Changes in the Composition of the Soil Bacterial Community in Heavy Metal-Contaminated Farmland
Abstract
:1. Introduction
2. Materials and Methods
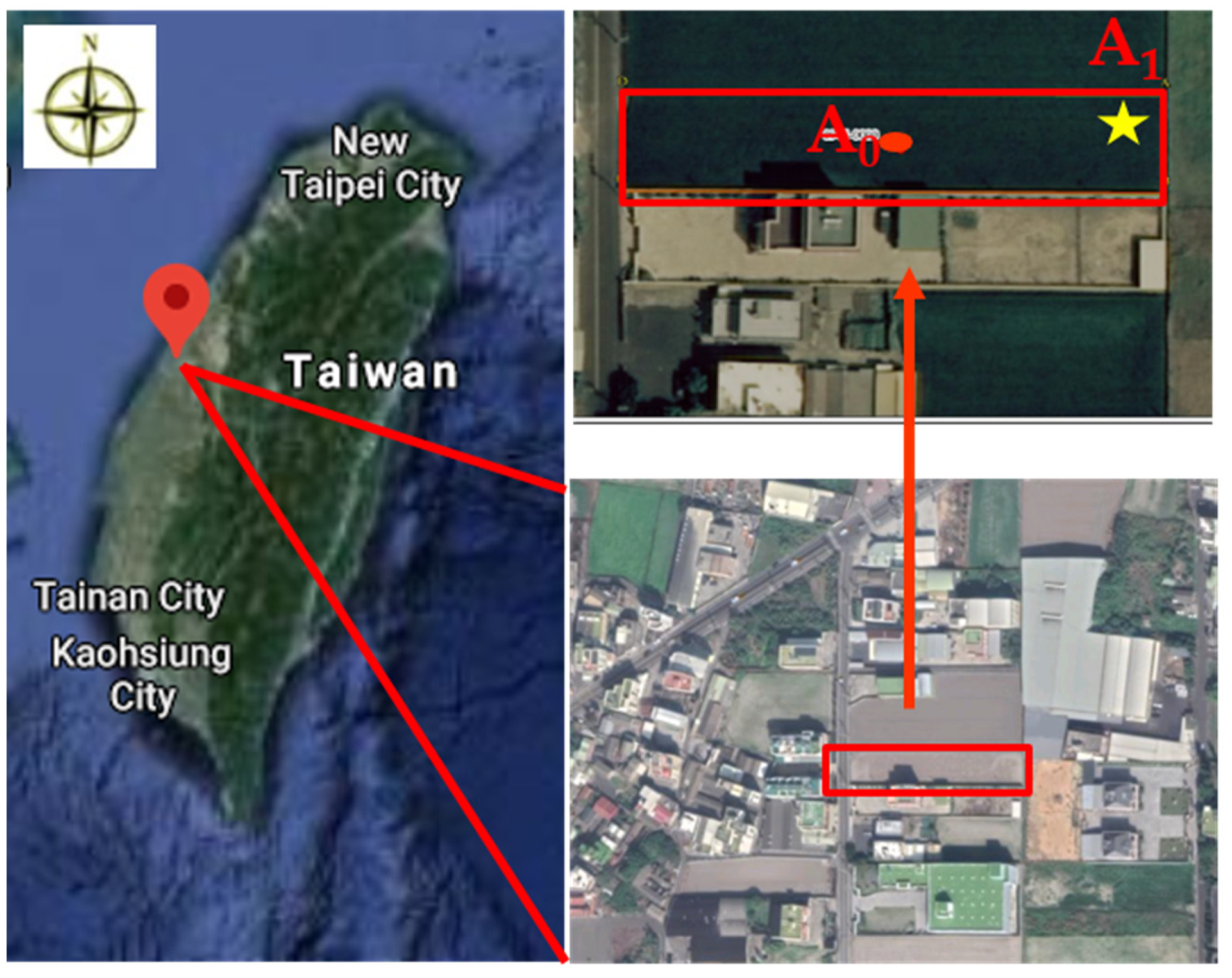
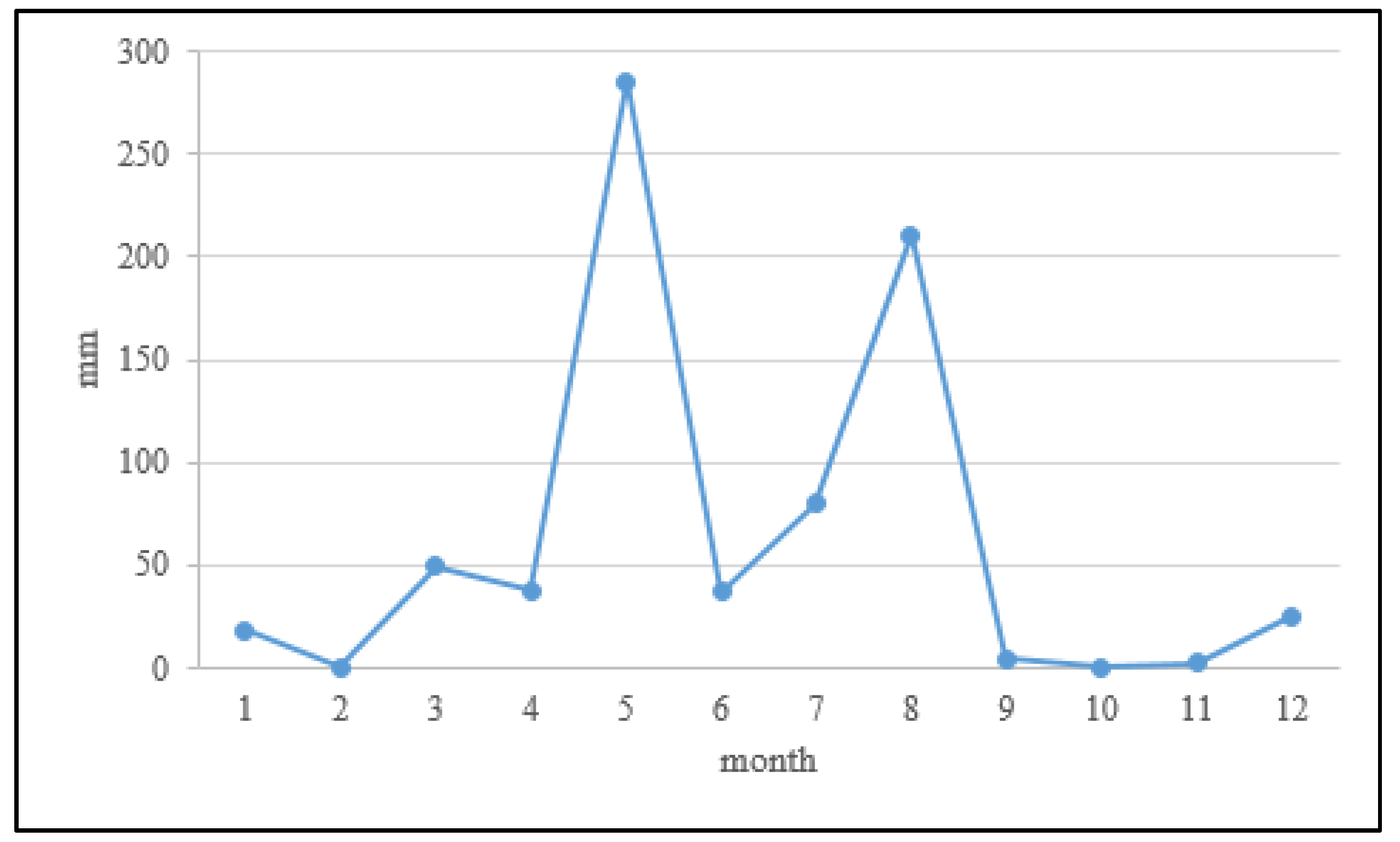
2.1. Soil Sampling
2.2. Analysis of Heavy Metals in Soil
2.3. DNA Extraction, PCR Amplification, and Sequencing
2.4. Diversity Analyses
2.5. Domestication, Separation and Purification of Heavy Metal-Tolerant Bacteria
2.6. Sequencing and Comparison of Bacterial Species
2.7. Heavy Metal Tolerance Test of Isolated Bacteria
3. Results and Discussion
3.1. Soil Element Content and Properties
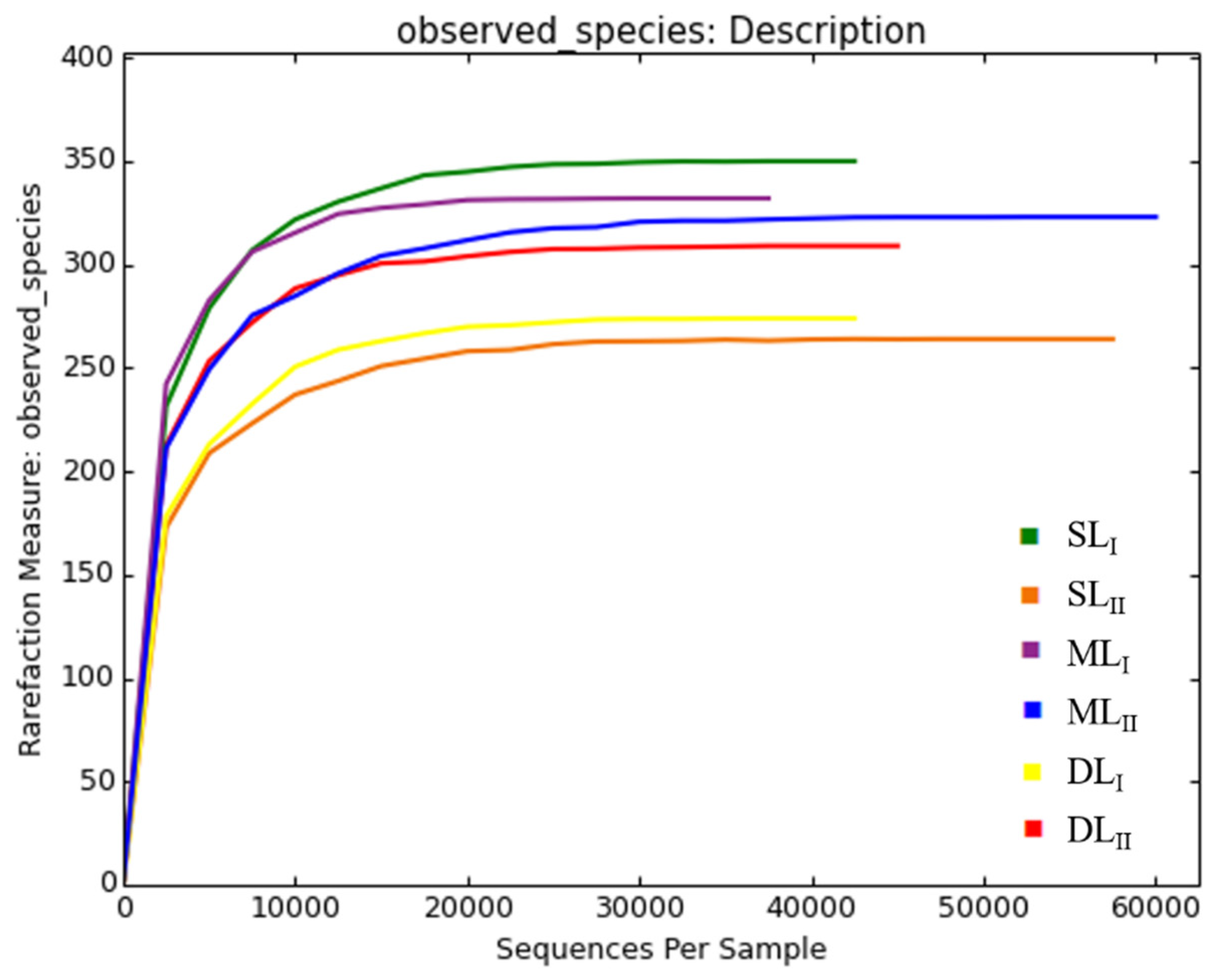
3.2. Sequence Data and Bacterial Taxonomic Richness
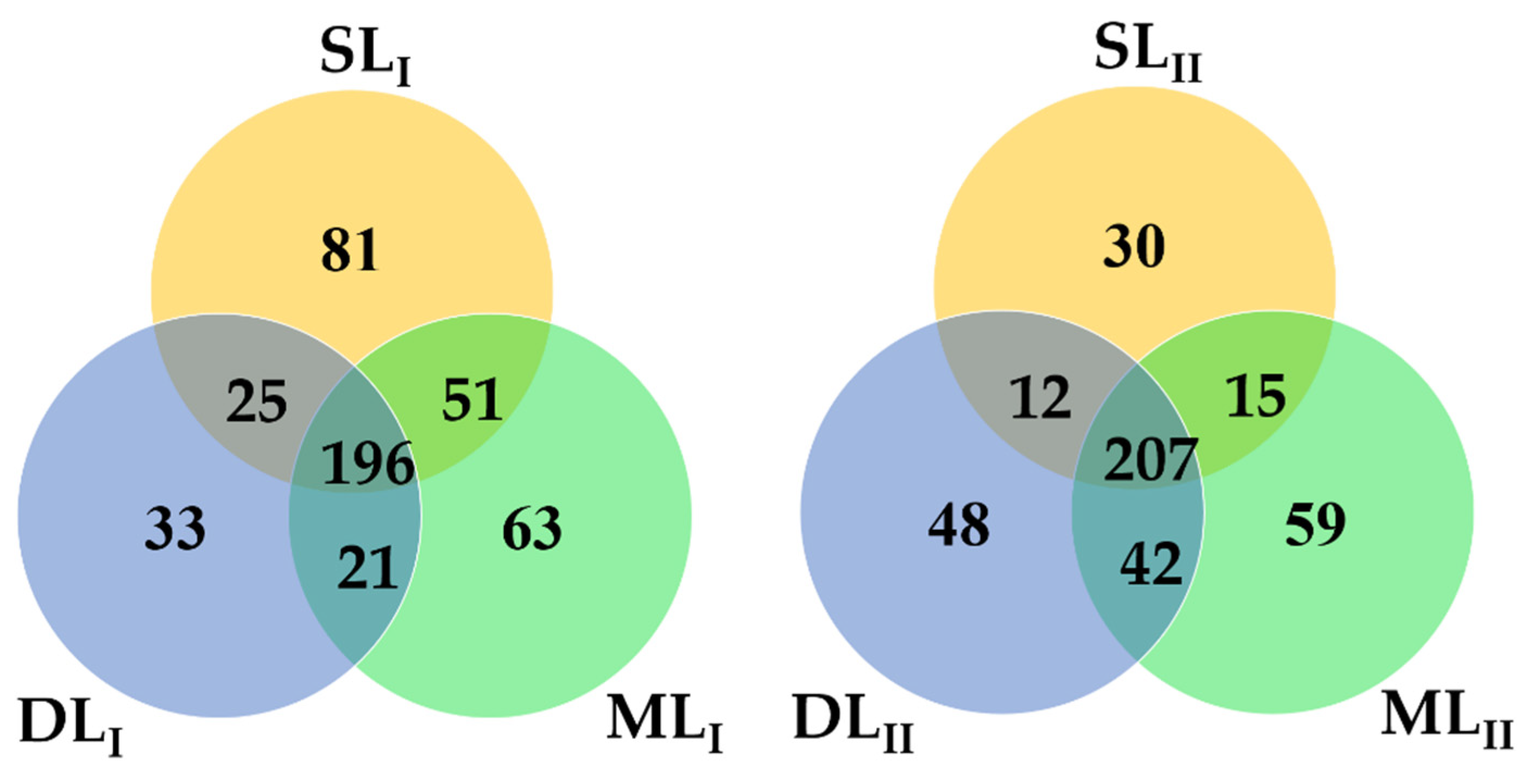
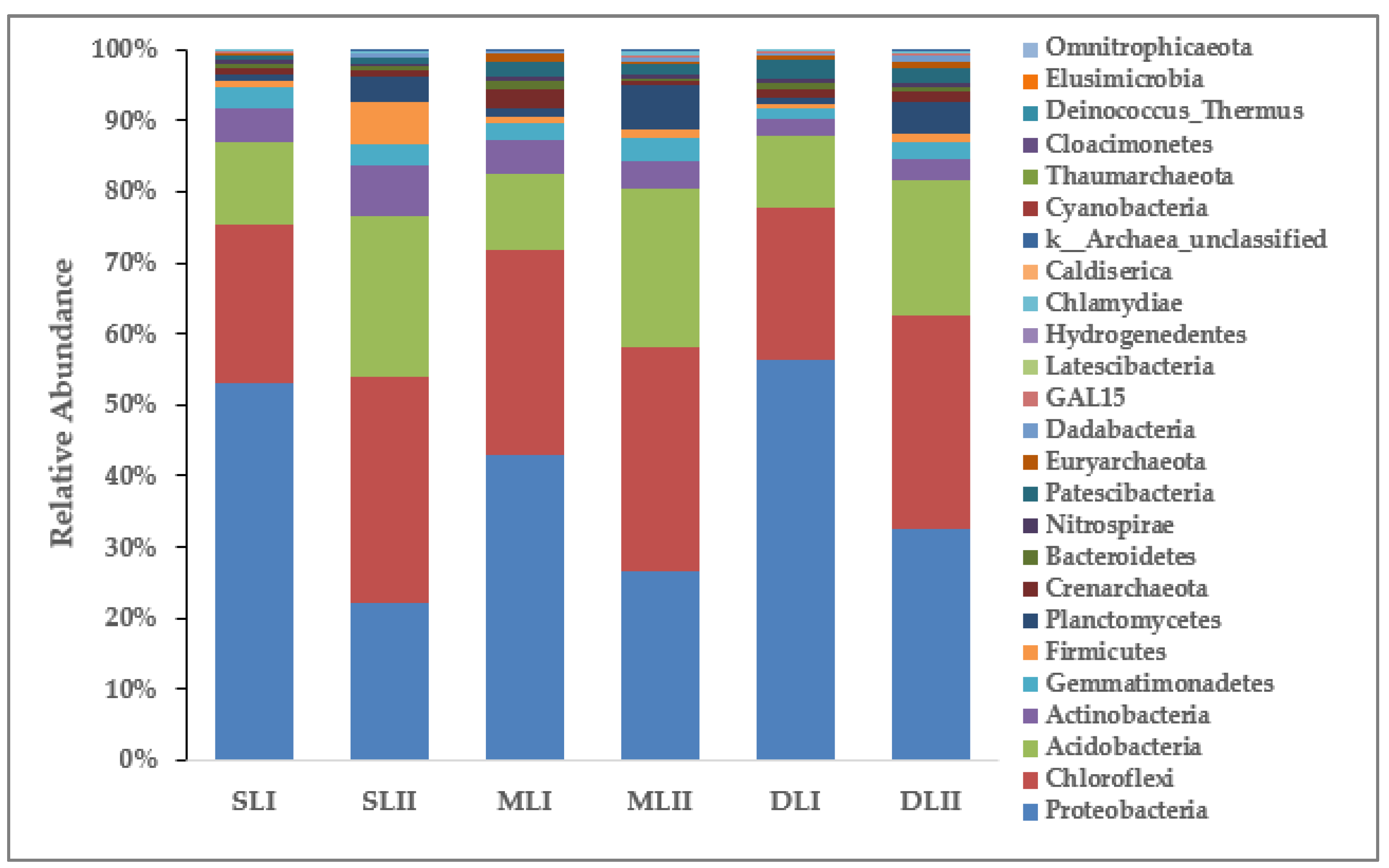
3.3. Analysis of the Bacterial Community in Soil
3.4. Bacteria Identification
3.5. Tolerance to Cu and Zn
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Li, Q.; Zhang, J.; Ge, W.; Sun, P.; Han, Y.; Qiu, H.; Zhou, S. Geochemical baseline establishment and source-oriented ecological risk assessment of heavy metals in lime concretion black soil from a typical agricultural area. Int. J. Environ. Res. Public Health 2021, 18, 6859. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, B.S.; Philippot, L. Insights into the resistance and resilience of the soil microbial community. FEMS Microbiol. Rev. 2013, 37, 112–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, X.; Zhang, Y.; Wommack, K.E.; Wilhelm, S.W.; DeBruyn, J.M.; Sherfy, A.C.; Zhuang, J.; Radosevich, M. Lysogenic reproductive strategies of viral communities vary with soil depth and are correlated with bacterial diversity. Soil Biol. Biochem. 2020, 144, 107767. [Google Scholar] [CrossRef]
- Alawiye, T.T.; Babalola, O.O. Metagenomic insight into the community structure and functional genes in the sunflower rhizosphere microbiome. Agriculture 2021, 11, 167. [Google Scholar] [CrossRef]
- Naveed, M.; Bukhari, S.S.; Mustafa, A.; Ditta, A.; Alamri, S.; El-Esawi, M.A.; Rafique, M.; Ashraf, S.; Siddiqui, M.H. Mitigation of nickel toxicity and growth promotion in sesame through the application of a bacterial endophyte and zeolite in nickel contaminated soil. Int. J. Environ. Res. Public Health 2020, 17, 8859. [Google Scholar] [CrossRef]
- Cui, H.; Liu, L.-L.; Dai, J.-R.; Yu, X.-N.; Guo, X.; Yi, S.-J.; Zhou, D.-Y.; Guo, W.-H.; Du, N. bacterial community shaped by heavy metals and contributing to health risks in cornfields. Ecotoxicol. Environ. Saf. 2018, 166, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Slimane, M.; El-hafid, N. Microbiome response under heavy metal stress. In Heavy Metals in the Environment; Kumar, V., Sharma, A., Cerdà, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 39–56. [Google Scholar] [CrossRef]
- Chodak, M.; Gołębiewski, M.; Morawska-Płoskonka, J.; Kuduk, K.; Niklińska, M. Diversity of microorganisms from forest soils differently polluted with heavy metals. Appl. Soil Ecol. 2013, 64, 7–14. [Google Scholar] [CrossRef]
- Mohammadian, E.; Babai Ahari, A.; Arzanlou, M.; Oustan, S.; Khazaei, S.H. Tolerance to heavy metals in filamentous fungi isolated from contaminated mining soils in the Zanjan Province, Iran. Chemosphere 2017, 185, 290–296. [Google Scholar] [CrossRef]
- Keiblinger, K.M.; Schneider, M.; Gorfer, M.; Paumann, M.; Deltedesco, E.; Berger, H.; Jöchlinger, L.; Mentler, A.; Zechmeister-Boltenstern, S.; Soja, G.; et al. Assessment of Cu applications in two contrasting soils—Effects on soil microbial activity and the fungal community structure. Ecotoxicology 2018, 27, 217–233. [Google Scholar] [CrossRef] [Green Version]
- Fagnano, M.; Agrelli, D.; Pascale, A.; Adamo, P.; Fiorentino, N.; Rocco, C.; Pepe, O.; Ventorino, V. Copper accumulation in agricultural soils: Risks for the food chain and soil microbial populations. Sci. Total Environ. 2020, 734, 139434. [Google Scholar] [CrossRef]
- Li, X.; Meng, D.; Li, J.; Yin, H.; Liu, H.; Liu, X.; Cheng, C.; Xiao, Y.; Liu, Z.; Yan, M. Response of soil microbial communities and microbial interactions to long-term heavy metal contamination. Environ. Pollut. 2017, 231, 908–917. [Google Scholar] [CrossRef]
- Ding, Z.; Wu, J.; You, A.; Huang, B.; Cao, C. Effects of heavy metals on soil microbial community structure and diversity in the rice (Oryza sativa L. subsp. Japonica, Food Crops Institute of Jiangsu Academy of Agricultural Sciences) rhizosphere. Soil Sci. Plant Nutr. 2017, 63, 75–83. [Google Scholar] [CrossRef] [Green Version]
- Soil and Groundwater Pollution Fund Management Board. 2018 Annual Report Soil and Groundwater Pollution Remediation. Available online: https://sgw.epa.gov.tw/public/download/annual-report/e845ec08-16c2-432d-942f-1a62b69f4f9a (accessed on 1 July 2021).
- Góes-Neto, A.; Loguercio-Leite, C.; Guerrero, R.T. DNA extraction from frozen fieldcollected and dehydrated herbarium fungal basidiomata: Performance of SDS and CTAB-based methods. Biotemas 2005, 18, 19–32. [Google Scholar] [CrossRef]
- Yamamoto, N.; Otawa, K.; Nakai, Y. Bacterial communities developing during composting processes in animal manure treatment facilities. Asian Australas. J. Anim. Sci. 2009, 22, 900–905. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.; Ye, Y.; Hu, Y.; Shi, H. The variation in microbial community structure under different heavy metal contamination levels in paddy soils. Ecotoxicol. Environ. Saf. 2019, 180, 557–564. [Google Scholar] [CrossRef]
- Chao, A. Estimating the population size for capture-recapture data with unequal catchability. Biometrics 1987, 43, 783–791. [Google Scholar] [CrossRef]
- Bandeira, B.; Jamet, J.-L.; Jamet, D.; Ginoux, J.-M. Mathematical convergences of biodiversity indices. Ecol. Indic. 2013, 29, 522–528. [Google Scholar] [CrossRef]
- Lane, D.J. 16S/23S rRNA sequencing. In Nucleic Acid Techniques in Bacterial Systematics; Stackebrandt, E., Goodfellow, M., Eds.; John Wiley & Sons: New York, NY, USA, 1911. [Google Scholar]
- Relman, D.A.; Schmidt, T.M.; MacDermott, R.P.; Falkow, S. Identification of the uncultured bacillus of whipple’s disease. N. Engl. J. Med. 1992, 327, 293–301. Available online: https://www.nejm:doi/full/10.1056/NEJM199207303270501 (accessed on 30 July 2021). [CrossRef]
- Wilbrink, B.; van der Heijden, I.M.; Schouls, L.M.; van Embden, J.D.A.; Hazes, J.M.W.; Breedveld, F.C.; Tak, P.P. Detection of bacterial DNA in joint samples from patients with undifferentiated arthritis and reactive arthritis, using polymerase chain reaction with universal 16S ribosomal RNA primers. Arthritis Rheum. 1998, 41, 535–543. [Google Scholar] [CrossRef]
- Soil Pollution Monitoring Standard—Article Content—Laws & Regulations Database of the Republic of China. Available online: https://law.moj.gov.tw/LawClass/LawAll.aspx?pcode=O0110012 (accessed on 28 June 2021).
- Soil Pollution ControlStandard—Article Content—Laws & Regulations Database of the Republic of China. Available online: https://law.moj.gov.tw/LawClass/LawAll.aspx?pcode=O0110005 (accessed on 28 June 2021).
- Bohn, H.L.; McNeal, B.L.; O’Connor, G.A. Soil Chemistry, 3rd ed.; Wiley: Hoboken, NJ, USA, 2001. [Google Scholar]
- Khadhar, S.; Sdiri, A.; Chekirben, A.; Azouzi, R.; Charef, A. Integration of sequential extraction, chemical analysis and statistical tools for the availability risk assessment of heavy metals in sludge amended soils. Environ. Pollut. 2020, 263, 114543. [Google Scholar] [CrossRef]
- Ayangbenro, A.S.; Babalola, O.O. A new strategy for heavy metal polluted environments: A review of microbial biosorbents. Int. J. Environ. Res. Public Health 2017, 14, 94. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Esteban, J.; Escolástico, C.; Ruiz-Fernández, J.; Masaguer, A.; Moliner, A. Bioavailability and extraction of heavy metals from contaminated soil by atriplex halimus. Environ. Exp. Bot. 2013, 88, 53–59. [Google Scholar] [CrossRef] [Green Version]
- Thavamani, P.; Malik, S.; Beer, M.; Megharaj, M.; Naidu, R. Microbial activity and diversity in long-term mixed contaminated soils with respect to polyaromatic hydrocarbons and heavy metals. J. Environ. Manag. 2012, 99, 10–17. [Google Scholar] [CrossRef]
- Song, J.; Shen, Q.; Wang, L.; Qiu, G.; Shi, J.; Xu, J.; Brookes, P.C.; Liu, X. Effects of Cd, Cu, Zn and their combined action on microbial biomass and bacterial community structure. Environ. Pollut. 2018, 243, 510–518. [Google Scholar] [CrossRef]
- Yin, H.; Niu, J.; Ren, Y.; Cong, J.; Zhang, X.; Fan, F.; Xiao, Y.; Zhang, X.; Deng, J.; Xie, M.; et al. An integrated insight into the response of sedimentary microbial communities to heavy metal contamination. Sci. Rep. 2015, 5, 14266. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.J.; Jeong, S.E.; Kim, P.J.; Madsen, E.L.; Jeon, C.O. High resolution depth distribution of bacteria, archaea, methanotrophs, and methanogens in the bulk and rhizosphere soils of a flooded rice PADDY. Front. Microbiol. 2015, 6. [Google Scholar] [CrossRef]
- Hu, W.; Zhang, Q.; Tian, T.; Li, D.; Cheng, G.; Mu, J.; Wu, Q.; Niu, F.; Stegen, J.C.; An, L.; et al. Relative roles of deterministic and stochastic processes in driving the vertical distribution of bacterial communities in a permafrost core from the Qinghai-Tibet Plateau, China. PLoS ONE 2015, 10, e0145747. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Huang, J.; Lu, J.; Sun, Y. Study on the influence of soil microbial community on the long-term heavy metal pollution of different land use types and depth layers in mine. Ecotoxicol. Environ. Saf. 2019, 170, 218–226. [Google Scholar] [CrossRef]
- Park, K.; Chess, B. Foundations in Microbiology; McGraw-Hill: New York, NY, USA, 2012. [Google Scholar]
- Li, C.; Wang, X.; Huang, H.; Wang, L.; Wei, F.; Zhang, C.; Rong, Q. Effect of multiple heavy metals pollution to bacterial diversity and community structure in farmland soils. Hum. Ecol. Risk Assess. Int. J. 2021, 27, 724–741. [Google Scholar] [CrossRef]
- Luo, L.Y.; Xie, L.L.; Jin, D.C.; Mi, B.B.; Wang, D.H.; Li, X.F.; Dai, X.Z.; Zou, X.X.; Zhang, Z.; Ma, Y.Q.; et al. Bacterial community response to cadmium contamination of agricultural paddy soil. Appl. Soil Ecol. 2019, 139, 100–106. [Google Scholar] [CrossRef]
- Yang, J.; Wang, S.; Guo, Z.; Deng, Y.; Xu, M.; Zhang, S.; Yin, H.; Liang, Y.; Liu, H.; Miao, B.; et al. Spatial distribution of toxic metal(loid)s and microbial community analysis in soil vertical profile at an abandoned nonferrous metal smelting site. Int. J. Environ. Res. Public Health 2020, 17, 7101. [Google Scholar] [CrossRef]
- Beattie, R.E.; Henke, W.; Campa, M.F.; Hazen, T.C.; McAliley, L.R.; Campbell, J.H. Variation in microbial community structure correlates with heavy-metal contamination in soils decades after mining ceased. Soil Biol. Biochem. 2018, 126, 57–63. [Google Scholar] [CrossRef] [Green Version]
- Bouskill, N.J.; Barker-Finkel, J.; Galloway, T.S.; Handy, R.D.; Ford, T.E. Temporal bacterial diversity associated with metal-contaminated river sediments. Ecotoxicology 2010, 19, 317–328. [Google Scholar] [CrossRef]
- Nunoura, T.; Hirai, M.; Miyazaki, M.; Kazama, H.; Makita, H.; Hirayama, H.; Furushima, Y.; Yamamoto, H.; Imachi, H.; Takai, K. Isolation and characterization of a thermophilic, obligately anaerobic and heterotrophic marine chloroflexi bacterium from a chloroflexi-dominated microbial community associated with a Japanese shallow hydrothermal system, and proposal for thermomarinilinea lacunofontalis gen. nov., sp. nov. microb. Microbes Environ. 2013, 28, 228–235. [Google Scholar] [CrossRef] [Green Version]
- Idris, R.; Trifonova, R.; Puschenreiter, M.; Wenzel, W.W.; Sessitsch, A. Bacterial communities associated with flowering plants of the Ni hyperaccumulator thlaspi goesingense. Appl. Environ. Microbiol. 2004, 70, 2667–2677. [Google Scholar] [CrossRef] [Green Version]
- Wolna-Maruwka, A.; Piechota, T.; Niewiadomska, A.; Kamiński, A.; Kayzer, D.; Grzyb, A.; Pilarska, A.A. The effect of biochar-based organic amendments on the structure of soil bacterial community and yield of maize (Zea mays L.). Agronomy 2021, 11, 1286. [Google Scholar] [CrossRef]
- Brandt, K.K.; Frandsen, R.J.N.; Holm, P.E.; Nybroe, O. Development of pollution-induced community tolerance is linked to structural and functional resilience of a soil bacterial community following a five-year field exposure to copper. Soil Biol. Biochem. 2010, 42, 748–757. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, J.; Li, Q.; Han, T.; Xie, J.; Hu, Y.; Chai, L. Phylogenetic analysis of bacterial community composition in sediment contaminated with multiple heavy metals from the xiangjiang river in China. Mar. Pollut. Bull. 2013, 70, 134–139. [Google Scholar] [CrossRef]
- Huang, F.; Dang, Z.; Guo, C.-L.; Lu, G.-N.; Gu, R.R.; Liu, H.-J.; Zhang, H. Biosorption of Cd(II) by live and dead cells of bacillus cereus RC-1 isolated from cadmium-contaminated soil. Colloids Surf. B Biointerfaces 2013, 107, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Jin, Y.; Zhang, C.; Gu, H.; Qu, J. Characteristics of bacillus Sp. PZ-1 and its biosorption to Pb(II). Ecotoxicol. Environ. Saf. 2015, 117, 141–148. [Google Scholar] [CrossRef]
- Ayangbenro, A.S.; Babalola, O.O. Genomic analysis of bacillus cereus NWUAB01 and its heavy metal removal from polluted soil. Sci. Rep. 2020, 10, 19660. [Google Scholar] [CrossRef]
- Hasan, H.A.; Abdullah, S.R.S.; Kofli, N.T.; Kamarudin, S.K. Isotherm equilibria of Mn2+ biosorption in drinking water treatment by locally isolated bacillus species and sewage activated sludge. J. Environ. Manag. 2012, 111, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Abu Hasan, H.; Sheikh Abdullah, S.R.; Tan Kofli, N.; Yeoh, S.J. Interaction of environmental factors on simultaneous biosorption of lead and manganese ions by locally isolated bacillus cereus. J. Ind. Eng. Chem. 2016, 37, 295–305. [Google Scholar] [CrossRef]


| No. | Primer Set | Size (bp) | Target | Reference(s) |
|---|---|---|---|---|
| 1. | 27F/1525R | 1500 | 16S rRNA gene | [21] |
| 2. | 8F2/806R | 802 | 16S rRNA gene | [22] |
| 3. | fD1modF/16S1RR-B | 568 | 16S rRNA gene | [23] |
| mg/kg | |||||||
|---|---|---|---|---|---|---|---|
| Cr | Ni | Cu | Zn | Cd | Pb | pH | |
| SLI | 160.796.36 | 133.784.95 | 557.0913.76 | 603.0629.49 | 0.670.12 | 68.038.27 | 6.520.01 |
| MLI | 165.998.37 | 139.8911.49 | 528.7416.32 | 586.6825.92 | 0.690.11 | 64.216.89 | 6.700.01 |
| DLI | 166.856.30 | 136.959.56 | 460.1315.92 | 544.8023.12 | 0.670.06 | 59.082.35 | 6.880.01 |
| SLII | 153.539.62 | 127.4412.70 | 583.1221.48 | 643.9524.26 | 0.680.11 | 70.089.78 | 6.090.01 |
| MLII | 153.369.11 | 123.9114.28 | 578.5621.77 | 594.2128.79 | 0.690.08 | 69.156.35 | 6.160.01 |
| DLII | 163.257.70 | 131.1015.14 | 588.8813.81 | 633.7716.60 | 0.840.11 | 68.395.95 | 6.230.01 |
| Variation in SL between two samplings | −4.5% | −4.7% | 4.7% | 6.8% | 1.5% | 3.0% | −6.6% |
| Variation in ML between two samplings | −7.6% | −11% | 9.4% | 1.3% | 0.0% | 7.7% | −8.1% |
| Variation in DL between two samplings | −2.2% | −4.3% | 28% | 16% | 25% | 16% | −9.4% |
| Monitoring value | 109 | 112 | 280 | 363 | <0.36 | 41.4 | |
| Monitoring standard a | 175 | 130 | 120 | 260 | 2.5 | 300 | |
| Control standard b | 250 | 200 | 200 | 600 | 5 | 500 | |
| OTUs | Observed_Species | Shannon | Simpson_Reciprocal | Chao1 | |
|---|---|---|---|---|---|
| SLI | 33,130 | 353 | 6.01 | 25.12 | 353 |
| MLI | 39,622 | 331 | 6.27 | 32.63 | 331 |
| DLI | 30,014 | 275 | 5.46 | 17.38 | 275 |
| SLII | 21,681 | 264 | 5.72 | 20.50 | 264 |
| MLII | 18,085 | 323 | 6.15 | 29.22 | 323 |
| DLII | 15,635 | 309 | 6.21 | 33.77 | 309 |
| Variation in DL between two samplings | −47.9% | 12.8% | 13.7% | 94.3% | 12.8% |
| LAB-ID | Closest NCBI xiaoshuangDatabase Match | Copper Enriched-Media Concentration (mg/L) | Zinc Enriched-Media Concentration (mg/L) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 50 | 100 | 150 | 200 | 400 | 50 | 100 | 150 | 200 | 400 | ||
| Cu_SLI-1 | Bacillus velezensis | ○ | ν | - | ○ | ○ | ν | - | |||
| Cu_MLI-1 | Bacillus albus | ν | - | ○ | ○ | ν | - | ||||
| Cu_SLII-1 | Bacillus marisflavi | ○ | ○ | ○ | ○ | - | ○ | ν | - | - | |
| Cu_SLII-2 | Bacillsu megaterium | ○ | ○ | ○ | ○ | - | ○ | ○ | ○ | ○ | - |
| Cu_SLII-3 | Bacillus velezensis | ○ | ν | - | ○ | ○ | ν | - | |||
| Cu_SLII-4 | Bacillus marisflavi | ν | - | ○ | ν | - | - | ||||
| Cu_MLII-1 | Bacillus amyloliquefaciens | ν | - | ○ | ○ | ν | - | ||||
| Cu_MLII-2 | Bacillus aryabhattai | ○ | ○ | ○ | ○ | - | ○ | ○ | ○ | ν | - |
| Cu_MLII-3 | Bacillus kribbensis | ν | - | ○ | ○ | ○ | ○ | - | |||
| Cu_MLII-4 | Bacillus velezensis | ○ | ○ | ○ | ○ | - | ○ | ○ | ν | - | |
| Cu_DLII-1 | Bacillus velezensis | ν | - | ○ | ○ | ν | - | ||||
| Cu_DLII-3 | Bacillus marisflavi | ○ | ○ | ○ | ○ | - | ○ | ○ | ν | ν | - |
| Zn_SLII-1 | Bacillus marisflavi | ν | - | ○ | ○ | ○ | ○ | - | |||
| Zn_SLII-2 | Bacillus albus | - | ○ | ○ | ○ | ○ | - | ||||
| Zn_MLII-1 | Bacillus albus | - | ○ | ○ | ○ | ○ | - | ||||
| Zn_DLII-1 | Bacillus albus | - | ○ | ν | ν | ν | - | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tseng, S.-c.; Liang, C.-m.; Chia, T.; Ton, S.-s. Changes in the Composition of the Soil Bacterial Community in Heavy Metal-Contaminated Farmland. Int. J. Environ. Res. Public Health 2021, 18, 8661. https://doi.org/10.3390/ijerph18168661
Tseng S-c, Liang C-m, Chia T, Ton S-s. Changes in the Composition of the Soil Bacterial Community in Heavy Metal-Contaminated Farmland. International Journal of Environmental Research and Public Health. 2021; 18(16):8661. https://doi.org/10.3390/ijerph18168661
Chicago/Turabian StyleTseng, Shu-chun, Chih-ming Liang, Taipau Chia, and Shan-shin Ton. 2021. "Changes in the Composition of the Soil Bacterial Community in Heavy Metal-Contaminated Farmland" International Journal of Environmental Research and Public Health 18, no. 16: 8661. https://doi.org/10.3390/ijerph18168661
APA StyleTseng, S.-c., Liang, C.-m., Chia, T., & Ton, S.-s. (2021). Changes in the Composition of the Soil Bacterial Community in Heavy Metal-Contaminated Farmland. International Journal of Environmental Research and Public Health, 18(16), 8661. https://doi.org/10.3390/ijerph18168661






