Acute Effects of Tecar Therapy on Skin Temperature, Ankle Mobility and Hyperalgesia in Myofascial Pain Syndrome in Professional Basketball Players: A Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design
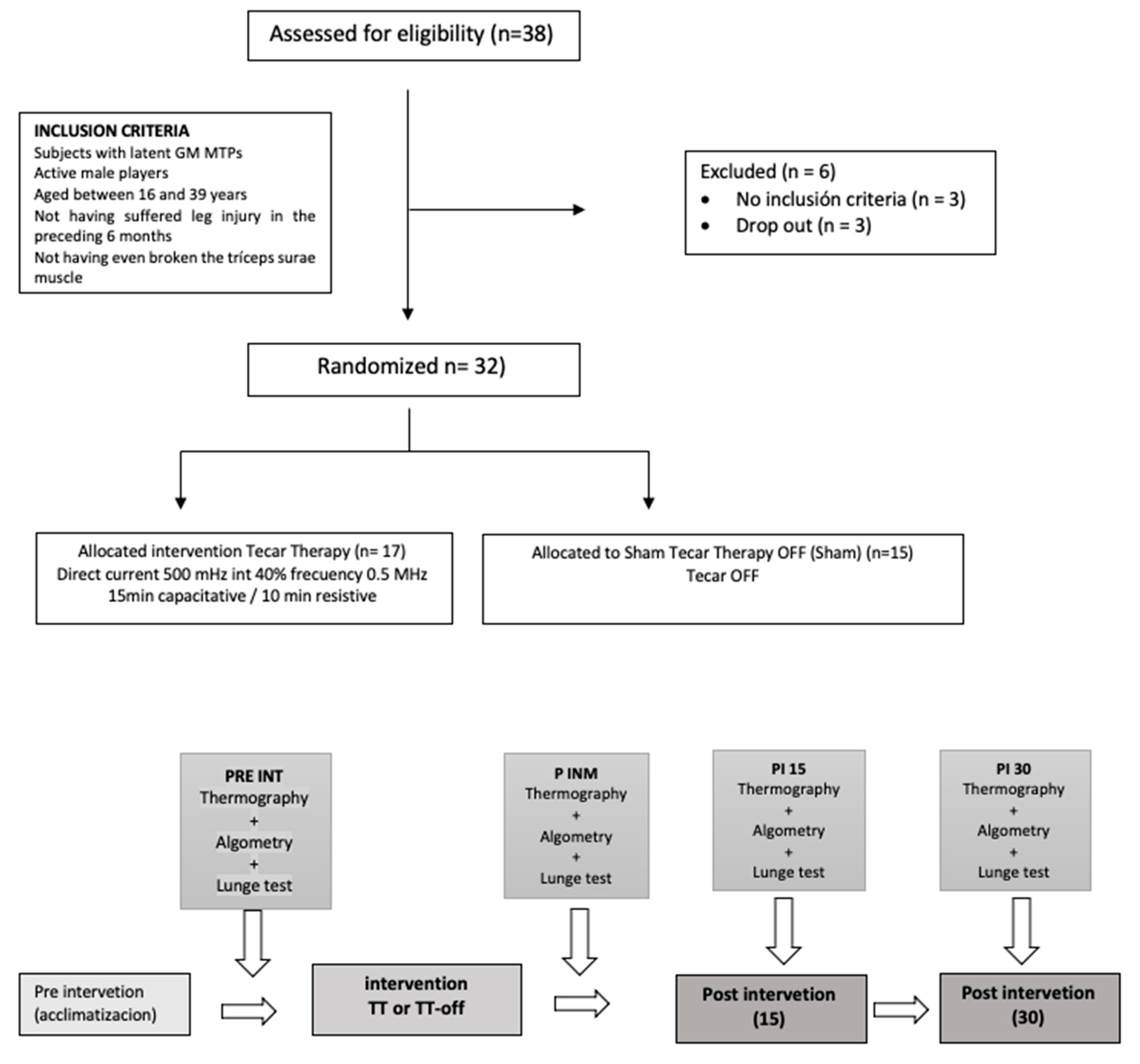
2.2. Sample
2.3. Instruments
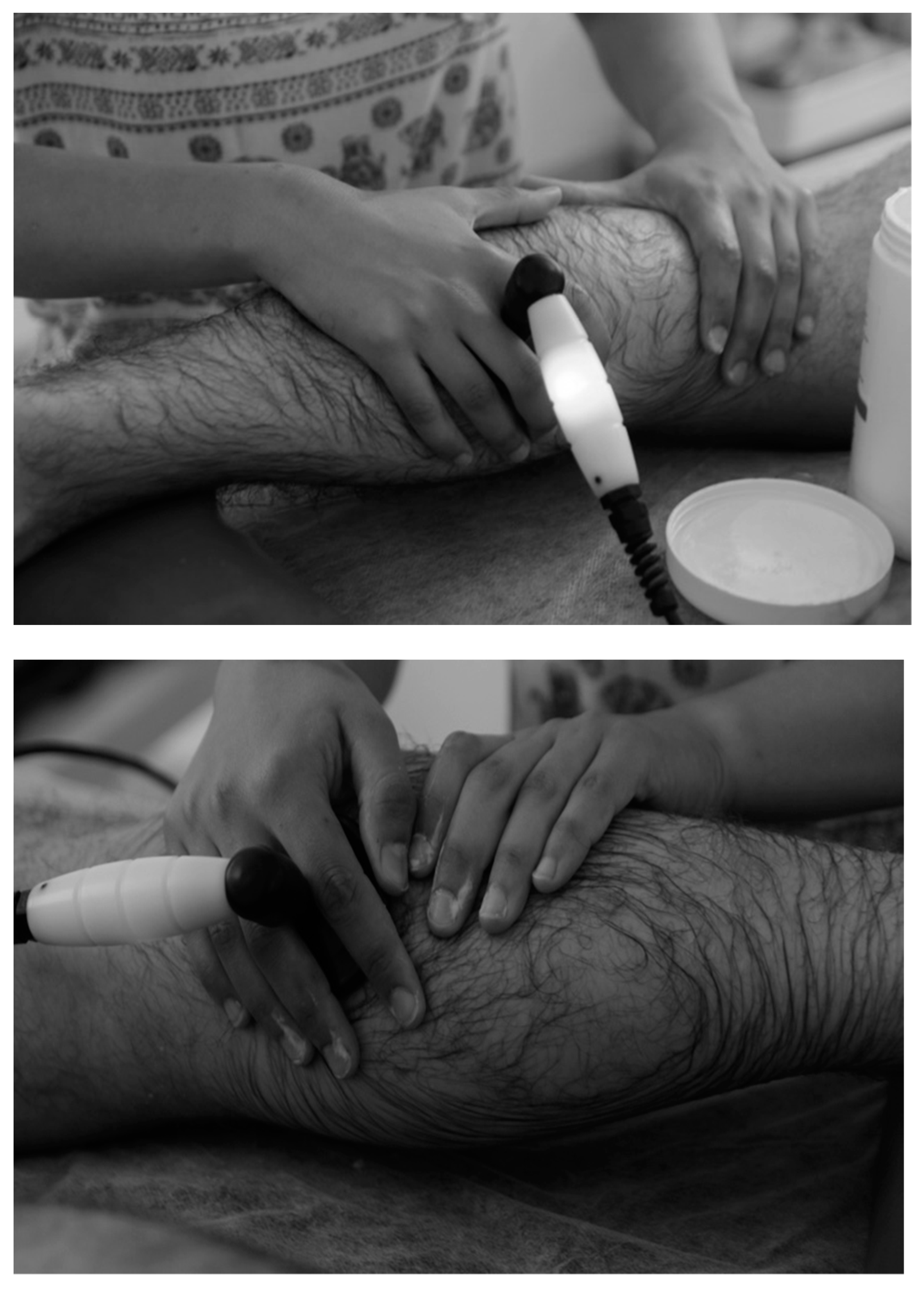
2.3.1. Tecar Therapy
2.3.2. Control Group
2.4. Procedures
2.4.1. Thermographic Assessment
2.4.2. Algometry Assessment
2.4.3. Lunge Test Protocol
2.5. Statistical Analyses
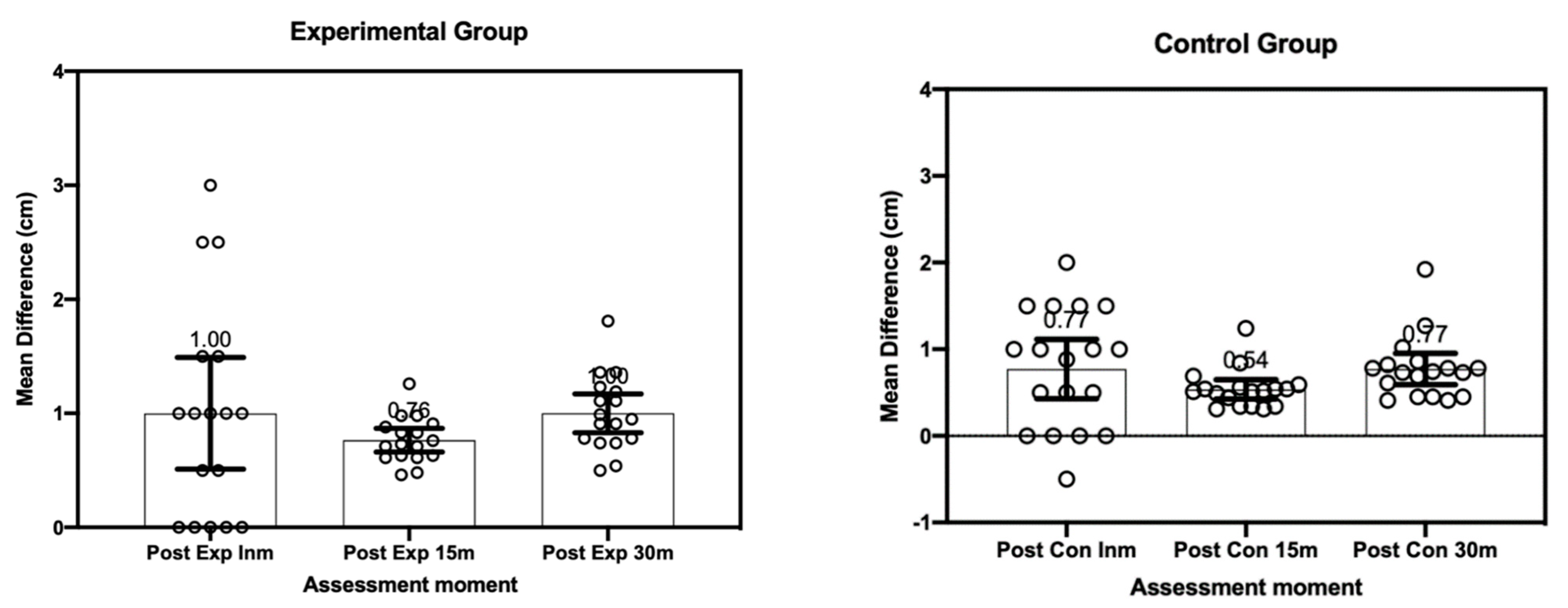
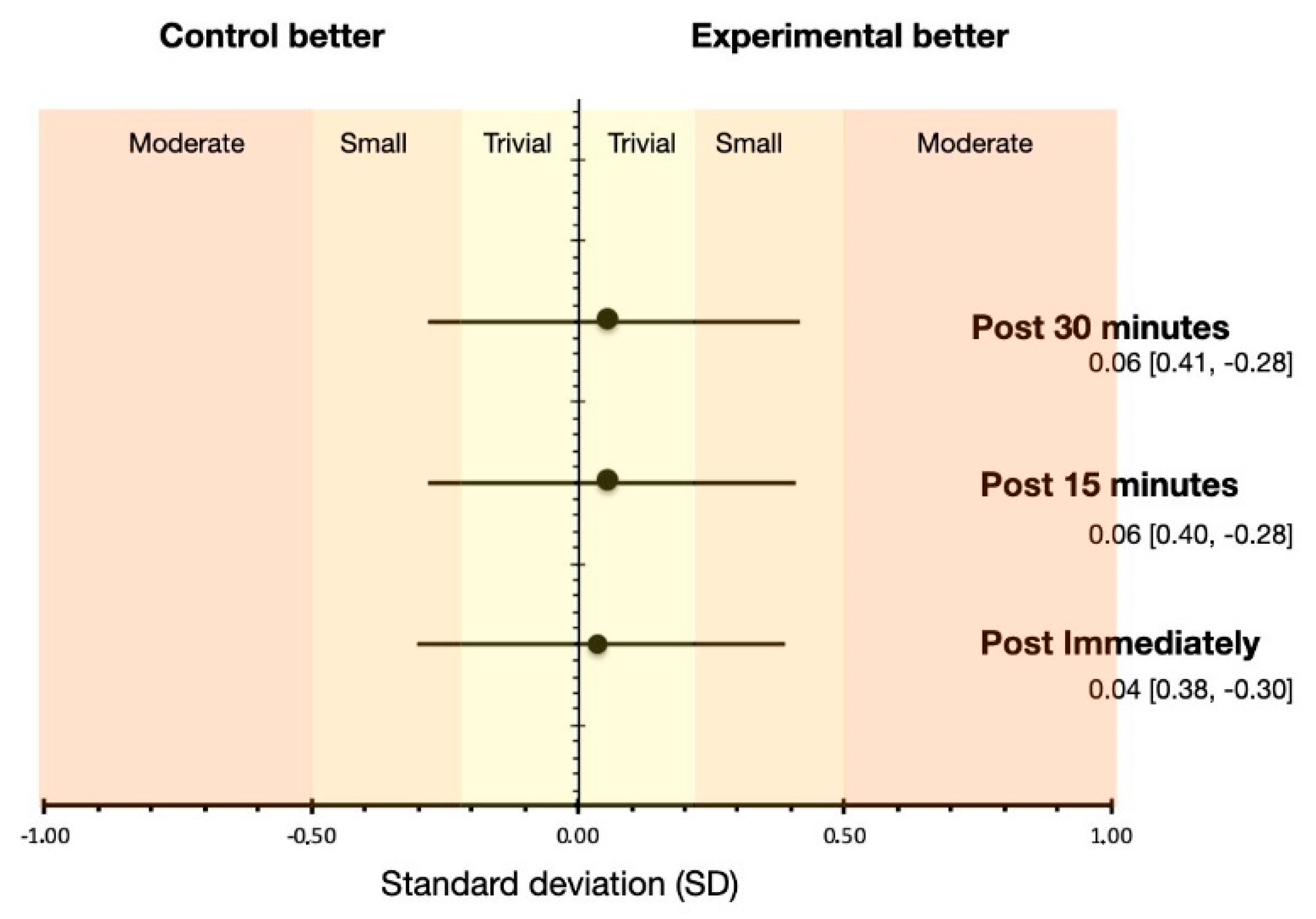
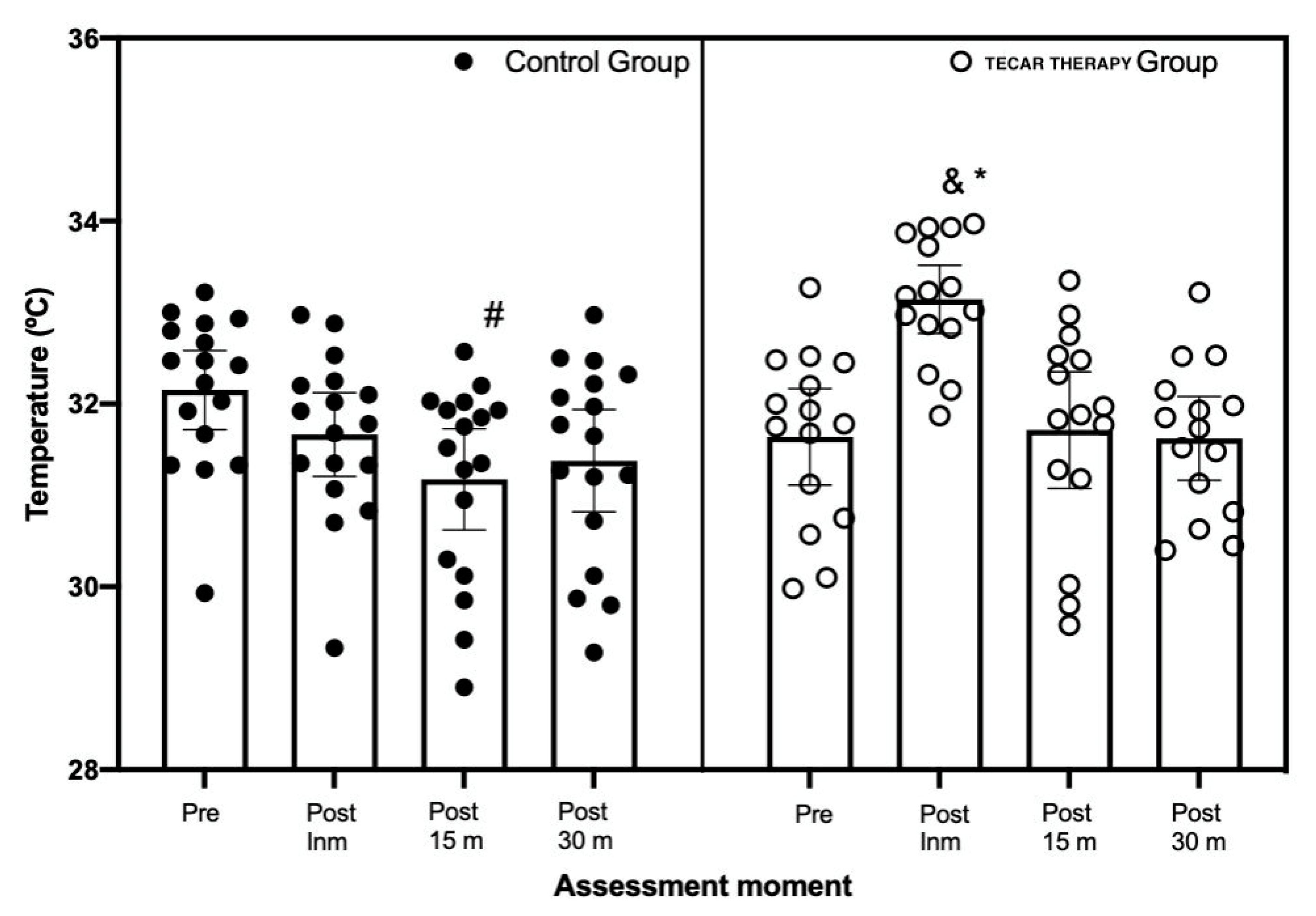
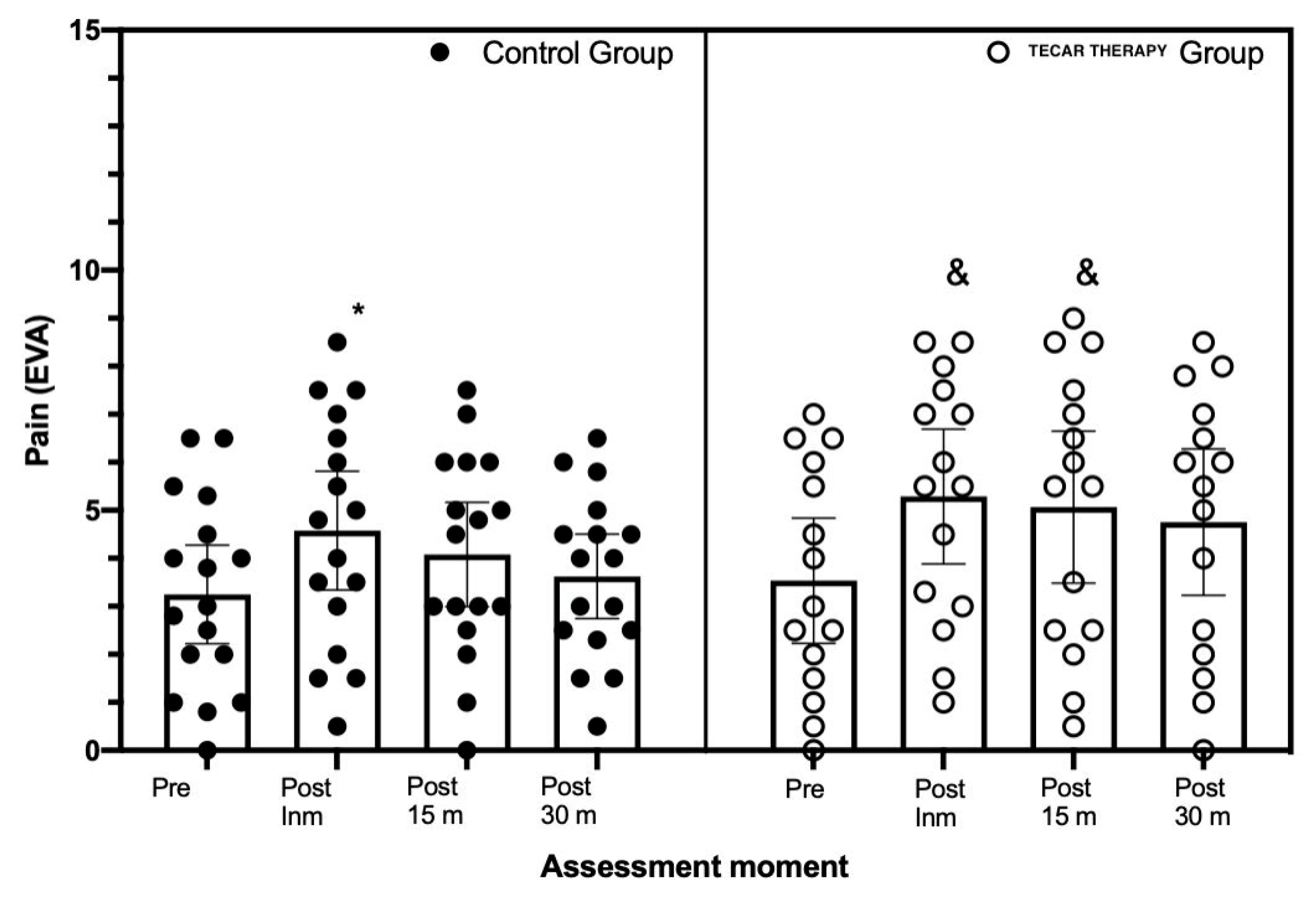
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Duñabeitia, I.; Arrieta, H.; Torres-Unda, J.; Gil, J.; Santos-Concejero, J.; Gil, S.M.; Irazusta, J.; Bidaurrazaga-Letona, I. Effects of a capacitive-resistive electric transfer therapy on physiological and biomechanical parameters in recreational runners: A randomized controlled crossover trial. Phys. Ther. Sport 2018, 32, 227–234. [Google Scholar] [CrossRef]
- Costantino, C.; Pogliacomi, F.; Vaienti, E. Cryoultrasound therapy and tendonitis in athletes: A comparative evaluation versus laser CO2 and t.e.ca.r. therapy. Acta Biomed. Atenei Parm. 2005, 76, 37–41. [Google Scholar]
- Diego, I.M.A.; Fernández-Carnero, J.; Val, S.L.; Cano-De-La-Cuerda, R.; Calvo-Lobo, C.; Piédrola, R.M.; Oliva, L.C.L.; Rueda, F.M. Analgesic effects of a capacitive-resistive monopolar radiofrequency in patients with myofascial chronic neck pain: A pilot randomized controlled trial. Rev. Assoc. Méd. Bras. 2019, 65, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Paolucci, T.; Pezzi, L.; Centra, M.; Porreca, A.; Barbato, C.; Bellomo, R.G.; Saggini, R. Effects of capacitive and resistive electric transfer therapy in patients with painful shoulder impingement syndrome: A comparative study. J. Int. Med Res. 2020, 48, 0300060519883090. [Google Scholar] [CrossRef] [Green Version]
- Bito, T.; Tashiro, Y.; Suzuki, Y.; Kajiwara, Y.; Zeidan, H.; Kawagoe, M.; Sonoda, T.; Nakayama, Y.; Yokota, Y.; Shimoura, K.; et al. Acute effects of capacitive and resistive electric transfer (CRet) on the Achilles tendon. Electromagn. Biol. Med. 2019, 38, 48–54. [Google Scholar] [CrossRef]
- Wostyn, V. La Tecarthérapie Appliquée a la Kinésithérapie: Evaluation de Leffet Antalgique Immédiat; Institut de formation on Masso-Kinésithérapie: Reims, France, 2015. [Google Scholar]
- Clijsen, R.; Leoni, D.; Schneebeli, A.; Cescon, C.; Soldini, E.; Li, L.; Barbero, M. Does the Application of Tecar Therapy Affect Temperature and Perfusion of Skin and Muscle Microcirculation? A Pilot Feasibility Study on Healthy Subjects. J. Altern. Complement. Med. 2020, 26, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Masiero, S.; Pignataro, A.; Piran, G.; Duso, M.; Mimche, P.; Ermani, M.; Del Felice, A. Short-wave diathermy in the clinical management of musculoskeletal disorders: A pilot observational study. Int. J. Biometeorol. 2020, 64, 981–988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozen, S.; Doganci, E.B.; Ozyuvali, A.; Yalcin, A.P. Effectiveness of continuous versus pulsed short-wave diathermy in the management of knee osteoarthritis: A randomized pilot study. Casp. J. Intern. Med. 2019, 10, 431–438. [Google Scholar]
- Pelfort, X.; Torres-Claramunt, R.; Sánchez-Soler, J.; Hinarejos, P.; Leal-Blanquet, J.; Valverde, D.; Monllau, J. Pressure algometry is a useful tool to quantify pain in the medial part of the knee: An intra- and inter-reliability study in healthy subjects. Orthop. Traumatol. Surg. Res. 2015, 101, 559–563. [Google Scholar] [CrossRef] [Green Version]
- Simons, D.G.; Travell, J.G.; Simons, L.S.; Cummings, B.D. Myofascial Pain and Dysfunction: The Trigger Point Manual; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 1998. [Google Scholar]
- Hernández, F.M.F. Síndromes miofasciales. Reumatol. Clín. 2009, 5, 36–39. [Google Scholar] [CrossRef]
- Montañez-Aguilera, F.J.; Valtueña-Gimeno, N.; Pecos-Martín, D.; Arnau-Masanet, R.; Barrios-Pitarque, C.; Bosch-Morell, F. Changes in a patient with neck pain after application of ischemic compression as a trigger point therapy. J. Back Musculoskelet. Rehabil. 2010, 23, 101–104. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Gao, Q.; Li, J.; Tian, Y.; Hou, J. Impact of Needle Diameter on Long-Term Dry Needling Treatment of Chronic Lumbar Myofascial Pain Syndrome. Am. J. Phys. Med. Rehabil. 2016, 95, 483–494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tough, E.A.; White, A.R.; Richards, S.; Campbell, J. Variability of Criteria Used to Diagnose Myofascial Trigger Point Pain Syndrome—Evidence From a Review of the Literature. Clin. J. Pain 2007, 23, 278–286. [Google Scholar] [CrossRef]
- Ge, H.-Y.; Arendt-Nielsen, L. Latent Myofascial Trigger Points. Curr. Pain Headache Rep. 2011, 15, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Benito-De-Pedro, M.; Becerro-de-Bengoa-Vallejo, R.; Losa-Iglesias, M.E.; Rodríguez-Sanz, D.; López-López, D.; Cosín-Matamoros, J.; Martínez-Jiménez, E.; Calvo-Lobo, C. Effectiveness between Dry Needling and Ischemic Compression in the Triceps Surae Latent Myofascial Trigger Points of Triathletes on Pressure Pain Threshold and Thermography: A Single Blinded Randomized Clinical Trial. J. Clin. Med. 2019, 8, 1632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gattie, E.; Cleland, J.A.; Snodgrass, S. The Effectiveness of Trigger Point Dry Needling for Musculoskeletal Conditions by Physical Therapists: A Systematic Review and Meta-analysis. J. Orthop. Sports Phys. Ther. 2017, 47, 133–149. [Google Scholar] [CrossRef]
- Dommerholt, J.; Chou, L.-W.; Finnegan, M.; Hooks, T. A critical overview of the current myofascial pain literature—April 2018. J. Bodyw. Mov. Ther. 2018, 22, 402–410. [Google Scholar] [CrossRef] [Green Version]
- Capó-Juan, M. Síndrome de dolor miofascial cervical: Revisión narrativa del tratamiento fisioterápico. An. Sist. Sanit. Navar. 2015, 38, 105–115. [Google Scholar] [CrossRef]
- Bengtsson, H.; Ekstrand, J.; Hägglund, M. Muscle injury rates in professional football increase with fixture congestion: An 11-year follow-up of the UEFA Champions League injury study. Br. J. Sports Med. 2013, 47, 743–747. [Google Scholar] [CrossRef]
- Orchard, J. Intrinsic and Extrinsic Risk Factors for Muscle Strains in Australian Football. Am. J. Sports Med. 2001, 29, 300–303. [Google Scholar] [CrossRef]
- Fields, K.B.; Rigby, M.D. Muscular Calf Injuries in Runners. Curr. Sports Med. Rep. 2016, 15, 320–324. [Google Scholar] [CrossRef] [PubMed]
- López-De-Celis, C.; Hidalgo-García, C.; Pérez-Bellmunt, A.; Fanlo-Mazas, P.; González-Rueda, V.; Tricás-Moreno, J.M.; Ortiz, S.; Rodríguez-Sanz, J. Thermal and non-thermal effects off capacitive-resistive electric transfer application on the Achilles tendon and musculotendinous junction of the gastrocnemius muscle: A cadaveric study. BMC Musculoskelet. Disord. 2020, 21, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zuil-Escobar, J.C.; Martínez-Cepa, C.B.; Martín-Urrialde, J.A.; Gómez-Conesa, A. The Prevalence of Latent Trigger Points in Lower Limb Muscles in Asymptomatic Subjects. PM&R 2016, 8, 1055–1064. [Google Scholar] [CrossRef]
- Fernández-De-Las-Peñas, C.; Dommerholt, J. International Consensus on Diagnostic Criteria and Clinical Considerations of Myofascial Trigger Points: A Delphi Study. Pain Med. 2018, 19, 142–150. [Google Scholar] [CrossRef] [Green Version]
- Amalu, W. International Academy of Clinical Thermology Medical Infrared Imaging Standards and Guidelines; International Academy of Clinical Thermology: Foster City, CA, USA, 2018. [Google Scholar] [CrossRef]
- Tranquilli, C.; Ganzit, G.P.; Ciufetti, A.; Bergamo, P.; Combi, F. Multicentre Study on Tecar ® Therapy in Sports Pathologies; FMSI Institute of Sports medicine: Milano, Italy, 2009. [Google Scholar]
- De Sousa, N.T.A.; Guirro, E.C.D.O.; Calió, J.G.; De Queluz, M.C.; Guirro, R.R.D.J. Application of shortwave diathermy to lower limb increases arterial blood flow velocity and skin temperature in women: A randomized controlled trial. Braz. J. Phys. Ther. 2017, 21, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Ring, E.F.J. The technique of infrared imaging in medicine. In Infrared Imaging: A Casebook in Clinical Medicine; IOP Publishing: Lancaster, UK, 2014. [Google Scholar]
- Garagio, U.; Giani, E. Use of Telethermography in the Management of Sports Injuries. Sports Med. 1990, 10, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Gomez Carmona, P.M. Influencia de la Información Termográfica Infrarroja en el Protocolo de Prevención de Lesiones de un Equipo de Fútbol Profesional Español. Ph.D. Thesis, Universidad Politécnica de Madrid, Madrid, Spain, March 2012. [Google Scholar]
- Sterling, M. Testing for Sensory Hypersensitivity or Central Hyperexcitability Associated with Cervical Spine Pain. J. Manip. Physiol. Ther. 2008, 31, 534–539. [Google Scholar] [CrossRef]
- Mutlu, E.K.; Ozdincler, A.R. Reliability and responsiveness of algometry for measuring pressure pain threshold in patients with knee osteoarthritis. J. Phys. Ther. Sci. 2015, 27, 1961–1965. [Google Scholar] [CrossRef] [Green Version]
- Chisholm, M.D.; Birmingham, T.B.; Brown, J.; MacDermid, J.; Chesworth, B.M. Reliability and Validity of a Weight-Bearing Measure of Ankle Dorsiflexion Range of Motion. Physiother. Can. 2012, 64, 347–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cejudo, A.; de Baranda, P.S.; Ayala, F.; Santonja, F. A simplified version of the weight-bearing ankle lunge test: Description and test–retest reliability. Man. Ther. 2014, 19, 355–359. [Google Scholar] [CrossRef]
- Falgarone, G.; Zerkak, D.; Bauer, C.; Messow, M.; Dougados, M. How to define a Minimal Clinically Individual State (MCIS) with pain VAS in daily practice for patients suffering from musculoskeletal disorders. Clin. Exp. Rheumatol. 2005, 23, 235–238. [Google Scholar]
- Prouza, O.; Gonzalez, A.C. Targeted Radiofrequency Therapy for Training Induced Muscle Fatigue—Effective or Not? Int. J. Physiother. 2016, 3, 707–710. [Google Scholar] [CrossRef]
- Kumaran, B.; Watson, T. Skin thermophysiological effects of 448 kHz capacitive resistive monopolar radiofrequency in healthy adults: A randomised crossover study and comparison with pulsed shortwave therapy. Electromagn. Biol. Med. 2018, 37, 1–12. [Google Scholar] [CrossRef]
- Takahashi, K.; Suyama, T.; Takakura, Y.; Hirabayashi, S.; Tsuzuki, N.; Li, Z.-S. Clinical Effects of Capacitive Electric Transfer Hyperthermia Therapy for Cervico-Omo-Brachial Pain. J. Phys. Ther. Sci. 2000, 12, 43–48. [Google Scholar] [CrossRef] [Green Version]
- Martín-Pintado-Zugasti, A.; Rodríguez-Fernández, Á.L.; Fernandez-Carnero, J. Postneedling soreness after deep dry needling of a latent myofascial trigger point in the upper trapezius muscle: Characteristics, sex differences and associated factors. J. Back Musculoskelet. Rehabil. 2016, 29, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Tabatabaiee, A.; Ebrahimi-Takamjani, I.; Ahmadi, A.; Sarrafzadeh, J.; Emrani, A. Comparison of pressure release, phonophoresis and dry needling in treatment of latent myofascial trigger point of upper trapezius muscle. J. Back Musculoskelet. Rehabil. 2019, 32, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Girasol, C.E.; Filho, A.V.D.; de Oliveira, A.K.; Guirro, R.R.D.J. Correlation Between Skin Temperature Over Myofascial Trigger Points in the Upper Trapezius Muscle and Range of Motion, Electromyographic Activity, and Pain in Chronic Neck Pain Patients. J. Manip. Physiol. Ther. 2018, 41, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Moraska, A.F.; Schmiege, S.J.; Mann, J.D.; Butryn, N.; Krutsch, J.P. Responsiveness of Myofascial Trigger Points to Single and Multiple Trigger Point Release Massages. Am. J. Phys. Med. Rehabil. 2017, 96, 639–645. [Google Scholar] [CrossRef]
- Mejuto-Vázquez, M.J.; Salom-Moreno, J.; Ortega-Santiago, R.; Truyols-Domínguez, S.; Fernández-De-Las-Peñas, C. Short-Term Changes in Neck Pain, Widespread Pressure Pain Sensitivity, and Cervical Range of Motion After the Application of Trigger Point Dry Needling in Patients With Acute Mechanical Neck Pain: A Randomized Clinical Trial. J. Orthop. Sports Phys. Ther. 2014, 44, 252–260. [Google Scholar] [CrossRef] [Green Version]
- Grieve, R.; Cranston, A.; Henderson, A.; John, R.; Malone, G.; Mayall, C. The immediate effect of triceps surae myofascial trigger point therapy on restricted active ankle joint dorsiflexion in recreational runners: A crossover randomised controlled trial. J. Bodyw. Mov. Ther. 2013, 17, 453–461. [Google Scholar] [CrossRef]
- Draper, D.O. Pulsed Shortwave Diathermy and Joint Mobilizations for Achieving Normal Elbow Range of Motion After Injury or Surgery With Implanted Metal: A Case Series. J. Athl. Train. 2014, 49, 851–855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamamoto, L.G.; Nomura, J.T.; Sato, R.L.; Ahern, R.M.; Snow, J.L.; Kuwaye, T.T. Minimum clinically significant VAS differences for simultaneous (paired) interval serial pain assessments. Am. J. Emerg. Med. 2003, 21, 176–179. [Google Scholar] [CrossRef]
- Mengshoel, A.M. Physiotherapy and the Placebo Effect. Phys. Ther. Rev. 2000, 5, 161–165. [Google Scholar] [CrossRef]
- Shahimoridi, D.; Shafiei, S.A.; Yousefian, B. The Effectiveness of the Polarized Low-Level Laser in the Treatment of Patients with Myofascial Trigger Points in the Trapezius Muscles. J. Lasers Med Sci. 2020, 11, 14–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuentes-Márquez, P.; Valenza, M.C.; Cabrera-Martos, I.; Ríos-Sánchez, A.; Ocón-Hernández, O. Trigger Points, Pressure Pain Hyperalgesia, and Mechanosensitivity of Neural Tissue in Women with Chronic Pelvic Pain. Pain Med. 2017, 20, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.A.; Tonomura, H.; Arai, Y.; Terauchi, R.; Honjo, K.; Hiraoka, N.; Hojo, T.; Kunitomo, T.; Kubo, T. Hyperthermia for the treatment of articular cartilage with osteoarthritis. Int. J. Hyperth. 2009, 25, 661–667. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeste-Fabregat, M.; Baraja-Vegas, L.; Vicente-Mampel, J.; Pérez-Bermejo, M.; Bautista González, I.J.; Barrios, C. Acute Effects of Tecar Therapy on Skin Temperature, Ankle Mobility and Hyperalgesia in Myofascial Pain Syndrome in Professional Basketball Players: A Pilot Study. Int. J. Environ. Res. Public Health 2021, 18, 8756. https://doi.org/10.3390/ijerph18168756
Yeste-Fabregat M, Baraja-Vegas L, Vicente-Mampel J, Pérez-Bermejo M, Bautista González IJ, Barrios C. Acute Effects of Tecar Therapy on Skin Temperature, Ankle Mobility and Hyperalgesia in Myofascial Pain Syndrome in Professional Basketball Players: A Pilot Study. International Journal of Environmental Research and Public Health. 2021; 18(16):8756. https://doi.org/10.3390/ijerph18168756
Chicago/Turabian StyleYeste-Fabregat, Mireia, Luis Baraja-Vegas, Juan Vicente-Mampel, Marcelino Pérez-Bermejo, Iker J. Bautista González, and Carlos Barrios. 2021. "Acute Effects of Tecar Therapy on Skin Temperature, Ankle Mobility and Hyperalgesia in Myofascial Pain Syndrome in Professional Basketball Players: A Pilot Study" International Journal of Environmental Research and Public Health 18, no. 16: 8756. https://doi.org/10.3390/ijerph18168756
APA StyleYeste-Fabregat, M., Baraja-Vegas, L., Vicente-Mampel, J., Pérez-Bermejo, M., Bautista González, I. J., & Barrios, C. (2021). Acute Effects of Tecar Therapy on Skin Temperature, Ankle Mobility and Hyperalgesia in Myofascial Pain Syndrome in Professional Basketball Players: A Pilot Study. International Journal of Environmental Research and Public Health, 18(16), 8756. https://doi.org/10.3390/ijerph18168756





