Recurrence and Driving Factors of Visceral Leishmaniasis in Central China
Abstract
:1. Introduction
2. Materials and Methods
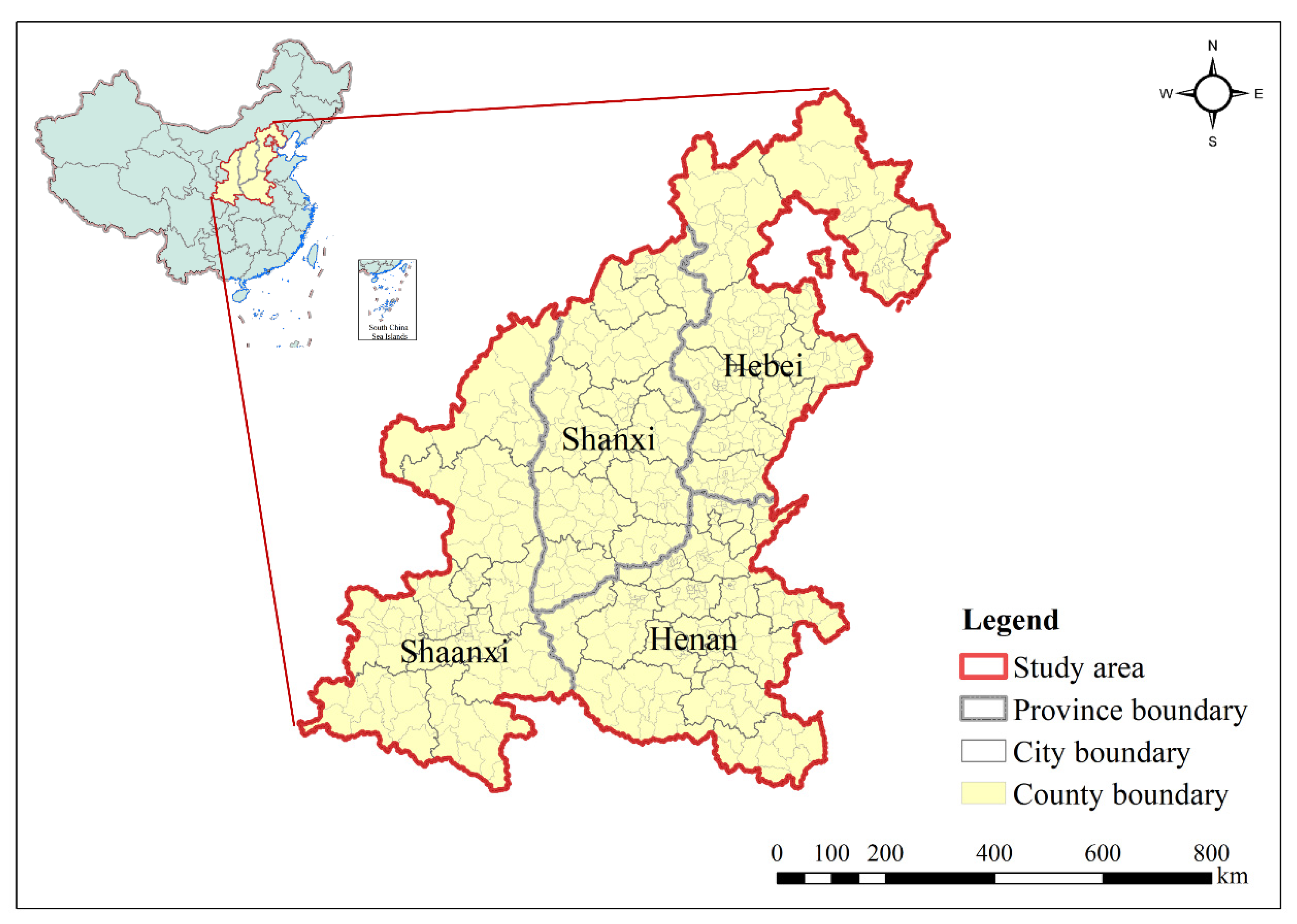
2.1. Study Area
2.2. Data Collection
2.2.1. Human Visceral Leishmaniasis
2.2.2. Driving Factors
Environmental Factors
Meteorological Factors
Socioeconomic Factors
2.3. Modelling Approach
3. Results
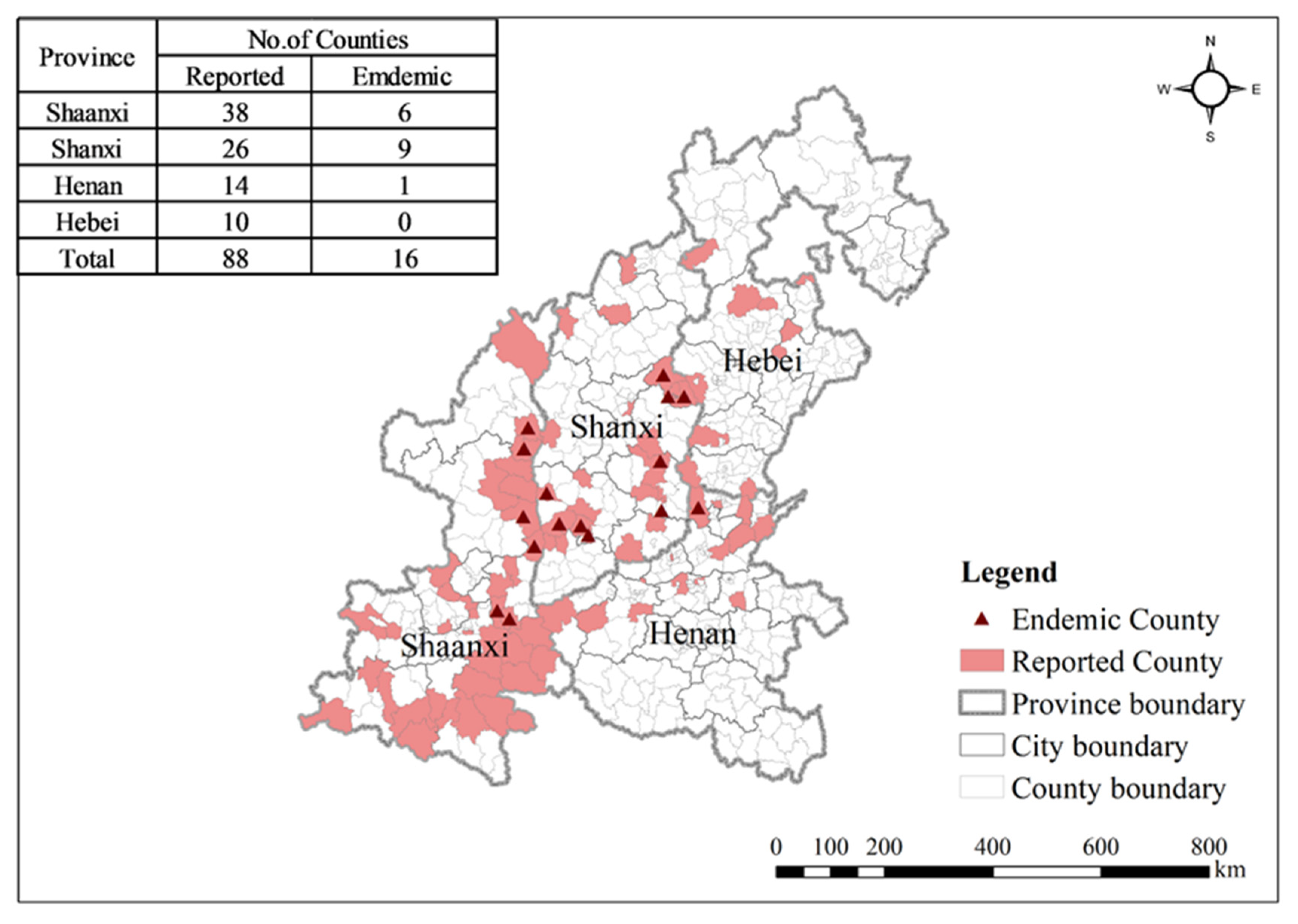
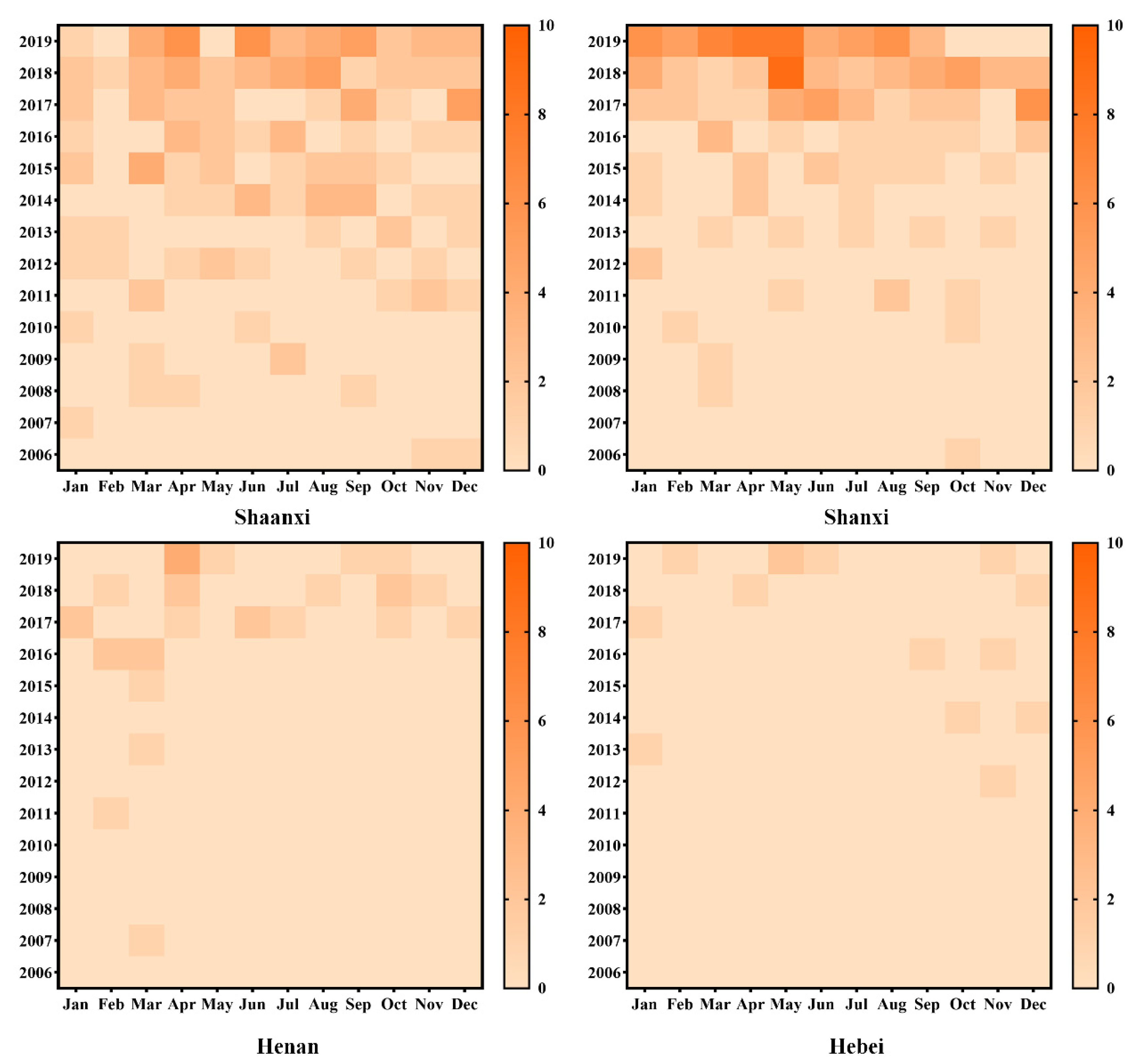
3.1. Spatiotemporal Distribution Characteristics
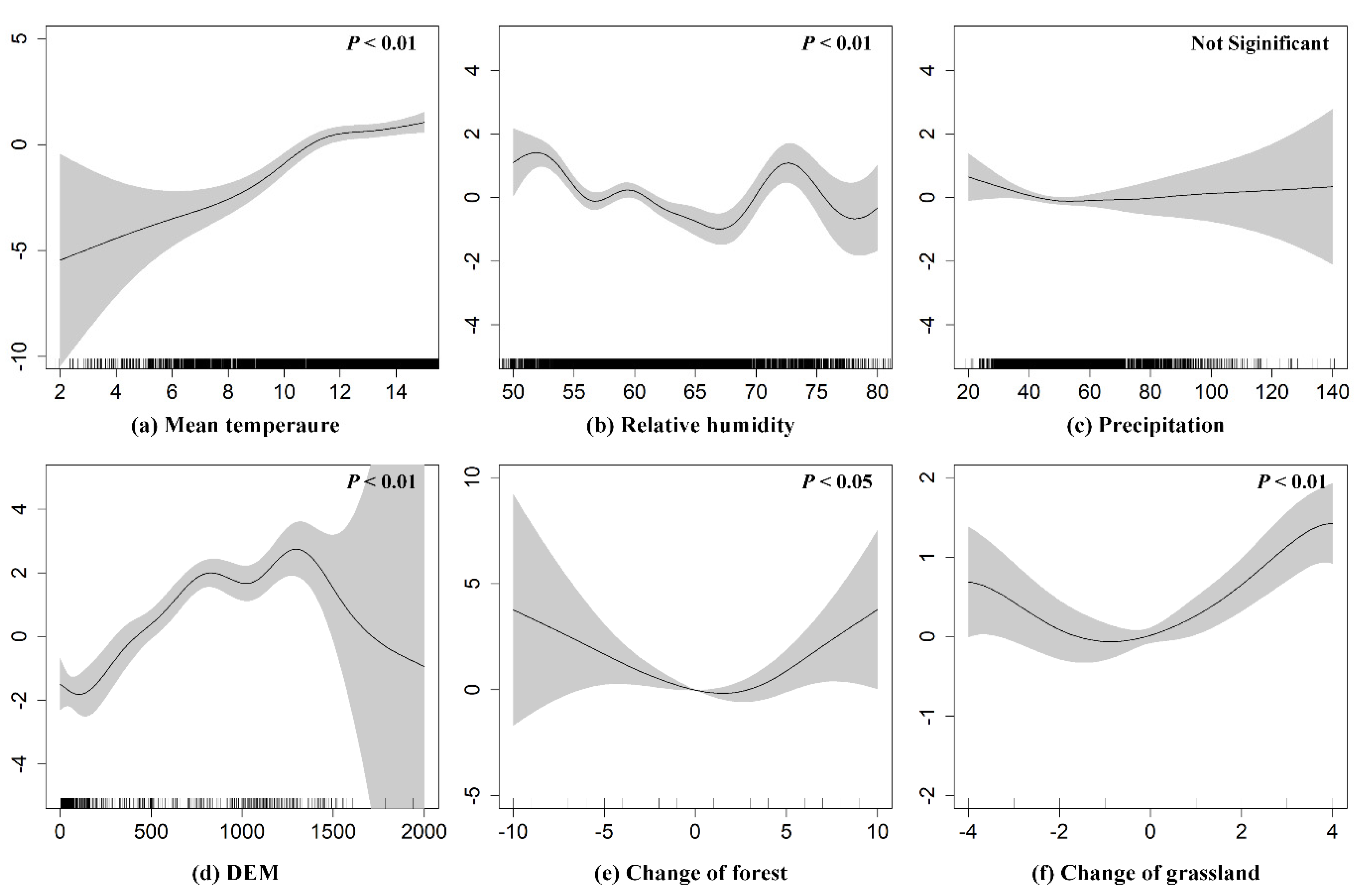
3.2. Driving Factors of VL Recurrence
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bi, K.; Chen, Y.; Zhao, S.; Kuang, Y.; John Wu, C.H. Current Visceral Leishmaniasis Research: A Research Review to Inspire Future Study. Biomed. Res. Int. 2018, 2018. [Google Scholar] [CrossRef] [Green Version]
- El Hajj, R.; El Hajj, H.; Khalifeh, I. Fatal Visceral Leishmaniasis Caused by Leishmania infantum, Lebanon. Emerg. Infect. Dis. 2018, 24, 906. [Google Scholar] [CrossRef] [Green Version]
- Sereno, D. Leishmania (Mundinia) spp.: From description to emergence as new human and animal Leishmania pathogens. New Microbes New Infect. 2019, 30, 20–22. [Google Scholar] [CrossRef]
- Akhoundi, M.; Kuhls, K.; Cannet, A.; Votýpka, J.; Marty, P.; Delaunay, P.; Sereno, D. A Historical Overview of the Classification, Evolution, and Dispersion of Leishmania Parasites and Sandflies. PLoS Negl. Trop. Dis. 2016, 10, e0004349. [Google Scholar] [CrossRef]
- Murray, H.W.; Berman, J.D.; Davies, C.R.; Saravia, N.G. Advances in leishmaniasis. Lancet 2005, 366, 1561–1577. [Google Scholar] [CrossRef]
- World Health Organization. Control of the leishmaniases. Wkly. Epidemiol. Rec. Relev. épidémiologique Hebd. 1991, 66, 88. [Google Scholar]
- Desjeux, P. Leishmaniasis: Current situation and new perspectives. Comp. Immunol. Microbiol. Infect. Dis. 2004, 27, 305–318. [Google Scholar] [CrossRef] [PubMed]
- Al-Salem, W.; Herricks, J.R.; Hotez, P.J. A review of visceral leishmaniasis during the conflict in South Sudan and the consequences for East African countries. Parasites Vectors 2016, 9, 1. [Google Scholar] [CrossRef] [Green Version]
- Jiang, D.; Ma, T.; Hao, M.; Qian, Y.; Chen, S.; Meng, Z.; Wang, L.; Zheng, C.; Qi, X.; Wang, Q.; et al. Spatiotemporal patterns and spatial risk factors for visceral leishmaniasis from 2007 to 2017 in Western and Central China: A modelling analysis. Sci. Total. Environ. 2021, 764, 144275. [Google Scholar] [CrossRef] [PubMed]
- Gomes, D.; Silva, G.; Sherlock, I.A. Horizontal stratification of the sand fly fauna (Diptera: Psychodidae) in a transitional vegetation between caatinga and tropical rain forest, state of Bahia, Brazil. Mem. Inst. Oswaldo Cruz 2003, 98, 733–737. [Google Scholar]
- Dos Reis, L.L.; Da Silva Balieiro, A.A.; Fonseca, F.R.; Gonçalves, M.J.F. Visceral leishmaniasis and its relationship with climate and environmental factors in the state of Tocantins, Brazil, from 2007 to 2014. Cad. Saude Publica 2019, 35, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Ready, P.D. Biology of phlebotomine sand flies as vectors of disease agents. Annu. Rev. Entomol. 2013, 58, 227–250. [Google Scholar] [CrossRef] [PubMed]
- Hlavacova, J.; Votypka, J.; Volf, P. The effect of temperature on Leishmania (Kinetoplastida: Trypanosomatidae) development in sand flies. J. Med. Entomol. 2013, 50, 955–958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alvar, J.; Yactayo, S.; Bern, C. Leishmaniasis and poverty. Trends Parasitol. 2006, 22, 552–557. [Google Scholar] [CrossRef] [PubMed]
- Lun, Z.R.; Wu, M.S.; Chen, Y.F.; Wang, J.Y.; Zhou, X.N.; Liao, L.F.; Chen, J.P.; Chow, L.M.; Chang, K.P. Visceral leishmaniasis in China: An endemic disease under Control. Clin. Microbiol. Rev. 2015, 28, 987–1004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiong, G.H. The status of the epidemiology of kala-azar in China. Endem. Dis. Bulletin. 1992, 7, 113–124. (In Chinese) [Google Scholar]
- Wang, Z.J.; Xiong, G.H.; Guan, L.R. Achievements in epidemiology and control of kala-azar in new China. Chin. J. Epidemiol. 2000, 21, 1–76. (In Chinese) [Google Scholar]
- Zhao, S.; Li, Z.; Zhou, S.; Zheng, C.; Ma, H. Epidemiological feature of visceral leishmaniasis in China, 2004–2012. Iran. J. Public Health 2015, 44, 51–59. [Google Scholar] [PubMed]
- Li, Y.F.; Zhong, W.X.; Zhao, G.H.; Wang, H.F. Epidemic situation and control status of kala-azar in China. J. Pathog. Biology 2011, 6, 629–631. (In Chinese) [Google Scholar]
- Han, S.; Wu, W.; Xue, C.; Wei, D.; Hou, Y.; Feng, Y. Endemic status of visceral leishmaniasis in China from 2004 to 2016. Chin. J. Parasitol Parasit Dis. 2019, 37, 189–195. (In Chinese) [Google Scholar] [CrossRef]
- Zheng, C.; Wang, L.; Li, Y.; Zhou, X.N. Visceral leishmaniasis in northwest China from 2004 to 2018: A spatio-temporal analysis. Infect. Dis. Poverty 2020, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Cruz, C.D.S.S.; Barbosa, D.S.; Oliveira, V.C.; Cardoso, D.T. Guimarães NS, Carneiro, M. Factors associated with human visceral leishmaniasis cases during urban epidemics in Brazil: A systematic review. Parasitology 2021, 148, 1–32. [Google Scholar] [CrossRef]
- Harrus, S.; Baneth, G. Drivers for the emergence and re-emergence of vector-borne protozoal and bacterial diseases. Int. J. Parasitol. 2005, 35, 1309–1318. [Google Scholar] [CrossRef]
- Ding, F.; Wang, Q.; Fu, J.; Chen, S.; Hao, M.; Ma, T.; Zheng, C.; Jiang, D. Risk factors and predicted distribution of visceral leishmaniasis in the Xinjiang Uygur Autonomous Region, China, 2005–2015. Parasites Vectors 2019, 12, 528. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Cao, Z. Meteorological conditions, elevation and land cover as predictors for the distribution analysis of visceral leishmaniasis in Sinkiang province, Mainland China. Sci. Total. Environ. 2019, 646, 1111–1116. [Google Scholar] [CrossRef]
- Elnaiem, D.E.A.; Schorscher, J.; Bendall, A.; Obsomer, V.; Osman, M.E.; Mekkawi, A.M.; Connor, S.J.; Ashford, R.W.; Thomson, M.C. Risk mapping of visceral leishmaniasis: The role of local variation in rainfall and altitude on the presence and incidence of kala-azar in eastern Sudan. Am. J. Trop. Med. Hyg. 2003, 68, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Werneck, G.L.; Costa, C.H.N.; Walker, A.M.; David, J.R.; Wand, M.; Maguire, J.H. Multilevel modelling of the incidence of visceral leishmaniasis in Teresina, Brazil. Epidemiol. Infect. 2007, 135, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Shah, H.A.; Huxley, P.; Elmes, J.; Murray, K.A. Agricultural land-uses consistently exacerbate infectious disease risks in Southeast Asia. Nat. Commun. 2019, 10, 1–13. [Google Scholar] [CrossRef]
- Dwyer-Lindgren, L.; Cork, M.A.; Sligar, A.; Steuben, K.M.; Wilson, K.F.; Provost, N.R.; Mayala, B.K.; VanderHeide, J.D.; Collison, M.L.; Hall, J.B.; et al. Mapping HIV prevalence in sub-Saharan Africa between 2000 and 2017. Nature 2019, 570, 189–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stoddard, S.T.; Morrison, A.C.; Vazquez-Prokopec, G.M.; Soldan, V.P.; Kochel, T.J.; Kitron, U.; Elder, J.P.; Scott, T.W. The role of human movement in the transmission of vector-borne pathogens. PLoS Negl. Trop. Dis. 2009, 3, e481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azevedo, T.S.; Lorenz, C.; Chiaravalloti-Neto, F. Risk mapping of visceral leishmaniasis in Brazil. Rev. Soc. Bras. Med. Trop. 2019, 52, e20190240. [Google Scholar] [CrossRef]
- Ding, F.; Li, Y.; Huang, B.; Edwards, J.; Robertson, I.D. Infection and risk factors of human and avian influenza in pigs in south China. Prev. Vet. Med. 2021, 190, 105317. [Google Scholar] [CrossRef]
- Ma, T.; Jiang, D.; Quzhen, G.; Xue, C.; Han, S.; Wu, W.; Zheng, C.; Ding, F. Factors influencing the spatial distribution of cystic echinococcosis in Tibet, China. Sci. Total. Environ. 2021, 754, 142229. [Google Scholar] [CrossRef]
- Desjeux, P. The increase in risk factors for leishmaniasis worldwide. Trans. R. Soc. Trop. Med. Hyg. 2001, 95, 239–243. [Google Scholar] [CrossRef]
- World Health Organization. Control of the Leishmaniases: Report of A Meeting of the WHO Expert Committee on the Control of Leishmaniases; Leishmaniases: Geneva, Switzerland, 2010. [Google Scholar]
- Kraemer, M.U.G.; Faria, N.R.; Reiner, R.C., Jr.; Golding, N.; Nikolay, B.; Stasse, S.; Johansson, M.A.; Salje, H.; Faye, O.; Wint, G.W.; et al. Spread of yellow fever virus outbreak in Angola and the Democratic Republic of the Congo 2015–16: A modelling study. Lancet Infect. Dis. 2017, 17, 330–338. [Google Scholar] [CrossRef]
- Ali, S.; Gugliemini, O.; Harber, S.; Harrison, A.; Houle, L.; Ivory, J.; Kersten, S.; Khan, R.; Kim, J.; LeBoa, C.; et al. Environmental and Social Change Drive the Explosive Emergence of Zika Virus in the Americas. PLoS Negl. Trop. Dis. 2017, 11, e0005135. [Google Scholar] [CrossRef]
- Jiang, D.; Hao, M.; Ding, F.; Fu, J.; Li, M. Mapping the Transmission Risk of Zika Virus using Machine Learning Models. Acta Trop. 2018, 185, 391–399. [Google Scholar] [CrossRef]
- Ding, F.; Fu, J.; Jiang, D.; Hao, M.; Lin, G. Mapping the spatial distribution of Aedes aegypti and Aedes albopictus. Acta Trop. 2018, 178, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Budke, C.M. WHO/OIE Manual on Echinococcosis in Humans and Animals: A Public Health Problem of Global Concern. Vet. Parasitol. 2002, 104, 357. [Google Scholar] [CrossRef]
- Li, R.; Xu, L.; Bjørnstad, O.N.; Liu, K.; Song, T.; Chen, A.; Xu, B.; Liu, Q.; Stenseth, N.C. Climate-driven variation in mosquito density predicts the spatiotemporal dynamics of dengue. Proc. Natl. Acad. Sci. USA 2019, 116, 3624–3629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, L.; Stige, L.C.; Chan, K.S.; Zhou, J.; Yang, J.; Sang, S.; Wang, M.; Yang, Z.; Yan, Z.; Jiang, T.; et al. Climate variation drives dengue dynamics. Proc. Natl. Acad. Sci. USA 2017, 114, 113–118. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Z.B.; Li, Y.Y.; Zhang, Y.; Li, S.Z. Prevalence of visceral leishmaniasis in China in 2018. Chin. J. Parasitol. Parasit. Dis. 2020, 38, 175–181. (In Chinese) [Google Scholar]
- Li, Y.; Zheng, C. Associations between Meteorological Factors and Visceral Leishmaniasis Outbreaks in Jiashi County, Xinjiang Uygur Autonomous Region, China, 2005-2015. Int. J. Environ. Res. Public Health 2019, 16, 1775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ivana, B.; Petr, V. Effect of temperature on metabolism of Phlebotomus papatasi (Diptera: Psychodidae). J. Med Entomol. 2007, 44, 150–154. [Google Scholar]
- Kasap, O.E.; Alten, B. Comparative demography of the sand fly Phlebotomus papatasi (Diptera: Psychodidae) at constant temperatures. J. Vector Ecol. 2006, 31, 378–385. [Google Scholar] [CrossRef]
- Moreno, J.; Alvar, J. Canine leishmaniasis: Epidemiological risk and the experimental model. Trends Parasitol. 2002, 18, 399–405. [Google Scholar] [CrossRef]
- Wang, F.P.; Chen, S.; Liu, D.L.; Li, G.Z.; Liu, G.; Zhang, Y.; Wang, T.H.; Wang, Y.Q.; Wang, W.H.; Zhang, Y. Epidemiological characteristics and risk factors of kala-azar in hancheng city, shaanxi province. Chin. J. Zoonoses 2018, 34, 756–760. (In Chinese) [Google Scholar]
| Factor | Variables | Data Source |
|---|---|---|
| Environmental | Elevation | Shuttle Radar Topography Mission (SRTM) |
| Change of forest | MODIS Land Cover Product (MCD12C1) | |
| Change of grassland | ||
| Meteorological | Mean temperature | China Meteorological Data Service Centre (CMDC) |
| Relative humidity | ||
| Precipitation | ||
| Socioeconomic | Population density | Socioeconomic Data and Applications Centre (SEDAC) |
| GDP | Global Change Research Data Publishing and Repository | |
| Urban accessibility | European Commission Joint Research Centre (ECJRC) |
| Province | City | County/District |
|---|---|---|
| Shanxi | Yangquan | Urban district (Pingding) |
| Mining district (Yu) | ||
| Suburban district | ||
| Changzhi | Suburban district | |
| Wuxiang | ||
| Linfen | Quwo | |
| Xiangfen | ||
| Xiangning | ||
| Daning | ||
| Shaanxi | Weinan | Linwei |
| Huazhou | ||
| Hancheng | ||
| Yanan | Yichuan | |
| Yulin | Suide | |
| Qingjian | ||
| Henan | Anyang | Linzhou |
| Year | Shaanxi | Shanxi | Henan | Hebei | Total |
|---|---|---|---|---|---|
| No. of Cases | |||||
| 2006 | 2 | 1 | 0 | 0 | 3 |
| 2007 | 1 | 0 | 1 | 0 | 2 |
| 2008 | 3 | 1 | 0 | 0 | 4 |
| 2009 | 3 | 1 | 0 | 0 | 4 |
| 2010 | 2 | 2 | 0 | 0 | 4 |
| 2011 | 6 | 4 | 1 | 0 | 11 |
| 2012 | 8 | 2 | 0 | 1 | 11 |
| 2013 | 6 | 5 | 1 | 1 | 13 |
| 2014 | 14 | 4 | 0 | 2 | 20 |
| 2015 | 15 | 9 | 1 | 0 | 25 |
| 2016 | 13 | 10 | 4 | 2 | 29 |
| 2017 | 20 | 29 | 8 | 1 | 58 |
| 2018 | 31 | 41 | 7 | 2 | 81 |
| 2019 | 37 | 52 | 7 | 5 | 101 |
| Total | 161 | 161 | 30 | 14 | 366 |
| Factor | Variables | Chi.sq/ Estimate | P Value |
|---|---|---|---|
| Environmental | Elevation | 99.618 a | 0.000 *** |
| Change of forest | 13.747 a | 0.010 * | |
| Change of grassland | 42.210 a | 0.000 *** | |
| Meteorological | Mean temperature | 72.594 a | 0.000 *** |
| Relative humidity | 87.193 a | 0.000 *** | |
| Precipitation | 4.322 a | 0.262 | |
| Socioeconomic | Population | 4.615 × 10−5 b | 0.000 *** |
| GDP | 4.322 × 10−5 b | 0.054 † | |
| Urban accessibility | −1.195 × 10−3 b | 0.038 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Jiang, D.; Ding, F.; Hao, M.; Wang, Q.; Chen, S.; Xie, X.; Zheng, C.; Ma, T. Recurrence and Driving Factors of Visceral Leishmaniasis in Central China. Int. J. Environ. Res. Public Health 2021, 18, 9535. https://doi.org/10.3390/ijerph18189535
Zhao Y, Jiang D, Ding F, Hao M, Wang Q, Chen S, Xie X, Zheng C, Ma T. Recurrence and Driving Factors of Visceral Leishmaniasis in Central China. International Journal of Environmental Research and Public Health. 2021; 18(18):9535. https://doi.org/10.3390/ijerph18189535
Chicago/Turabian StyleZhao, Yingze, Dong Jiang, Fangyu Ding, Mengmeng Hao, Qian Wang, Shuai Chen, Xiaolan Xie, Canjun Zheng, and Tian Ma. 2021. "Recurrence and Driving Factors of Visceral Leishmaniasis in Central China" International Journal of Environmental Research and Public Health 18, no. 18: 9535. https://doi.org/10.3390/ijerph18189535






