Bioavailability Assessment of Heavy Metals Using Various Multi-Element Extractants in an Indigenous Zinc Smelting Contaminated Site, Southwestern China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Soil Sampling and Preparation
2.3. The Determination of Total Metal Concentration
2.4. Soil Bioavailable Metal Extraction
2.5. Speciation Distribution of Heavy Metals
2.6. Quality Assurance and Data Analyses
3. Results and Discussion
3.1. Soil Metal Concentration
3.2. Soil Bioavailable Metal Fraction
3.3. Soil Metal Fractionation
3.4. Impact of Heavy Metals Speciations on Their Bioavailability
3.5. Soil Metal Release Kinetics
4. Conclusions and Environmental Implication
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Park, S.-C.; Boyanov, M.I.; Kemner, K.M.; O’Loughlin, E.J.; Kwon, M.J. Distribution and speciation of Sb and toxic metal(loid)s near an antimony refinery and their effects on indigenous microorganisms. J. Hazard. Mater. 2020, 403, 123625. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, B.; Wang, S.; Chen, J.; Jiang, B.; Xing, Y. Spatiotemporal vanadium distribution in soils with microbial community dynamics at vanadium smelting site. Environ. Pollut. 2020, 265, 114782. [Google Scholar] [CrossRef]
- Yan, D.; Guo, Z.; Xiao, X.; Peng, C.; He, Y.; Yang, A.; Wang, X.; Hu, Y.; Li, Z. Cleanup of arsenic, cadmium, and lead in the soil from a smelting site using N,N-bis(carboxymethyl)-L-glutamic acid combined with ascorbic acid: A lab-scale experiment. J. Environ. Manag. 2021, 296, 113174. [Google Scholar] [CrossRef]
- Xiao, R.; Shen, F.; Du, J.; Li, R.; Lahori, A.H.; Zhang, Z. Screening of native plants from wasteland surrounding a Zn smelter in Feng County China, for phytoremediation. Ecotoxicol. Environ. Saf. 2018, 162, 178–183. [Google Scholar] [CrossRef]
- Lopes, G.; Li, W.; Siebecker, M.G.; Sparks, D.L.; Guilherme, L.R.G. Combining zinc desorption with EXAFS speciation analysis to understand Zn mobility in mining and smelting affected soils in Minas Gerais, Brazil. Sci. Total Environ. 2020, 754, 142450. [Google Scholar] [CrossRef]
- Tuhý, M.; Hrstka, T.; Ettler, V. Automated mineralogy for quantification and partitioning of metal(loid)s in particulates from mining/smelting-polluted soils. Environ. Pollut. 2020, 266, 115118. [Google Scholar] [CrossRef]
- Lomaglio, T.; Hattab-Hambli, N.; Miard, F.; Lebrun, M.; Nandillon, R.; Trupiano, D.; Scippa, G.S.; Gauthier, A.; Motelica-Heino, M.; Bourgerie, S.; et al. Cd, Pb, and Zn mobility and (bio)availability in contaminated soils from a former smelting site amended with biochar. Environ. Sci. Pollut. Res. 2017, 25, 25744–25756. [Google Scholar] [CrossRef] [Green Version]
- Yin, N.; Li, Y.; Yang, Y.; Fan, C.; Li, Y.; Du, X.; Sun, G.; Cui, Y. Human health risk assessment in aluminium smelting site: Soil fluoride bioaccessibility and relevant mechanism in simulated gastrointestinal tract. J. Hazard. Mater. 2021, 416, 125899. [Google Scholar] [CrossRef]
- Remon, E.; Bouchardon, J.L.; Le Guedard, M.; Bessoule, J.J.; Conord, C.; Faure, O. Are plants useful as accumulation indicators of metal bioavailability? Environ. Pollut. 2013, 175, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Kim, R.-Y.; Yoon, J.-K.; Kim, T.-S.; Yang, J.E.; Owens, G.; Kim, K.-R. Bioavailability of heavy metals in soils: Definitions and practical implementation—A critical review. Environ. Geochem. Health 2015, 37, 1041–1061. [Google Scholar] [CrossRef] [PubMed]
- Pelfrêne, A.; Sahmer, K.; Waterlot, C.; Glorennec, P.; Douay, F.; Le Bot, B. Evaluation of single-extraction methods to estimate the oral bioaccessibility of metal(loid)s in soils. Sci. Total Environ. 2020, 727, 138553. [Google Scholar] [CrossRef]
- He, B.; Wang, W.; Geng, R.; Ding, Z.; Luo, D.; Qiu, J.; Zheng, G.; Fan, Q. Exploring the fate of heavy metals from mining and smelting activities in soil-crop system in Baiyin, NW China. Ecotoxicol. Environ. Saf. 2020, 207, 111234. [Google Scholar] [CrossRef]
- Liu, B.; Ai, S.; Zhang, W.; Huang, D.; Zhang, Y. Assessment of the bioavailability, bioaccessibility and transfer of heavy metals in the soil-grain-human systems near a mining and smelting area in NW China. Sci. Total Environ. 2017, 609, 822–829. [Google Scholar] [CrossRef]
- Krauße, T.; Schütze, E.; Phieler, R.; Fürst, D.; Merten, D.; Büchel, G.; Kothe, E. Changes in element availability induced by sterilization in heavy metal contaminated substrates: A comprehensive study. J. Hazard. Mater. 2019, 370, 70–79. [Google Scholar] [CrossRef]
- Galhardi, J.A.; Leles, B.P.; de Mello, J.W.; Wilkinson, K. Bioavailability of trace metals and rare earth elements (REE) from the tropical soils of a coal mining area. Sci. Total Environ. 2019, 717, 134484. [Google Scholar] [CrossRef] [PubMed]
- Lewis, C.; Lennon, A.M.; Eudoxie, G.; Sivapatham, P.; Umaharan, P. Plant metal concentrations in Theobroma cacao as affected by soil metal availability in different soil types. Chemosphere 2020, 262, 127749. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.-M.; Fu, R.-B.; Tong, Y.-H.; Shen, D.-L.; Guo, X.-P. The potential environmental risk implications of heavy metals based on their geochemical and mineralogical characteristics in the size-segregated zinc smelting slags. J. Clean. Prod. 2021, 315, 128199. [Google Scholar] [CrossRef]
- Wu, T.; Bi, X.; Li, Z.; Sun, G.; Feng, X.; Shang, L.; Zhang, H.; He, T.; Chen, J. Contaminations, Sources, and Health Risks of Trace Metal(loid)s in Street Dust of a Small City Impacted by Artisanal Zn Smelting Activities. Int. J. Environ. Res. Public Health 2017, 14, 961. [Google Scholar] [CrossRef] [PubMed]
- Li, G.H.; Feng, X.B.; Qiu, G.L.; Bi, X.Y.; Li, Z.G.; Zhang, C.; Wang, D.Y.; Shang, L.H.; Guo, Y.N. Environmental mercury con-tamination of an artisanal zinc smelting area in Weining County, Guizhou, China. Environ. Pollut. 2008, 154, 21–31. [Google Scholar] [CrossRef]
- Luo, Y.; Wu, Y.; Xing, R.; Wang, H.; Shu, J.; Wu, Z.; Wan, Z. Assessment of chemical, biochemical, and microbiological properties in an artisanal Zn-smelting waste slag site revegetated with four native woody plant species. Appl. Soil Ecol. 2018, 124, 17–26. [Google Scholar] [CrossRef]
- Luo, Y.; Wu, Y.; Xing, R.; Yao, C.; Shu, J.; Wu, Z. Effects of plant litter decomposition on chemical and microbiological characteristics of artisanal zinc smelting slag using indigenous methods. J. Geochem. Explor. 2018, 190, 292–301. [Google Scholar] [CrossRef]
- Yang, Y.; Li, S.; Bi, X.; Wu, P.; Liu, T.; Li, F.; Liu, C. Lead, Zn, and Cd in slags, stream sediments, and soils in an abandoned Zn smelting region, southwest of China, and Pb and S isotopes as source tracers. J. Soils Sediments 2010, 10, 1527–1539. [Google Scholar] [CrossRef]
- Luo, Y.; Wu, Y.; Qiu, J.; Wang, H.; Yang, L. Suitability of four woody plant species for the phytostabilization of a zinc smelting slag site after 5 years of assisted revegetation. J. Soils Sediments 2018, 19, 702–715. [Google Scholar] [CrossRef]
- Ma, L.; Duan, T.; Hu, J. Application of a universal soil extractant for determining the available NPK: A case study of crop planting zones in central China. Sci. Total Environ. 2020, 704, 135253. [Google Scholar] [CrossRef]
- Zhang, W.; Yang, J.; Li, Z.; Zhou, D.; Dang, F. Assessment of the availability of As and Pb in soils after in situ stabilization. Environ. Sci. Pollut. Res. 2017, 24, 23153–23160. [Google Scholar] [CrossRef]
- Tessier, A.; Campbell, P.G.C.; Bisson, M. Sequential extraction procedure for the speciation of particulate trace metals. Anal. Chem. 1979, 51, 844–851. [Google Scholar] [CrossRef]
- Ministry of Ecology and Environment of the People’s Republic of China (MEEPRC). Soil Environmental Quality-Risk Control Standard for Soil Contamination of Agricultural Land. GB15618—2018 (2018). (In Chinese). Available online: http://english.mee.gov.cn/Resources/laws/environmental_laws/202011/t20201113_807786.shtml (accessed on 18 July 2021).
- Lee, P.-K.; Kang, M.-J.; Yu, S.; Kwon, Y.K. Assessment of trace metal pollution in roof dusts and soils near a large Zn smelter. Sci. Total Environ. 2020, 713, 136536. [Google Scholar] [CrossRef] [PubMed]
- Amnai, A.; Radola, D.; Choulet, F.; Buatier, M.; Gimbert, F. Impact of ancient iron smelting wastes on current soils: Legacy contamination, environmental availability and fractionation of metals. Sci. Total Environ. 2021, 776, 145929. [Google Scholar] [CrossRef]
- Xu, D.-M.; Fu, R.-B.; Liu, H.-Q.; Guo, X.-P. Current knowledge from heavy metal pollution in Chinese smelter contaminated soils, health risk implications and associated remediation progress in recent decades: A critical review. J. Clean. Prod. 2020, 286, 124989. [Google Scholar] [CrossRef]
- Li, S.; Bi, X.; Li, Z.; Wang, H.; Li, X.; Feng, X.; Sun, G.; Chen, J.; Meng, B. Heavy Metal(loid)s Contamination in Ground Dust and Associated Health Risks at a Former Indigenous Zinc Smelting Area. Int. J. Environ. Res. Public Health 2021, 18, 893. [Google Scholar] [CrossRef]
- Tang, J.; Zhang, L.; Zhang, J.; Ren, L.; Zhou, Y.; Zheng, Y.; Luo, L.; Yang, Y.; Huang, H.; Chen, A. Physicochemical features, metal availability and enzyme activity in heavy metal-polluted soil remediated by biochar and compost. Sci. Total Environ. 2019, 701, 134751. [Google Scholar] [CrossRef]
- Zhong, X.; Chen, Z.; Li, Y.; Ding, K.; Liu, W.; Liu, Y.; Yuan, Y.; Zhang, M.; Baker, A.J.M.; Yang, W.; et al. Factors influencing heavy metal availability and risk assessment of soils at typical metal mines in Eastern China. J. Hazard. Mater. 2020, 400, 123289. [Google Scholar] [CrossRef]
- Li, W.C.; Wong, M.-H. Effects of bacteria on metal bioavailability, speciation, and mobility in different metal mine soils: A column study. J. Soils Sediments 2010, 10, 313–325. [Google Scholar] [CrossRef]
- Golui, D.; Datta, S.P.; Rattan, R.K.; Dwivedi, B.S.; Meena, M.C. Predicting bioavailability of metals from sludge-amended soils. Environ. Monit. Assess. 2014, 186, 8541–8553. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Geng, Y.; Sun, R.; Xie, M.; Feng, X.; Li, X.; Cui, Z. Distribution, speciation and ecological risk assessment of heavy metals in Jinan Iron & Steel Group soils from China. J. Clean. Prod. 2021, 295, 126504. [Google Scholar] [CrossRef]
- Sundaray, S.K.; Nayak, B.B.; Lin, S.; Bhatta, D. Geochemical speciation and risk assessment of heavy metals in the river estuarine sediments—A case study: Mahanadi basin, India. J. Hazard. Mater. 2011, 186, 1837–1846. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.-M.; Zhan, C.-L.; Liu, H.-X.; Lin, H.-Z. A critical review on environmental implications, recycling strategies, and ecological remediation for mine tailings. Environ. Sci. Pollut. Res. 2019, 26, 35657–35669. [Google Scholar] [CrossRef]
- Munir, M.A.M.; Irshad, S.; Yousaf, B.; Ali, M.U.; Dan, C.; Abbas, Q.; Liu, G.; Yang, X. Interactive assessment of lignite and bamboo-biochar for geochemical speciation, modulation and uptake of Cu and other heavy metals in the copper mine tailing. Sci. Total Environ. 2021, 779, 146536. [Google Scholar] [CrossRef]
- Liu, H.-B.; Liu, S.-J.; He, X.-S.; Dang, F.; Tang, Y.-Y.; Xi, B.-D. Effects of landfill refuse on the reductive dechlorination of pentachlorophenol and speciation transformation of heavy metals. Sci. Total Environ. 2020, 760, 144122. [Google Scholar] [CrossRef]
- Mohamadi, S.; Saeedi, M.; Mollahosseini, A. Desorption kinetics of heavy metals (Lead, Zinc, and Nickel) coexisted with phe-nanthrene from a natural high buffering doil. Int. J. Eng. 2019, 32, 1716–1725. [Google Scholar]
- Mishra, S.R.; Chandra, R.; Kaila, A.J.; Darshi, B.S. Kinetics and isotherm studies for the adsorption of metal ions onto two soil types. Environ. Tech. Innov. 2017, 7, 87–101. [Google Scholar] [CrossRef]
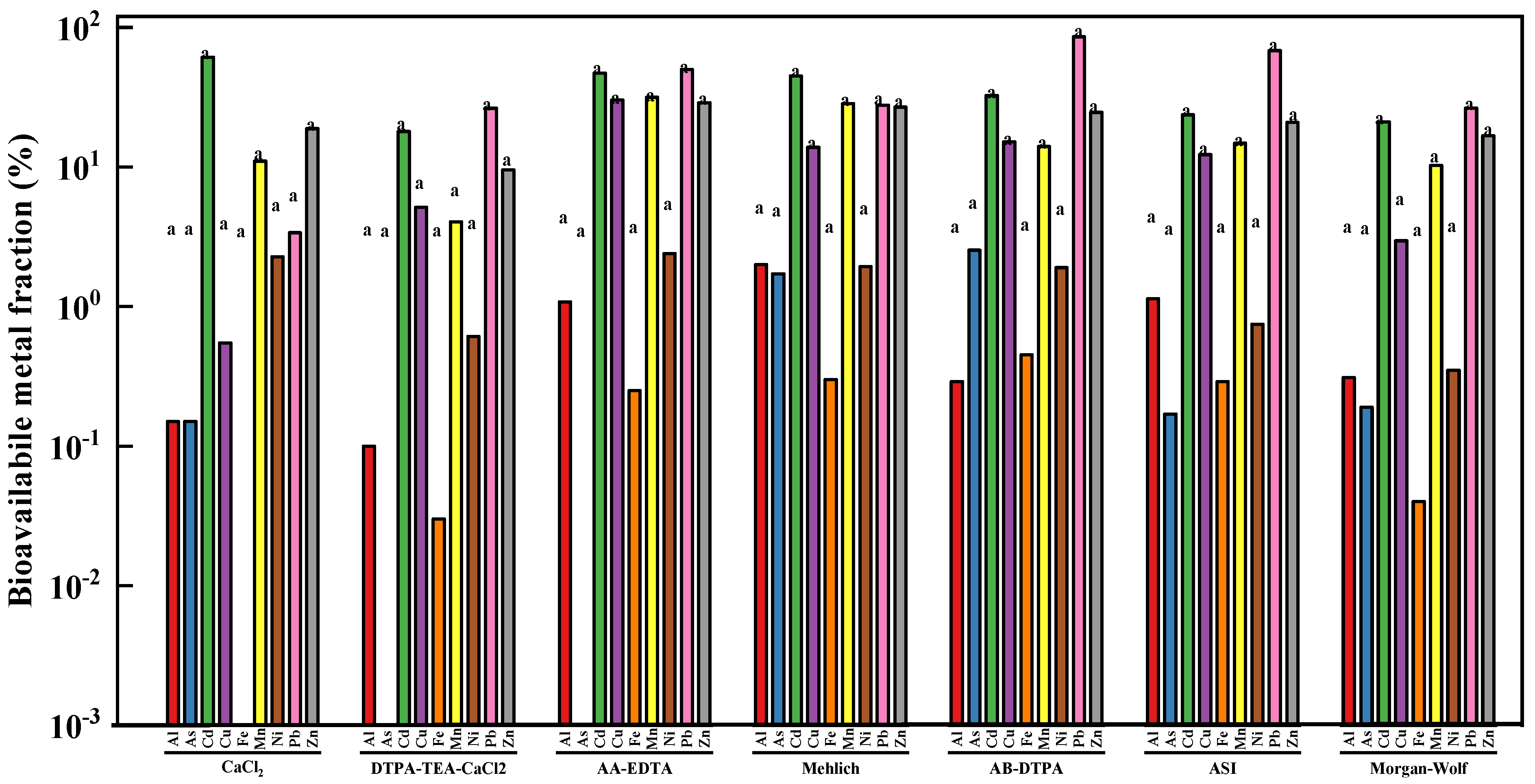
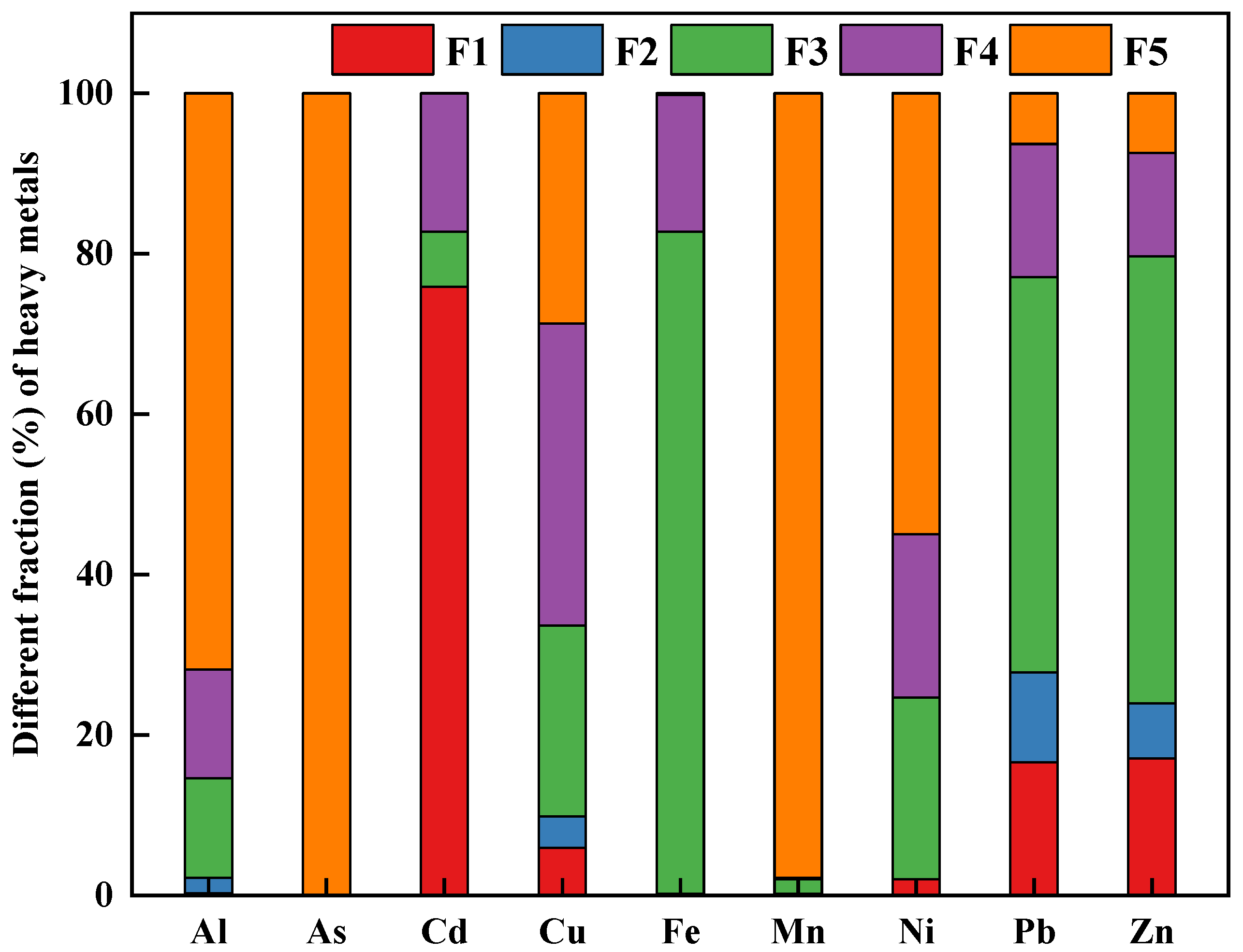
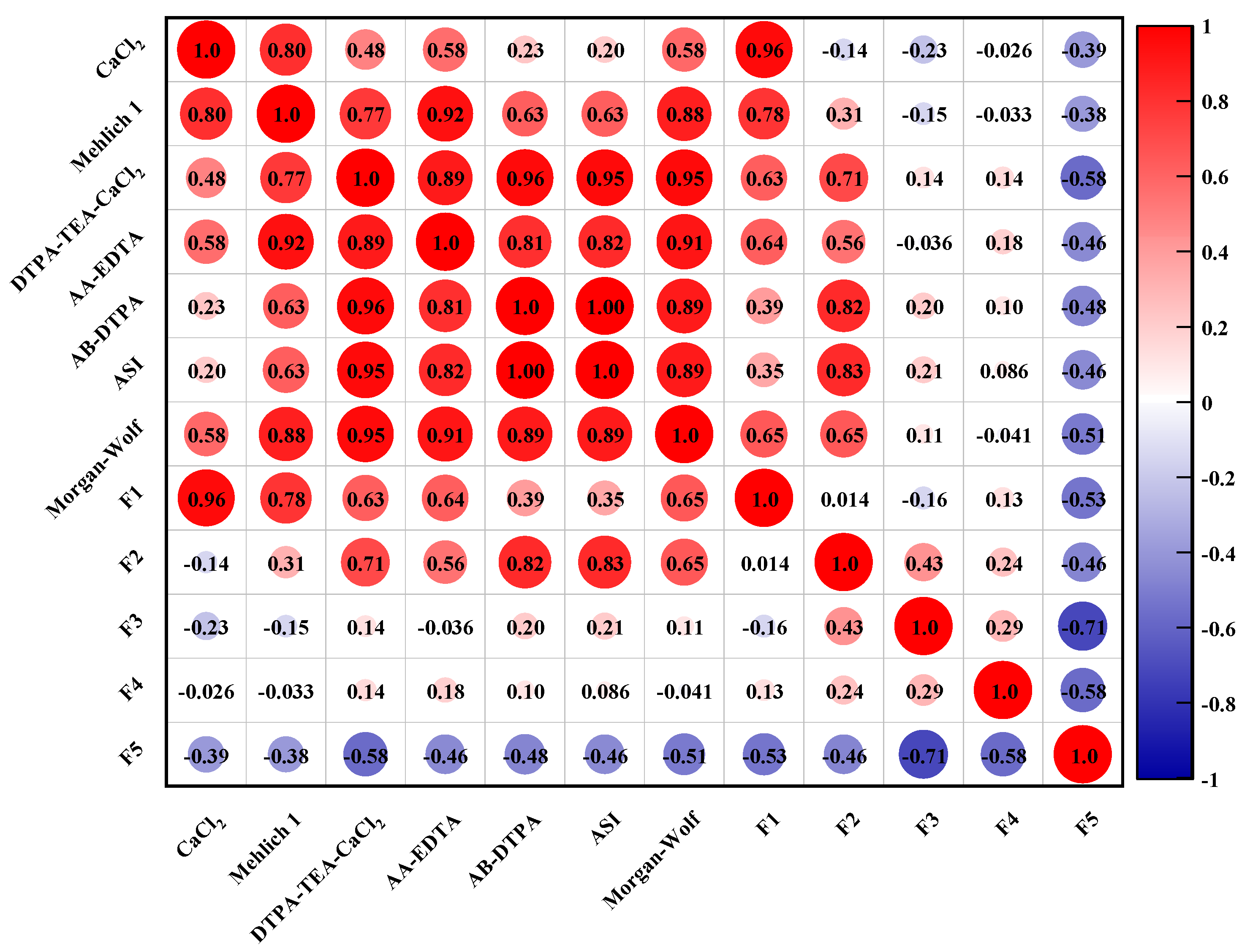
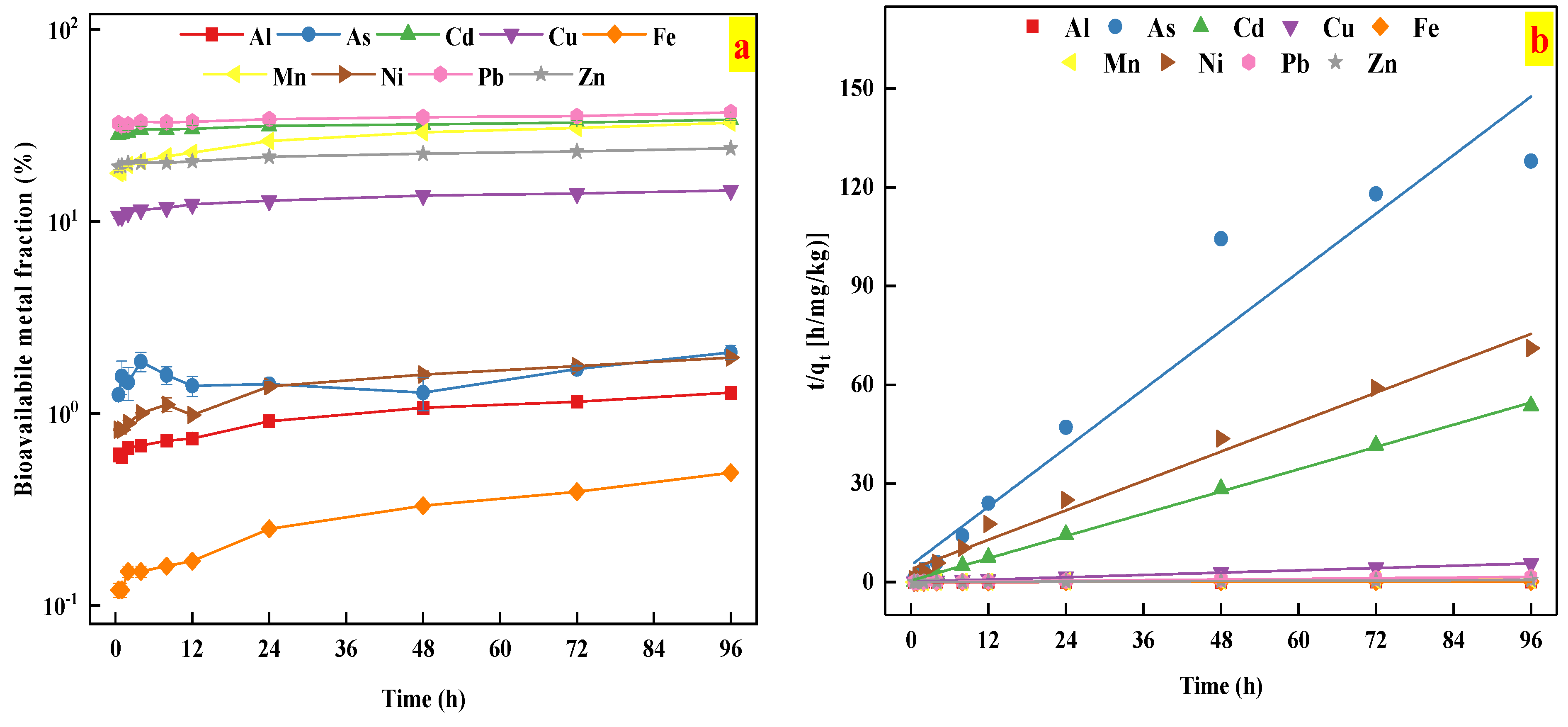
| Extractants | Chemical Reagents | pH | Soil Types | The Extracted Elements |
|---|---|---|---|---|
| Calcium chloride | 0.1 mol/L CaCl2 | - | - | Al, P, As, K, Mg, Ge, Na, Ni, B, Cu, Fe and Pb |
| Mehlich 1 | 0.05 mol/L HCl + 0.025 mol/L H2SO4 | 2.5 | Neutral to alkaline soils | P, K, Ca, Mg, Na, Mn and Zn |
| DTPA-TEA-CaCl2 | 0.005 mol/L DTPA +0.1 mol/L TEA + 0.01 mol/LCaCl2 | 7.3 | Calcareous soils | Cu, Fe, Mn and Zn |
| AA-EDTA | 0.02 mol/L EDTA +0.5 mol/L CH3COONH4 | 4.65 | Acidic soils | Cu, Fe, Mn and Zn |
| ASI | 0.25 mol/L NaHCO3 + 0.01 mol/L EDTA + 0.01 mol/L NH4F | - | Acid to alkaline soils | P, K, Cu, Fe, Mn and Zn |
| AB-DTPA | 1 mol/L NH4HCO3 + 0.005 mol/L DTPA | 7.6 | Alkaline soils | P, K, Na, Mn, Zn, As and Cd |
| Morgan-Wolf | 0.073 mol/L CH3COONa + 0.52 mol/L CH3COOH + 0.0001 mol/L DTPA | 4.8 | Acidic soils | Al, P, As, K, Ca, Mg, B, Cu, Fe, Mn and Zn |
| Extractants | Elements | Al | As | Cd | Cu | Fe | Mn | Ni | Pb | Zn | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total concentration | HCl + HNO3 + HF | Mean | 69,967.68 | 35.98 | 5.28 | 119.37 | 11,0511.76 | 402.07 | 69.35 | 168.67 | 645.81 |
| SD | 4310.95 | 6.84 | 0.39 | 6.67 | 3572.14 | 15.11 | 2.86 | 10.10 | 34.95 | ||
| Bioavailable concentrations | CaCl2 | Mean | 105.26 | 0.05 | 3.23 | 0.66 | 0.11 | 44.35 | 1.57 | 5.70 | 121.57 |
| SD | 10.67 | 0.02 | 0.24 | 0.00 | 0.01 | 3.01 | 0.16 | 0.03 | 11.29 | ||
| DTPA-TEA-CaCl2 | Mean | 67.58 | ND | 0.95 | 6.14 | 33.28 | 16.26 | 0.42 | 44.49 | 61.35 | |
| SD | 1.46 | ND | 0.01 | 0.57 | 1.22 | 1.51 | 0.01 | 2.54 | 5.20 | ||
| AA-EDTA | Mean | 756.36 | ND | 2.49 | 36.06 | 271.29 | 126.81 | 1.66 | 84.34 | 186.55 | |
| SD | 23.34 | ND | 0.05 | 0.43 | 8.56 | 1.16 | 0.02 | 1.38 | 2.49 | ||
| Mehlich 1 | Mean | 1398.35 | 0.62 | 2.37 | 16.48 | 329.20 | 114.01 | 1.34 | 46.75 | 173.45 | |
| SD | 26.22 | 0.03 | 0.08 | 0.17 | 7.52 | 3.02 | 0.01 | 0.75 | 1.66 | ||
| AB-DTPA | Mean | 200.43 | 0.91 | 1.72 | 18.10 | 500.05 | 56.48 | 1.32 | 144.60 | 158.86 | |
| SD | 9.13 | 0.01 | 0.05 | 0.55 | 6.60 | 1.68 | 0.06 | 3.50 | 2.50 | ||
| ASI | Mean | 799.14 | 0.06 | 1.25 | 14.62 | 321.65 | 59.43 | 0.52 | 115.13 | 134.65 | |
| SD | 1.69 | 0.03 | 0.01 | 0.16 | 6.63 | 0.68 | 0.10 | 1.04 | 1.08 | ||
| Morgan-Wolf | Mean | 214.12 | 0.07 | 1.11 | 3.53 | 43.11 | 41.33 | 0.24 | 44.66 | 108.38 | |
| SD | 3.22 | 0.04 | 0.04 | 0.06 | 0.05 | 0.32 | 0.01 | 2.13 | 1.46 | ||
| Elements | Second Order Model | R2 | SE | Constants | |
|---|---|---|---|---|---|
| qe | k | ||||
| Al | 0.986 | 1.69 × 10−4 | 884.95 | 2.49 × 10−4 | |
| As | 0.937 | 1343.59 | 0.67 | 0.43 | |
| Cd | 0.999 | 2.72 | 1.77 | 0.70 | |
| Cu | 0.999 | 0.045 | 17.19 | 0.043 | |
| Fe | 0.919 | 0.0028 | 531.91 | 1.64 × 10−4 | |
| Mn | 0.995 | 0.0027 | 130.38 | 2.67 × 10−3 | |
| Ni | 0.982 | 96.05 | 1.34 | 0.14 | |
| Pb | 0.999 | 0.0027 | 61.46 | 0.020 | |
| Zn | 0.999 | 5.30 × 10−4 | 153.61 | 5.81 × 10−3 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.-X.; Xu, D.-M.; Fu, R.-B.; Chen, J.-P. Bioavailability Assessment of Heavy Metals Using Various Multi-Element Extractants in an Indigenous Zinc Smelting Contaminated Site, Southwestern China. Int. J. Environ. Res. Public Health 2021, 18, 8560. https://doi.org/10.3390/ijerph18168560
Wang J-X, Xu D-M, Fu R-B, Chen J-P. Bioavailability Assessment of Heavy Metals Using Various Multi-Element Extractants in an Indigenous Zinc Smelting Contaminated Site, Southwestern China. International Journal of Environmental Research and Public Health. 2021; 18(16):8560. https://doi.org/10.3390/ijerph18168560
Chicago/Turabian StyleWang, Jun-Xian, Da-Mao Xu, Rong-Bing Fu, and Jia-Peng Chen. 2021. "Bioavailability Assessment of Heavy Metals Using Various Multi-Element Extractants in an Indigenous Zinc Smelting Contaminated Site, Southwestern China" International Journal of Environmental Research and Public Health 18, no. 16: 8560. https://doi.org/10.3390/ijerph18168560
APA StyleWang, J.-X., Xu, D.-M., Fu, R.-B., & Chen, J.-P. (2021). Bioavailability Assessment of Heavy Metals Using Various Multi-Element Extractants in an Indigenous Zinc Smelting Contaminated Site, Southwestern China. International Journal of Environmental Research and Public Health, 18(16), 8560. https://doi.org/10.3390/ijerph18168560





