Evaluation of an Environmental Transport Medium for Legionella pneumophila Recovery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Culture Method
2.3. Molecular Methods
2.4. Data Analysis
3. Results
3.1. Culture Methods
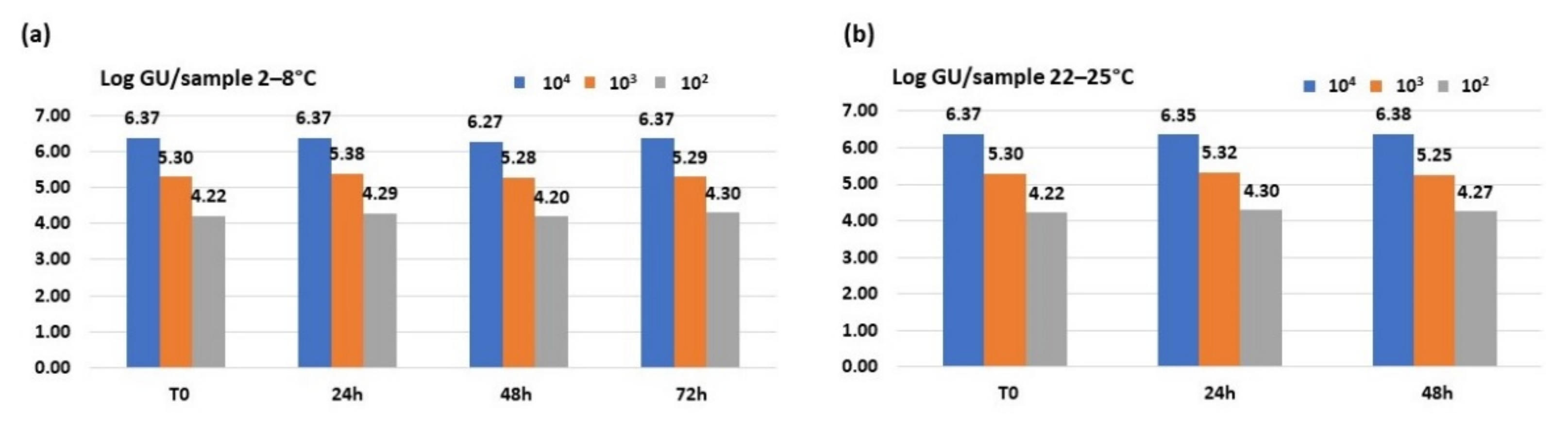
3.2. Molecular Methods
3.2.1. iQ-Check® Quanti Legionella Spp. Real-Time Assay
3.2.2. Qualyfast® Legionella qPCR Detection Kit
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Abdel-Nour, M.; Duncan, C.; Low, D.E.; Guyard, C. Biofilms: The stronghold of Legionella pneumophila. Int. J. Mol. Sci. 2013, 14, 21660–21675. [Google Scholar] [CrossRef] [Green Version]
- European Centre for Disease Prevention and Control (ECDC). Annual Epidemiological Report for 2018. Stockholm: ECDC. 2021. Available online: https://www.ecdc.europa.eu/sites/default/files/documents/AER-legionnaires-2019.pdf (accessed on 7 August 2021).
- Rota, M.C.; Caporali, M.G.; Bella, A.; Scaturro, M.; Giannitelli, S.; Ricci, M.L. Il sistema di Sorveglianza della Legionellosi in Italia: I risultati del 2019. Boll. Epidemiol. Naz. 2020, 1, 32–38. [Google Scholar]
- Ballard, A.L.; Fry, N.K.; Chan, L.; Surman, S.B.; Lee, J.V.; Harrison, T.G.; Towner, K.J. Detection of Legionella pneumophila using a real-time PCR hybridization assay. J. Clin. Microbiol. 2000, 38, 4215–4218. [Google Scholar] [CrossRef] [Green Version]
- Leoni, E.; Legnani, P.P. Comparison of selective procedures for isolation and enumeration of Legionella species from hot water systems. J. Appl. Microbiol. 2001, 90, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Italian Ministry of Health. “Linee Guida Per la Prevenzione ed il Controllo Della Legionellosi” (79/CSR of 07 of May 2015) Date of Publication: 13 May 2015, Last Updated: 31 October 2016. Available online: https://www.salute.gov.it/imgs/C_17_pubblicazioni_2362_allegato.pdf (accessed on 7 August 2021).
- De Filippis, P.; Mozzetti, C.; Messina, A.; D’Alò, G.L. Prevalence of Legionella in retirement homes and group homes water distribution systems. Sci. Total Environ. 2018, 643, 715–724. [Google Scholar] [CrossRef] [PubMed]
- Ditommaso, S.; Giacomuzzi, M.; Gentile, M.; Moiraghi, A.R.; Zotti, C.M. Effective environmental sampling strategies for monitoring Legionella spp contamination in hot water systems. Am. J. Infect. Control 2010, 38, 344–349. [Google Scholar] [CrossRef]
- Casini, B.; Baggiani, A.; Totaro, M.; Mansi, A.; Costa, A.L.; Aquino, F.; Miccoli, M.; Valentini, P.; Bruschi, F.; Lopalco, P.L.; et al. Detection of viable but non-culturable legionella in hospital water network following monochloramine disinfection. J. Hosp. Infect. 2018, 98, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Azeredo, J.; Azevedo, N.F.; Briandet, R.; Cerca, N.; Coenye, T.; Costa, A.R.; Desvaux, M.; Di Bonaventura, G.; Hébraud, M.; Jaglic, Z.; et al. Critical Review on Biofilm Methods. Crit. Rev. Microbiol. 2017, 43, 313–351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ECDC. European Technical Guidelines for the Prevention, Control and Investigation, of Infections Caused by Legionella Species. Available online: https://www.ecdc.europa.eu/en/publications-data/european-technical-guidelines-prevention-control-and-investigation-infections (accessed on 24 June 2021).
- Ta, A.C.; Stout, J.E.; Yu, V.L.; Wagener, M.M. Comparison of culture methods for monitoring Legionella species in hospital potable water systems and recommendations for standardization of such methods. J. Clin. Microbiol. 1995, 33, 2118–2123. [Google Scholar] [CrossRef] [Green Version]
- Pereira, A.C.; Silva, A.R.; Melo, L.F. Legionella and Biofilms-Integrated Surveillance to Bridge Science and Real-Field Demands. Microorganisms 2021, 9, 1212. [Google Scholar] [CrossRef]
- Levi, K.; Smedley, J.; Towner, K.J. Evaluation of a real-time PCR hybridization assay for rapid detection of Legionella pneumophila in hospital and environmental water samples. Clin. Microbiol. Infect. 2003, 9, 754–758. [Google Scholar] [CrossRef] [Green Version]
- Reischl, U.; Linde, H.J.; Lehn, N.; Landt, O.; Barratt, K.; Wellinghausen, N. Direct detection and differentiation of Legionella spp. and Legionella pneumophila in clinical specimens by dual-color real-time PCR and melting curve analysis. J. Clin. Microbiol. 2002, 40, 3814–3817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wellinghausen, N.; Frost, C.; Marre, R. Detection of legionellae in hospital water samples by quantitative real-time LightCycler PCR. Appl. Environ. Microbiol. 2001, 67, 3985–3993. [Google Scholar] [CrossRef] [Green Version]
- ISO (International Standards Organisation). ISO 11731: Water Quality-Detection and Enumeration of Legionella; ISO: Geneva, Switzerland, 2017. [Google Scholar]
- Cunha, B.A.; Burillo, A.; Bouza, E. Legionnaires’ disease. Lancet 2016, 387, 376–385. [Google Scholar] [CrossRef]
- Fields, B.S.; Benson, R.F.; Besser, R.E. Legionella and Legionnaires’ disease: 25 years of investigation. Clin. Microbiol. Rev. 2002, 15, 506–526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.V.; Lai, S.; Exner, M.; Lenz, J.; Gaia, V.; Casati, S.; Hartemann, P.; Lück, C.; Pangon, B.; Ricci, M.L.; et al. An international trial of quantitative PCR for monitoring Legionella in artificial water systems. J. Appl. Microbiol. 2011, 110, 1032–1044. [Google Scholar] [CrossRef] [Green Version]
- Joly, P.; Falconnet, P.A.; André, J.; Weill, N.; Reyrolle, M.; Vandenesch, F.; Maurin, M.; Etienne, J.; Jarraud, S. Quantitative real-time Legionella PCR for environmental water samples: Data interpretation. Appl. Environ. Microbiol. 2006, 72, 2801–2808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ditommaso, S.; Ricciardi, E.; Giacomuzzi, M.; Arauco Rivera, S.R.; Zotti, C.M. Legionella in water samples: How can you interpret the results obtained by quantitative PCR? Mol. Cell Probes 2015, 29, 7–12. [Google Scholar] [CrossRef]
- Bonetta, S.; Bonetta, S.; Ferretti, E.; Balocco, F.; Carraro, E. Evaluation of Legionella pneumophila contamination in Italian hotel water systems by quantitative real-time PCR and culture methods. J. Appl. Microbiol. 2010, 108, 1576–1583. [Google Scholar] [CrossRef] [PubMed]
- Scaturro, M.; Fontana, S.; Dell’eva, I.; Helfer, F.; Marchio, M.; Stefanetti, M.V.; Cavallaro, M.; Miglietta, M.; Montagna, M.T.; de Giglio, O.; et al. A multicenter study of viable PCR using propidium monoazide to detect Legionella in water samples. Diag. Microbiol. Infect. Dis. 2016, 85, 283–288. [Google Scholar] [CrossRef]
- Kontchou, J.A.; Nocker, A. Optimization of viability qPCR for selective detection of membrane-intact Legionella pneumophila. J. Microbiol. Methods 2019, 156, 68–76. [Google Scholar] [CrossRef]

| T (°C) | Agar | T0 | 24 h | 48 h | 72 h | Expected Colonies |
|---|---|---|---|---|---|---|
| 20–25 | GVPC | 23 (+/−2) | 29 (+/−9) | 22 (+/−10) | / | 21 (+/−2) |
| 20–25 | BCYE | 99 (+/−54) | 67 (+/−19) | 75 (+/−16) | / | 63 (+/−9) |
| 2–8 | GVPC | 23 (+/−2) | 43 (+/−22) | 48 (+/−9) | 28 (+/−11) | 21 (+/−2) |
| 2–8 | BCYE | 99 (+/−54) | 63 (+/−36) | 72 (+/−30) | 82 (+/−51) | 63 (+/−9) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinelli, M.; Calaresu, E.; Musumeci, R.; Giubbi, C.; Perdoni, F.; Frugoni, S.; Castriciano, S.; Scaturro, M.; Ricci, M.L.; Cocuzza, C.E. Evaluation of an Environmental Transport Medium for Legionella pneumophila Recovery. Int. J. Environ. Res. Public Health 2021, 18, 8551. https://doi.org/10.3390/ijerph18168551
Martinelli M, Calaresu E, Musumeci R, Giubbi C, Perdoni F, Frugoni S, Castriciano S, Scaturro M, Ricci ML, Cocuzza CE. Evaluation of an Environmental Transport Medium for Legionella pneumophila Recovery. International Journal of Environmental Research and Public Health. 2021; 18(16):8551. https://doi.org/10.3390/ijerph18168551
Chicago/Turabian StyleMartinelli, Marianna, Enrico Calaresu, Rosario Musumeci, Chiara Giubbi, Federica Perdoni, Sergio Frugoni, Santina Castriciano, Maria Scaturro, Maria Luisa Ricci, and Clementina E. Cocuzza. 2021. "Evaluation of an Environmental Transport Medium for Legionella pneumophila Recovery" International Journal of Environmental Research and Public Health 18, no. 16: 8551. https://doi.org/10.3390/ijerph18168551
APA StyleMartinelli, M., Calaresu, E., Musumeci, R., Giubbi, C., Perdoni, F., Frugoni, S., Castriciano, S., Scaturro, M., Ricci, M. L., & Cocuzza, C. E. (2021). Evaluation of an Environmental Transport Medium for Legionella pneumophila Recovery. International Journal of Environmental Research and Public Health, 18(16), 8551. https://doi.org/10.3390/ijerph18168551








