Curt, A. Reorganization and preservation of motor control of the brain in spinal cord injury: A systematic review. J. Neurotrauma 2009, 26, 2113–2126. [Google Scholar] [CrossRef] [Green Version]
- Lenggenhager, B.; Pazzaglia, M.; Scivoletto, G.; Molinari, M.; Aglioti, S.M. The Sense of the Body in Individuals with Spinal Cord Injury. PLoS ONE 2012, 7, e50757. [Google Scholar] [CrossRef] [Green Version]
- Tidoni, E.; Grisoni, L.; Liuzza, M.T.; Aglioti, S.M. Rubber hand illusion highlights massive visual capture and sensorimotor face-hand remapping in a tetraplegic man. Restor. Neurol. Neurosci. 2014, 32, 611–622. [Google Scholar] [CrossRef] [Green Version]
- Scandola, M.; Togni, R.; Tieri, G.; Avesani, R.; Brambilla, M.; Aglioti, S.M.; Moro, V. Embodying their own wheelchair modifies extrapersonal space perception in people with spinal cord injury. Exp. Brain Res. 2019, 237, 2621–2632. [Google Scholar] [CrossRef] [PubMed]
- Scandola, M.; Aglioti, S.M.; Avesani, R.; Bertagnoni, G.; Marangoni, A.; Moro, V. Corporeal illusions in chronic spinal cord injuries. Conscious. Cogn. 2017, 49, 278–290. [Google Scholar] [CrossRef] [PubMed]
- Fusco, G.; Tidoni, E.; Barone, N.; Pilati, C.; Aglioti, S.M. Illusion of arm movement evoked by tendon vibration in patients with spinal cord injury. Restor. Neurol. Neurosci. 2016, 34, 815–826. [Google Scholar] [CrossRef]
- Arrighi, R.; Cartocci, G.; Burr, D. Reduced perceptual sensitivity for biological motion in paraplegia patients. Curr. Biol. 2011, 21, R910–R911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scandola, M.; Aglioti, S.M.; Avesani, R.; Bertagnoni, G.; Marangoni, A.; Moro, V. Anticipation of wheelchair and rollerblade actions in spinal cord injured people, rollerbladers, and physiotherapists. PLoS ONE 2019, 14, e0213838. [Google Scholar] [CrossRef] [Green Version]
- Scandola, M.; Aglioti, S.M.; Bonente, C.; Avesani, R.; Moro, V. Spinal cord lesions shrink peripersonal space around the feet, passive mobilization of paraplegic limbs restores it. Sci. Rep. 2016, 6, 24126. [Google Scholar] [CrossRef] [Green Version]
- Scandola, M.; Aglioti, S.M.; Lazzeri, G.; Avesani, R.; Ionta, S.; Moro, V. Visuo-motor and interoceptive influences on peripersonal space representation following spinal cord injury. Sci. Rep. 2020, 10, 5162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sedda, A.; Ambrosini, E.; Dirupo, G.; Tonin, D.; Valsecchi, L.; Redaelli, T.; Spinelli, M.; Costantini, M.; Bottini, G. Affordances after spinal cord injury. J. Neuropsychol. 2018, 13, 354–369. [Google Scholar] [CrossRef] [Green Version]
- Thomschewski, A.; Ströhlein, A.; Langthaler, P.B.; Schmid, E.; Potthoff, J.; Höller, P.; Leis, S.; Trinka, E.; Höller, Y. Imagine There Is No Plegia. Mental Motor Imagery Difficulties in Patients with Traumatic Spinal Cord Injury. Front. Neurosci. 2017, 11, 689. [Google Scholar] [CrossRef] [Green Version]
- Scandola, M.; Aglioti, S.M.; Pozeg, P.; Avesani, R.; Moro, V. Motor imagery in spinal cord injured people is modulated by somatotopic coding, perspective taking, and post-lesional chronic pain. J. Neuropsychol. 2017, 11, 305–326. [Google Scholar] [CrossRef]
- Fiori, F.; Sedda, A.; Ferrè, E.R.; Toraldo, A.; Querzola, M.; Pasotti, F.; Ovadia, D.; Piroddi, C.; Dell’Aquila, R.; Redaelli, T.; et al. Motor imagery in spinal cord injury patients: Moving makes the difference. J. Neuropsychol. 2014, 8, 199–215. [Google Scholar] [CrossRef]
- LaCourse, M. Cortical potentials during imagined movements in individuals with chronic spinal cord injuries. Behav. Brain Res. 1999, 104, 73–88. [Google Scholar] [CrossRef]
- Alkadhi, H.; Brugger, P.; Boendermaker, S.H.; Crelier, G.; Curt, A.; Hepp-Reymond, M.-C.; Kollias, S.S. What Disconnection Tells about Motor Imagery: Evidence from Paraplegic Patients. Cereb. Cortex 2005, 15, 131–140. [Google Scholar] [CrossRef] [Green Version]
- Cramer, S.C.; Orr, E.L.R.; Cohen, M.J.; LaCourse, M.G. Effects of motor imagery training after chronic, complete spinal cord injury. Exp. Brain Res. 2007, 177, 233–242. [Google Scholar] [CrossRef] [Green Version]
- Hotz, S.; Funk, M.; Summers, P.; Brugger, P.; Hepp-Reymond, M.-C.; Curt, A.; Kollias, S.S. Preservation of motor programs in paraplegics as demonstrated by attempted and imagined foot movements. NeuroImage 2008, 39, 383–394. [Google Scholar] [CrossRef]
- Athanasiou, A.; Terzopoulos, N.; Pandria, N.; Xygonakis, I.; Foroglou, N.; Polyzoidis, K.; Bamidis, P.D. Functional Brain Connectivity during Multiple Motor Imagery Tasks in Spinal Cord Injury. Neural Plast. 2018, 2018, 9354207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conson, M.; Volpicella, F.; De Bellis, F.; Orefice, A.; Trojano, L. “Like the palm of my hands”: Motor imagery enhances implicit and explicit visual recognition of one‘s own hands. Acta Psychol. 2017, 180, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Zeugin, D.; Notter, M.P.; Knebel, J.-F.; Ionta, S. Temporo-parietal contribution to the mental representations of self/other face. Brain Cogn. 2020, 143, 105600. [Google Scholar] [CrossRef]
- Serino, A. Peripersonal space (PPS) as a multisensory interface between the individual and the environment, defining the space of the self. Neurosci. Biobehav. Rev. 2019, 99, 138–159. [Google Scholar] [CrossRef] [PubMed]
- Bufacchi, R.; Iannetti, G.D. An Action Field Theory of Peripersonal Space. Trends Cogn. Sci. 2018, 22, 1076–1090. [Google Scholar] [CrossRef] [Green Version]
- Biggio, M.; Bisio, A.; Avanzino, L.; Ruggeri, P.; Bove, M. Familiarity with a Tool Influences Peripersonal Space and Primary Motor Cortex Excitability of Muscles Involved in Haptic Contact. Cereb. Cortex Commun. 2020, 1. [Google Scholar] [CrossRef] [PubMed]
- Zapparoli, L.; Sacheli, L.M.; Seghezzi, S.; Preti, M.; Stucovitz, E.; Negrini, F.; Pelosi, C.; Ursino, N.; Banfi, G.; Paulesu, E. Motor imagery training speeds up gait recovery and decreases the risk of falls in patients submitted to total knee arthroplasty. Sci. Rep. 2020, 10, 8917. [Google Scholar] [CrossRef]
- Debarnot, U.; Di Rienzo, F.; Daligault, S.; Schwartz, S. Motor Imagery Training During Arm Immobilization Prevents Corticomotor Idling: An EEG Resting-State Analysis. Brain Topogr. 2020, 33, 327–335. [Google Scholar] [CrossRef]
- Guerra, Z.F.; Lucchetti, A.L.G.; Lucchetti, G. Motor Imagery Training After Stroke: A Systematic Review and Meta-analysis of Randomized Controlled Trials. J. Neurol. Phys. Ther. 2017, 41, 205–214. [Google Scholar] [CrossRef]
- Emateo, S.; Rienzo, F.E.; Ebergeron, V.; Eguillot, A.; Ecollet, C.; Erode, G. Motor imagery reinforces brain compensation of reach-to-grasp movement after cervical spinal cord injury. Front. Behav. Neurosci. 2015, 9, 234. [Google Scholar] [CrossRef]
- Di Rienzo, F.; Guillot, A.; Mateo, S.; Daligault, S.; Delpuech, C.; Rode, G.; Collet, C. Neuroplasticity of prehensile neural networks after quadriplegia. Neuroscience 2014, 274, 82–92. [Google Scholar] [CrossRef]
- Opsommer, E.; Chevalley, O.; Korogod, N. Motor imagery for pain and motor function after spinal cord injury: A systematic review. Spinal Cord 2020, 58, 262–274. [Google Scholar] [CrossRef]
- Scandola, M.; Dodoni, L.; Lazzeri, G.; Arcangeli, C.A.; Avesani, R.; Moro, V.; Ionta, S. Neurocognitive Benefits of Physiotherapy for Spinal Cord Injury. J. Neurotrauma 2019, 36, 2028–2035. [Google Scholar] [CrossRef]
- Ditunno, J.F.; Young, W.; Donovan, W.H.; Creasey, G. The International Standards Booklet for Neurological and Functional Classification of Spinal Cord Injury. Spinal Cord 1994, 32, 70–80. [Google Scholar] [CrossRef] [Green Version]
- Penn, R.D.; Savoy, S.M.; Corcos, D.; Latash, M.; Gottlieb, G.; Parke, B.; Kroin, J.S. Intrathecal Baclofen for Severe Spinal Spasticity. N. Engl. J. Med. 1989, 320, 1517–1521. [Google Scholar] [CrossRef]
- Ashworth, B. Preliminary trial of carisoprodal in multiple sclerosis. Practitioner 1964, 192, 540–542. [Google Scholar]
- Florence, J.M.; Pandya, S.; King, W.M.; Robison, J.D.; Baty, J.; Miller, J.P.; Schierbecker, J.; Signore, L.C. Intrarater Reliability of Manual Muscle Test (Medical Research Council Scale) Grades in Duchenne’s Muscular Dystrophy. Phys. Ther. 1992, 72, 115–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tessari, A.; Ottoboni, G.; Baroni, G.; Symes, E.; Nicoletti, R. Is access to the body structural description sensitive to a body part’s significance for action and cognition? A study of the sidedness effect using feet. Exp. Brain Res. 2012, 218, 515–525. [Google Scholar] [CrossRef]
- Ottoboni, G.; Tessari, A.; Cubelli, R.; Umiltà, C. Is Handedness Recognition Automatic? A Study Using a Simon-Like Paradigm. J. Exp. Psychol. Hum. Percept. Perform. 2005, 31, 778–789. [Google Scholar] [CrossRef]
- Schicke, T.; Bauer, F.; Röder, B.; Heed, T. Interactions of different body parts in peripersonal space: How vision of the foot influences tactile perception at the hand. Exp. Brain Res. 2009, 192, 703–715. [Google Scholar] [CrossRef]
- Roberts, R.; Callow, N.; Hardy, L.; Markland, D.; Bringer, J. Movement Imagery Ability: Development and Assessment of a Revised Version of the Vividness of Movement Imagery Questionnaire. J. Sport Exerc. Psychol. 2008, 30, 200–221. [Google Scholar] [CrossRef] [Green Version]
- Isaac, A.; Marks, D.F.; Russell, D.G. An instrument for assessing imagery of movement: The Vividness of Movement Imagery Questionnaire (VMIQ). J. Ment. Imag. 1986, 10, 23–30. [Google Scholar] [CrossRef]
- Ionta, S.; Fourkas, A.D.; Fiorio, M.; Aglioti, S.M. The influence of hands posture on mental rotation of hands and feet. Exp. Brain Res. 2007, 183, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Ionta, S.; Fourkas, A.D.; Aglioti, S.M. Egocentric and object-based transformations in the laterality judgement of human and animal faces and of non-corporeal objects. Behav. Brain Res. 2010, 207, 452–457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moro, V.; Pernigo, S.; Zapparoli, P.; Cordioli, Z.; Aglioti, S.M. Phenomenology and neural correlates of implicit and emergent motor awareness in patients with anosognosia for hemiplegia. Behav. Brain Res. 2011, 225, 259–269. [Google Scholar] [CrossRef]
- Pavani, F.; Spence, C.; Driver, J. Visual Capture of Touch: Out-of-the-Body Experiences with Rubber Gloves. Psychol. Sci. 2000, 11, 353–359. [Google Scholar] [CrossRef]
- Maravita, A.; Spence, C.; Kennett, S.; Driver, J. Tool-use changes multimodal spatial interactions between vision and touch in normal humans. Cognition 2002, 83, B25–B34. [Google Scholar] [CrossRef]
- Marini, F.; Romano, D.; Maravita, A. The contribution of response conflict, multisensory integration, and body-mediated attention to the crossmodal congruency effect. Exp. Brain Res. 2017, 235, 873–887. [Google Scholar] [CrossRef]
- Spence, C.; Pavani, F.; Driver, J. Spatial constraints on visual-tactile cross-modal distractor congruency effects. Cogn. Affect. Behav. Neurosci. 2004, 4, 148–169. [Google Scholar] [CrossRef]
- Maravita, A.; Husain, M.; Clarke, K.; Driver, J. Reaching with a tool extends visual–tactile interactions into far space: Evidence from cross-modal extinction. Neuropsychologia 2001, 39, 580–585. [Google Scholar] [CrossRef]
- Maravita, A.; Iriki, A. Tools for the body (schema). Trends Cogn. Sci. 2004, 8, 79–86. [Google Scholar] [CrossRef]
- Spence, C.; Pavani, F.; Maravita, A.; Holmes, N.P. Multi-sensory interactions. In Haptic Rendering: Foundations, Algorithms, and Applications; Lin, M., Otaduy, M.A., Eds.; AK Peters: Wellesley, MA, USA, 2008; pp. 21–52. [Google Scholar] [CrossRef]
- Simon, J.R. Reactions toward the source of stimulation. J. Exp. Psychol. 1969, 81, 174–176. [Google Scholar] [CrossRef] [Green Version]
- de Laplace, P.-S. Essai Philosophique sur les Probabilités, 5th ed.; Bachelier: Paris, France, 1825; p. 288. [Google Scholar] [CrossRef] [Green Version]
- Gelman, A.; Carlin, J.B.; Stern, H.S.; Dunson, D.B.; Vehtari, A.; Rubin, D.B. Bayesian Data Analysis; CRC Press: Boca, FL, USA, 2013; p. 625. [Google Scholar] [CrossRef]
- Richard, M. Statistical Rethinking: A Bayesian Course with Examples in R and Stan, 1st ed.; Chapman and Hall/CRC: Boca Raton, FL, USA, 2016; p. 505. [Google Scholar] [CrossRef]
- Albert, J. Bayesian Computation with R; Springer: New York, NY, USA, 2009; ISBN 978-0-387-92297-3. [Google Scholar]
- Kruschke, J.K. Doing Bayesian Data Analysis: A Tutorial with R, JAGS, and Stan, 2nd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2014; ISBN 9780124058880. [Google Scholar]
- Dickey, J.M.; Lientz, B.P. The Weighted Likelihood Ratio, Sharp Hypotheses about Chances, the Order of a Markov Chain. Ann. Math. Stat. 1970, 41, 214–226. [Google Scholar] [CrossRef]
- Wagenmakers, E.-J.E. A practical solution to the pervasive problems of p values. Psychon. Bull. Rev. 2007, 14, 779–804. [Google Scholar] [CrossRef] [Green Version]
- Kooperberg, C. logspline: Routines for Logspline Density Estimation. R package version 2.1.15. 2019. Available online: https://CRAN.R-project.org/package=logspline (accessed on 8 September 2021).
- Raftery, A.E. Bayesian Model Selection in Social Research. In Sociological Methodology; Marsden, P.V., Ed.; Blackwells: Cambridge, UK, 1995; Volume 25, pp. 111–163. [Google Scholar]
- Schönbrodt, F.D.; Wagenmakers, E.-J.; Zehetleitner, M.; Perugini, M. Sequential hypothesis testing with Bayes factors: Efficiently testing mean differences. Psychol. Methods 2017, 22, 322–339. [Google Scholar] [CrossRef] [Green Version]
- Gelman, A. Two simple examples for understanding posterior p-values whose distributions are far from uniform. Electron. J. Stat. 2013, 7, 2595–2602. [Google Scholar] [CrossRef]
- Dienes, Z. How Do I Know What My Theory Predicts? Adv. Methods Pract. Psychol. Sci. 2019, 2. [Google Scholar] [CrossRef]
- Lodewyckx, T.; Kim, W.; Lee, M.D.; Tuerlinckx, F.; Kuppens, P.; Wagenmakers, E.-J. A tutorial on Bayes factor estimation with the product space method. J. Math. Psychol. 2011, 55, 331–347. [Google Scholar] [CrossRef] [Green Version]
- Palmer, E.M.; Horowitz, T.S.; Torralba, A.; Wolfe, J.M. What are the Shapes of Response Time Distributions in Visual Search? J. Exp. Psychol. Hum. Percept. Perform. 2011, 37, 58–71. [Google Scholar] [CrossRef] [Green Version]
- Ionta, S.; Villiger, M.; Jutzeler, C.R.; Freund, P.; Curt, A.; Gassert, R. Spinal cord injury affects the interplay between visual and sensorimotor representations of the body. Sci. Rep. 2016, 6, 20144. [Google Scholar] [CrossRef]
- Brozzoli, C.; Cardinali, L.; Pavani, F.; Farne, A. Action-specific remapping of peripersonal space. Neuropsychologia 2010, 48, 796–802. [Google Scholar] [CrossRef]
- Brozzoli, C.; Pavani, F.; Urquizar, C.; Cardinali, L.; Farnè, A. Grasping actions remap peripersonal space. NeuroReport 2009, 20, 913–917. [Google Scholar] [CrossRef]
- Serino, A.; Bassolino, M.; Farne’, A.; Ladavas, E. Extended Multisensory Space in Blind Cane Users. Psychol. Sci. 2007, 18, 642–648. [Google Scholar] [CrossRef]
- Zopf, R.; Savage, G.; Williams, M.A. The crossmodal congruency task as a means to obtain an objective behavioral measure in the rubber hand illusion paradigm. J. Vis. Exp. 2013, e50530. [Google Scholar] [CrossRef] [Green Version]
- Bassolino, M.; Serino, A.; Ubaldi, S.; Làdavas, E. Everyday use of the computer mouse extends peripersonal space representation. Neuropsychologia 2010, 48, 803–811. [Google Scholar] [CrossRef]
- Mine, D.; Yokosawa, K. Disconnected hand avatar can be integrated into the peripersonal space. Exp. Brain Res. 2021, 239, 237–244. [Google Scholar] [CrossRef]
- Bassolino, M.; Finisguerra, A.; Canzoneri, E.; Serino, A.; Pozzo, T. Dissociating effect of upper limb non-use and overuse on space and body representations. Neuropsychologia 2014, 70, 385–392. [Google Scholar] [CrossRef]
- Toussaint, L.; Wamain, Y.; Bidet-Ildei, C.; Coello, Y. Short-term upper-limb immobilization alters peripersonal space representation. Psychol. Res. 2020, 84, 907–914. [Google Scholar] [CrossRef] [PubMed]
- Canzoneri, E.; Marzolla, M.; Amoresano, A.; Verni, G.; Serino, A. Amputation and prosthesis implantation shape body and peripersonal space representations. Sci. Rep. 2013, 3, 2844. [Google Scholar] [CrossRef] [Green Version]
- Buxbaum, L.J.; Coslett, H.B. Specialised structural descriptions for human body parts: Evidence from autotopagnosia. Cogn. Neuropsychol. 2001, 18, 289–306. [Google Scholar] [CrossRef]
- Sirigu, A.; Grafman, J.; Bressler, K.; Sunderland, T. Multiple Representations Contribute to Body Knowledge Processing. Brain 1991, 114, 629–642. [Google Scholar] [CrossRef] [PubMed]
- Schwoebel, J.; Coslett, H.B. Evidence for Multiple, Distinct Representations of the Human Body. J. Cogn. Neurosci. 2005, 17, 543–553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ataria, Y.; Tanaka, S. When Body Image Takes over the Body Schema: The Case of Frantz Fanon. Hum. Stud. 2020, 43, 653–665. [Google Scholar] [CrossRef]
- Caggiano, P.; Cocchini, G. The functional body: Does body representation reflect functional properties? Exp. Brain Res. 2020, 238, 153–169. [Google Scholar] [CrossRef]
- Fuentes, C.T.; Pazzaglia, M.; Longo, M.; Scivoletto, G.; Haggard, P. Body image distortions following spinal cord injury. J. Neurol. Neurosurg. Psychiatry 2013, 84, 201–207. [Google Scholar] [CrossRef] [Green Version]
- Berlucchi, G.; Aglioti, S.M. The body in the brain revisited. Exp. Brain Res. 2010, 200, 25–35. [Google Scholar] [CrossRef]
- D’Imperio, D.; Bulgarelli, C.; Bertagnoli, S.; Avesani, R.; Moro, V. Modulating anosognosia for hemiplegia: The role of dangerous actions in emergent awareness. Cortex 2017, 92, 187–203. [Google Scholar] [CrossRef] [PubMed]
- Tsakiris, M. The multisensory basis of the self: From body to identity to others. Q. J. Exp. Psychol. 2017, 70, 597–609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van De Schoot, R.; Miocević, M. Small Sample Size Solutions: A Guide for Applied Researchers and Practitioners; Taylor & Francis: Milton Park, UK, 2020; p. 266. [Google Scholar]
- Plummer, M. JAGS: A Program For Analysis Of Bayesian Graphical Models Using Gibbs Sampling, Ver4.3.0. Available online: http://mcmc-jags.sourceforge.net/ (accessed on 8 September 2021).
- R Core Team. R: A Language and Environment for Statistical Computing Version 4.0.0. R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 8 September 2021).
- Kellner, K. jagsUI: A Wrapper Around ‘rjags’ to Streamline ‘JAGS’ Analyses. R Package Version 1.5.1. 2019. Available online: https://CRAN.R-project.org/package=jagsUI (accessed on 8 September 2021).
- Gilks, W.R.; Markov, M.; Carlo, M. Encyclopedia of Biostatistics; John Wiley & Sons, Ltd.: New York, NY, USA, 2005; ISBN 9780470011812. [Google Scholar]
- Gelman, A.; Rubin, D.B. Inference from Iterative Simulation Using Multiple Sequences. Stat. Sci. 1992, 7, 457–472. [Google Scholar] [CrossRef]
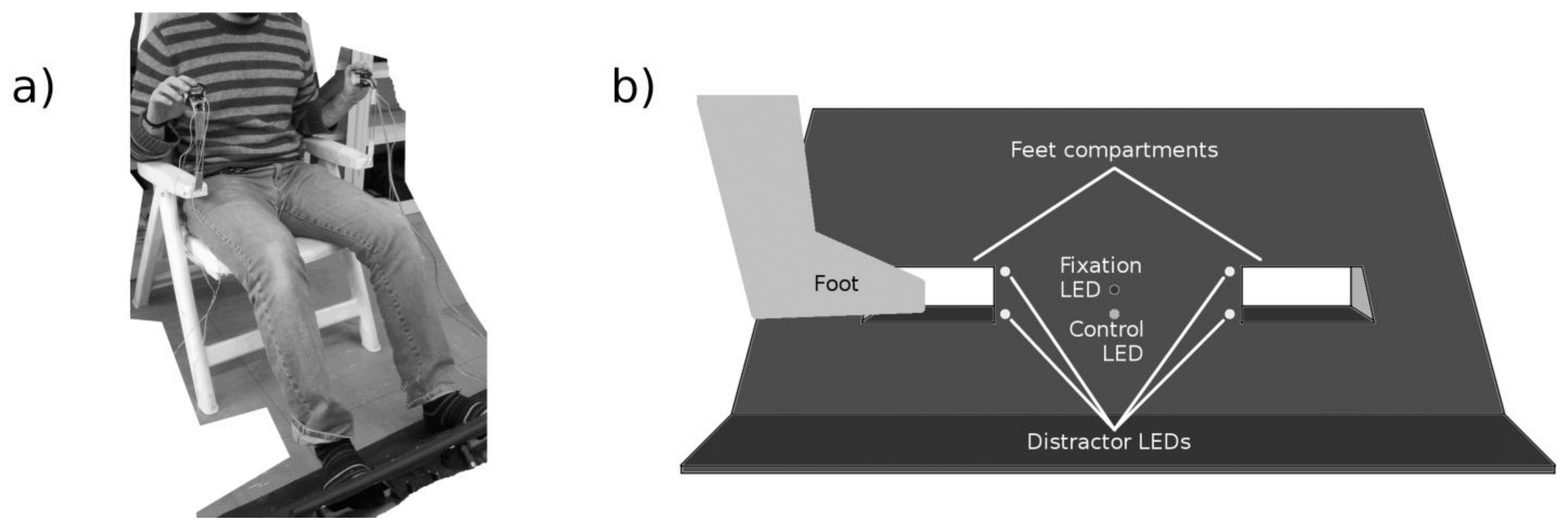
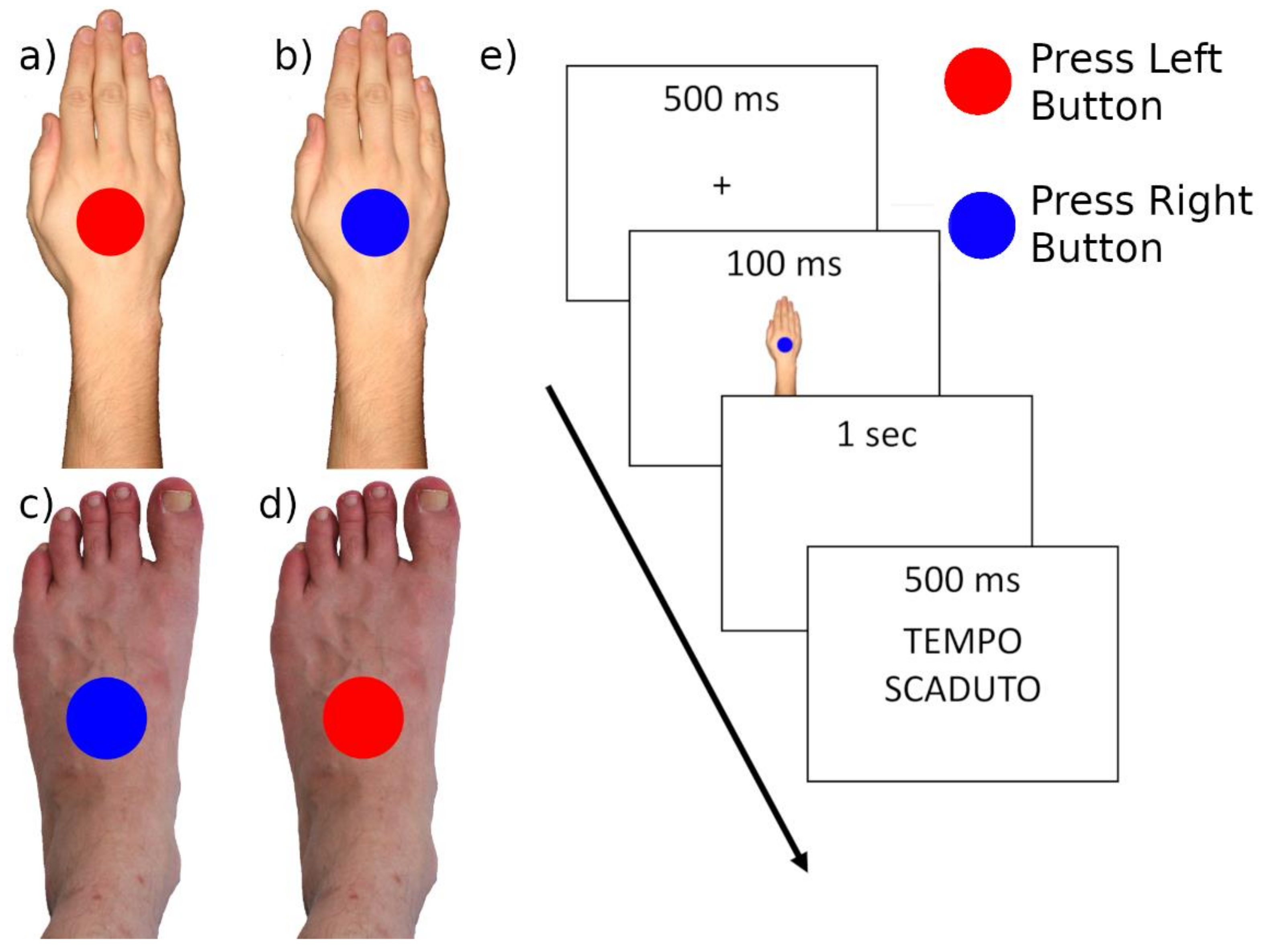
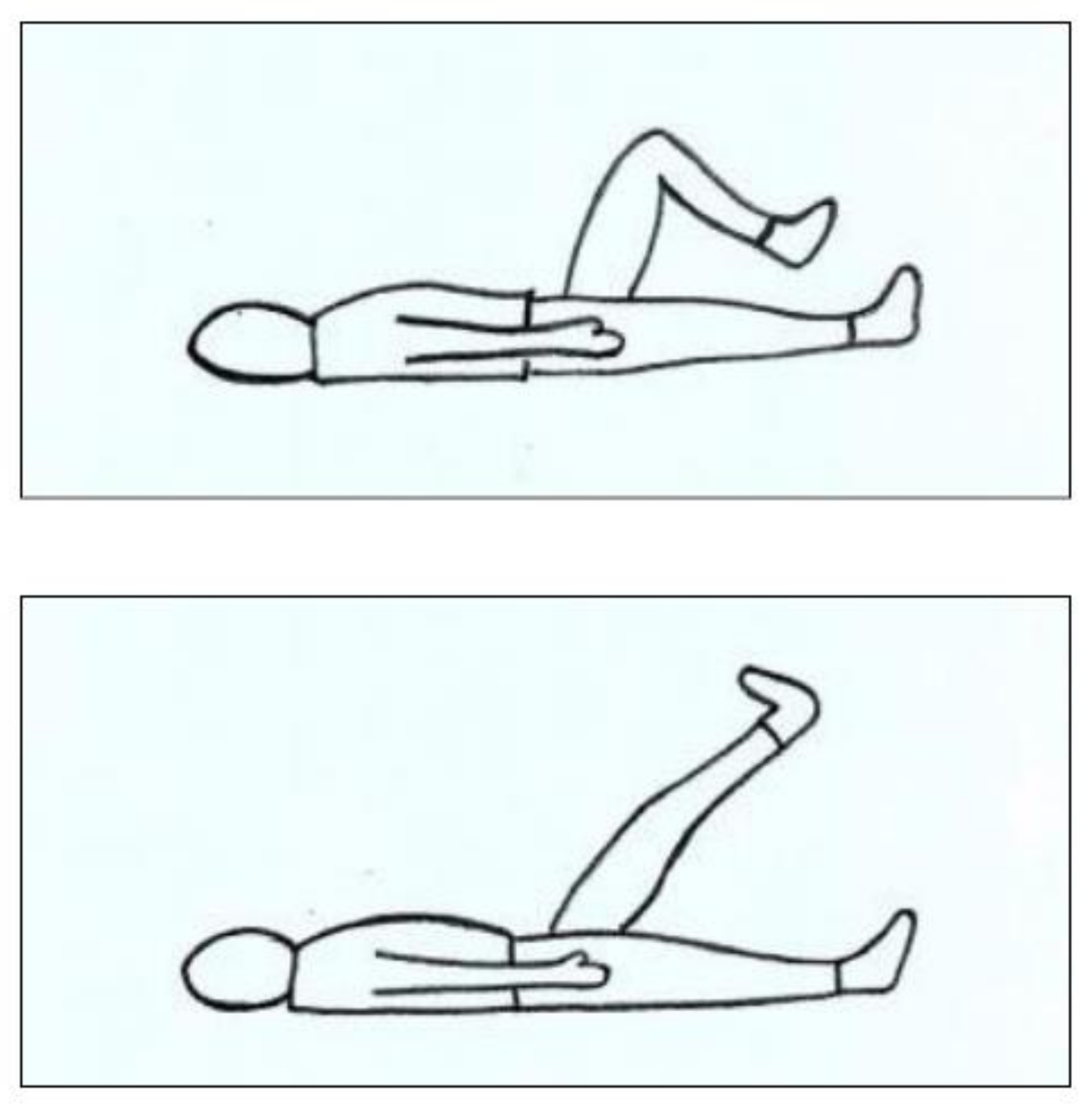
| ID | Age (Years) a | Lesion Onset (Years) b | N. Treat. c | NLI d | AIS e | Group f | Motor g | Gender h |
|---|---|---|---|---|---|---|---|---|
| Subj01 | 43 | 26.82 | 10 | T4 | A | Motor | EKSO | M |
| Subj02 | 37 | 8.83 | 10 | T4 | A | Motor + MI | EKSO | M |
| Subj03 | 54 | 30.05 | 10 | L1 | D | Motor | EKSO | M |
| Subj04 | 65 | 29.05 | 10 | T6 | A | Motor + MI | EKSO | M |
| Subj05 | 44 | 18.24 | 8 | T6 | A | Motor | EKSO | M |
| Subj06 | 44 | 28.31 | 8 | T7 | A | Motor | EKSO | M |
| Subj07 | 65 | 29.35 | 10 | T4 | A | Motor + MI | EKSO | M |
| Subj08 | 57 | 1.63 | 10 | C5 | C | Motor | EKSO | M |
| Subj09 | 44 | 27.40 | 8 | T4 | A | Motor + MI | Mobilisation | M |
| Subj10 | 65 | 29.54 | 9 | T6 | A | Motor | Mobilisation | M |
| Subj11 | 54 | 30.58 | 9 | L1 | D | Motor + MI | Mobilisation | M |
| Subj12 | 39 | 5.58 | 9 | T7 | A | Motor | Mobilisation | M |
| Subj13 | 44 | 26.58 | 8 | T6 | A | Motor | Mobilisation | F |
| Subj14 | 49 | 15.64 | 10 | T4 | A | Motor + MI | Mobilisation | F |
| Subj15 | 65 | 10.64 | 10 | T5 | A | Motor + MI | Mobilisation | M |
| Mean | 51.22 | 21.22 | 9.33 | T = 12 | A = 12 | Motor = 8 | EKSO = 8 | M = 13 |
| St. Dev. | 9.88 | 9.81 | 0.94 | L = 2 | C = 1 | Motor + MI = 7 | Mobilisation = 7 | F = 2 |
| C = 1 | D = 2 |
| (A) REAL Condition | Mode a | HDI b | neff c | Ȓ d | BF10 e | ||
|---|---|---|---|---|---|---|---|
| (Intercept) | 11.065 | 7.238 | 14.390 | 50 | 1.065 | >150 | H1 g |
| Space | 0.902 | −2.510 | 4.101 | 221 | 1.016 | 0.409 | |
| Training | −5.116 | −8.177 | −1.961 | 82 | 1.040 | >150 | H1 |
| Time | 11.022 | 6.572 | 16.483 | 208 | 1.014 | >150 | H1 |
| Time2 f | −15.906 | −22.293 | −11.308 | 135 | 1.019 | >150 | H1 |
| Space:Group | 1.051 | −2.027 | 5.046 | 36 | 1.088 | 0.422 | |
| Space:Time | 1.285 | −3.052 | 6.456 | 171 | 1.017 | 0.543 | |
| Space:Time2 f | 3.766 | −1.567 | 9.287 | 363 | 1.008 | 1.472 | |
| Group:Time | −8.082 | −12.878 | −3.068 | 51 | 1.058 | 53.021 | H1 |
| Group:Time2 f | −6.824 | −11.620 | −0.643 | 140 | 1.038 | 7.306 | H1 |
| Space:Group:Time | −0.748 | −6.254 | 3.970 | 92 | 1.036 | 0.642 | |
| Space:Group:Time2 f | −12.339 | −17.981 | −6.478 | 92 | 1.034 | >150 | H1 |
| (B) VOID Condition | Mode | HDI | neff | Ȓ | BF10 | ||
| (Intercept) | 15.081 | 11.298 | 18.621 | 49 | 1.075 | >150 | H1 |
| Space | 6.492 | 3.393 | 9.863 | 69 | 1.045 | >150 | H1 |
| Group | 21.305 | 18.552 | 25.146 | 44 | 1.073 | >150 | H1 |
| Time | −0.797 | −5.915 | 4.551 | 438 | 1.007 | 0.551 | |
| Time2 f | 3.886 | −1.416 | 9.363 | 306 | 1.009 | 1.3 | |
| Space:Group | 0.030 | −3.360 | 3.379 | 95 | 1.031 | 0.383 | |
| Space:Time | −3.198 | −8.014 | 1.354 | 307 | 1.011 | 1.341 | |
| Space:Time2 f | −2.388 | −7.872 | 2.251 | 36 | 1.086 | 0.911 | |
| Group:Time | −5.268 | −10.403 | −0.286 | 252 | 1.019 | 4.224 | |
| Group:Time2 f | −8.540 | −14.874 | −3.619 | 563 | 1.011 | 28.343 | H1 |
| Space:Group:Time | −9.152 | −14.173 | −4.731 | 109 | 1.031 | >150 | H1 |
| Space:Group:Time2 f | 6.894 | 2.015 | 11.763 | 208 | 1.014 | 16.964 | H1 |
| Mode a | HDI b | neff c | Ȓ d | BF10 e | |||
|---|---|---|---|---|---|---|---|
| (Intercept) | 78.867 | 69.795 | 87.956 | 73 | 1.045 | >150 | H1 |
| Group | 2.704 | −7.591 | 10.886 | 190 | 1.016 | 1.11 | |
| NLI f | −0.684 | −8.971 | 9.640 | 52 | 1.059 | 0.911 | |
| Lesion Onset | 0.349 | −9.810 | 9.942 | 30 | 1.100 | 1.038 | |
| PSFS–Frequency g | −0.733 | −10.907 | 7.794 | 67 | 1.045 | 1.013 | |
| PSFS–Intensity h | −1.163 | −9.808 | 9.617 | 32 | 1.093 | 1.031 | |
| VMIQ2 i | −0.481 | −11.213 | 9.385 | 151 | 1.022 | 1.035 | |
| Group: NLI | 25.995 | 16.680 | 34.995 | 23 | 1.152 | >150 | H1 |
| Group: Lesion Onset | −3.457 | −12.678 | 7.139 | 33 | 1.090 | 1.218 | |
| Group: PSFS–Frequency | 43.598 | 35.118 | 54.388 | 37 | 1.081 | >150 | H1 |
| Group: PSFS–Intensity | 10.226 | −0.297 | 19.338 | 18 | 1.199 | 5.574 | H1 |
| Group: VMIQ2 | −4.562 | −14.945 | 5.441 | 17 | 1.204 | 1.552 | |
| Mode a | HDI b | neff c | Ȓ d | BF10 e | |||
|---|---|---|---|---|---|---|---|
| Intercept | 3.103 | 1.046 | 4.636 | 9224 | 1.009 | 14.217 | H1 g |
| Background | −0.195 | −1.970 | 1.584 | 123 | 1.022 | 0.179 | H0 g |
| Group | 0.871 | −1.154 | 2.671 | 111 | 1.026 | 0.285 | |
| Time | 0.489 | −2.539 | 3.773 | 461 | 1.008 | 0.339 | |
| Time2 f | −3.917 | −6.656 | −0.300 | 1498 | 1.003 | 3.509 | |
| Background:Group | 3.771 | 1.920 | 5.579 | 1118 | 1.002 | >150 | H1 |
| Background:Time | −0.071 | −3.136 | 2.918 | 254 | 1.012 | 0.301 | |
| Background:Time2 f | −5.058 | −8.205 | −1.844 | 128 | 1.023 | 40.947 | H1 |
| Group:Time | −2.231 | −5.506 | 0.727 | 89 | 1.035 | 0.939 | |
| Group:Time2 f | 3.846 | 0.795 | 7.472 | 484 | 1.009 | 6.759 | H1 |
| Background:Group:Time | −1.705 | −4.785 | 1.386 | 186 | 1.014 | 0.554 | |
| Background:Group:Time2 f | −0.807 | −3.958 | 2.202 | 113 | 1.024 | 0.367 | |
| Mode a | HDI b | neff c | Ȓ d | BF10 e | |||
|---|---|---|---|---|---|---|---|
| (Intercept) | 58.370 | 50.798 | 66.666 | 227 | 1.015 | >150 | H1 |
| Group | 19.090 | 12.301 | 27.211 | 139 | 1.023 | >150 | H1 |
| NLI f | −16.028 | −23.255 | −7.589 | 474 | 1.007 | >150 | H1 |
| Lesion Onset | −4.523 | −12.051 | 3.437 | 38 | 1.079 | 1.455 | |
| PSFS–Frequency g | −10.990 | −18.301 | −3.227 | 82 | 1.034 | 31.577 | H1 |
| PSFS–Intensity h | −0.584 | −8.313 | 7.232 | 87 | 1.032 | 0.84 | |
| VMIQ2 i | −2.562 | −10.511 | 4.970 | 70 | 1.040 | 1.009 | |
| Group: NLI | 1.654 | −6.306 | 9.293 | 269 | 1.013 | 0.858 | |
| Group: Lesion Onset | 7.846 | 0.517 | 16.269 | 39 | 1.077 | 6.497 | H1 |
| Group: PSFS–Frequency | 9.857 | 1.792 | 17.298 | 72 | 1.038 | 16.731 | H1 |
| Group: PSFS–Intensity | −16.872 | −25.035 | −9.497 | 236 | 1.011 | >150 | H1 |
| Group: VMIQ2 | −7.348 | −15.040 | 0.324 | 93 | 1.031 | 4.232 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moro, V.; Corbella, M.; Ionta, S.; Ferrari, F.; Scandola, M. Cognitive Training Improves Disconnected Limbs’ Mental Representation and Peripersonal Space after Spinal Cord Injury. Int. J. Environ. Res. Public Health 2021, 18, 9589. https://doi.org/10.3390/ijerph18189589
Moro V, Corbella M, Ionta S, Ferrari F, Scandola M. Cognitive Training Improves Disconnected Limbs’ Mental Representation and Peripersonal Space after Spinal Cord Injury. International Journal of Environmental Research and Public Health. 2021; 18(18):9589. https://doi.org/10.3390/ijerph18189589
Chicago/Turabian StyleMoro, Valentina, Michela Corbella, Silvio Ionta, Federico Ferrari, and Michele Scandola. 2021. "Cognitive Training Improves Disconnected Limbs’ Mental Representation and Peripersonal Space after Spinal Cord Injury" International Journal of Environmental Research and Public Health 18, no. 18: 9589. https://doi.org/10.3390/ijerph18189589
APA StyleMoro, V., Corbella, M., Ionta, S., Ferrari, F., & Scandola, M. (2021). Cognitive Training Improves Disconnected Limbs’ Mental Representation and Peripersonal Space after Spinal Cord Injury. International Journal of Environmental Research and Public Health, 18(18), 9589. https://doi.org/10.3390/ijerph18189589









