Adsorption Characteristics of Activated Carbon Fibers in Respirator Cartridges for Toluene
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
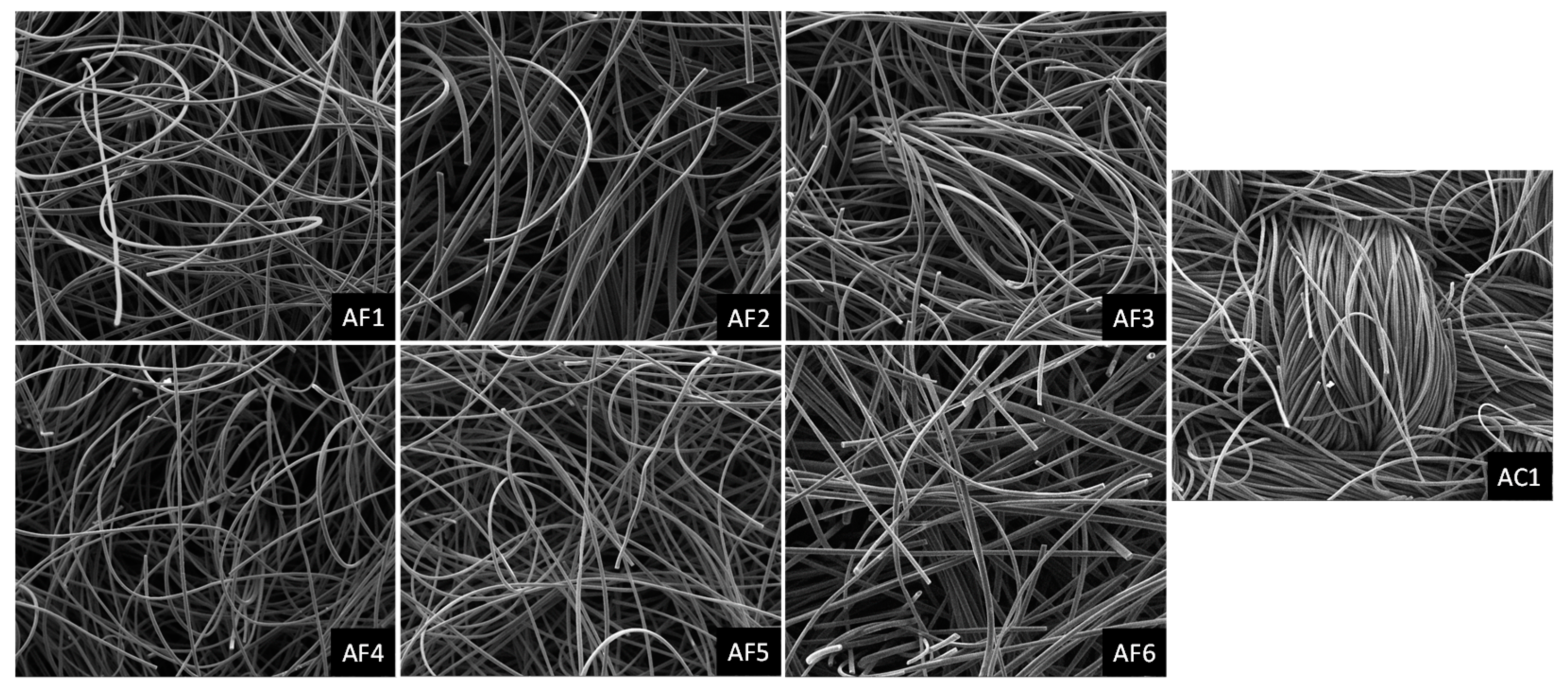
2.2. Characterization of ACF Structural Properties
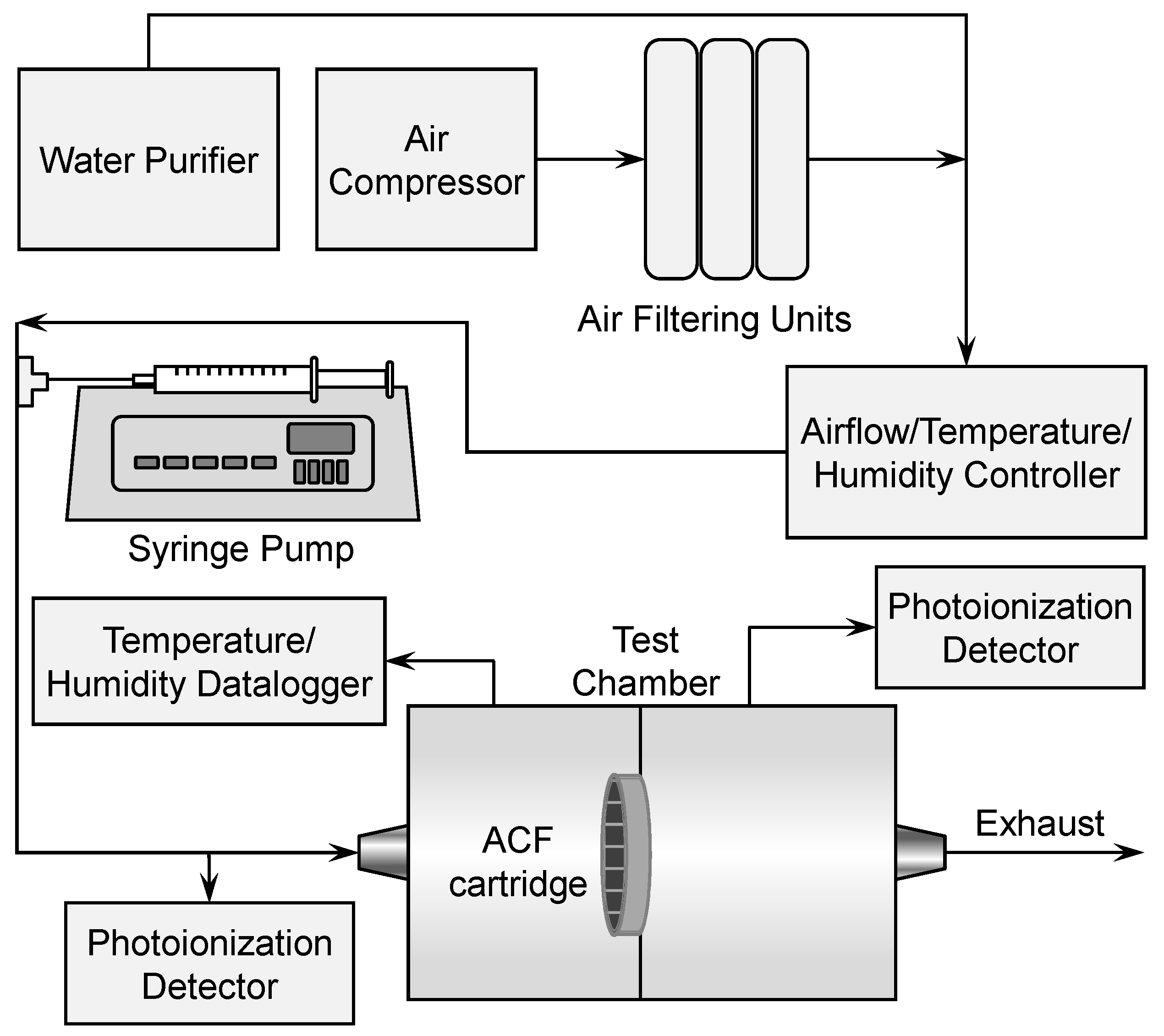
2.3. Breakthrough Determination
2.4. Determination of Adsorption Capacity
2.5. Comparison of Adsorption Characteristics by ACF Type
3. Results and Discussion
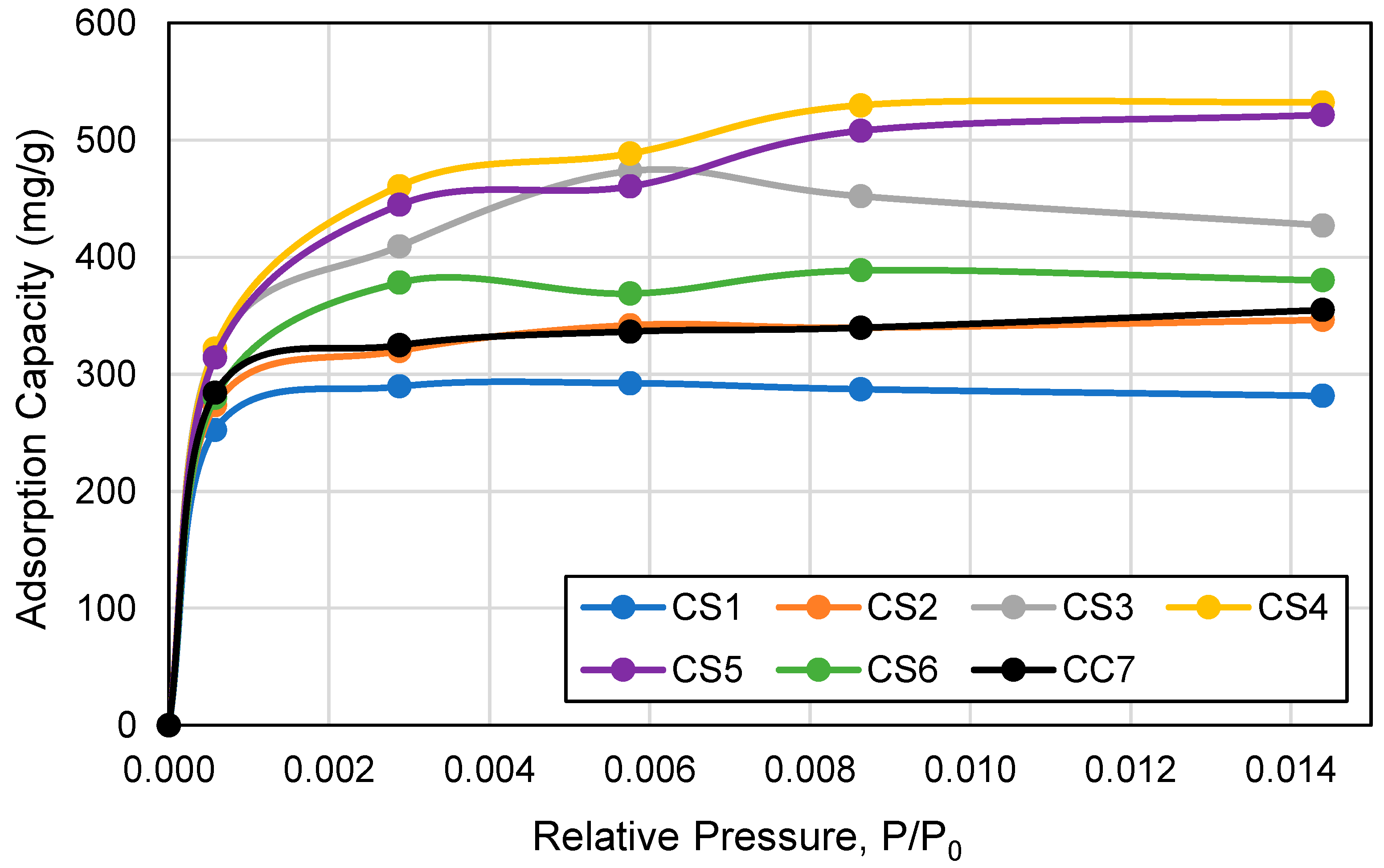
3.1. ACF Structural Properties
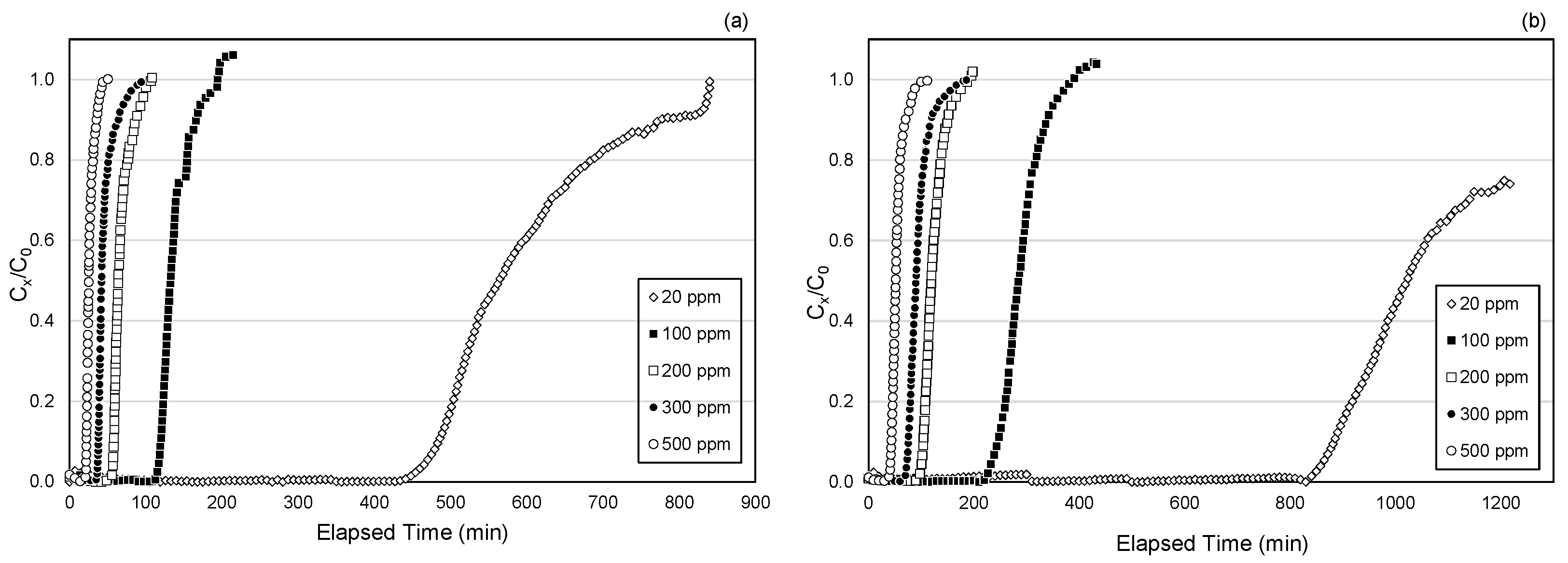
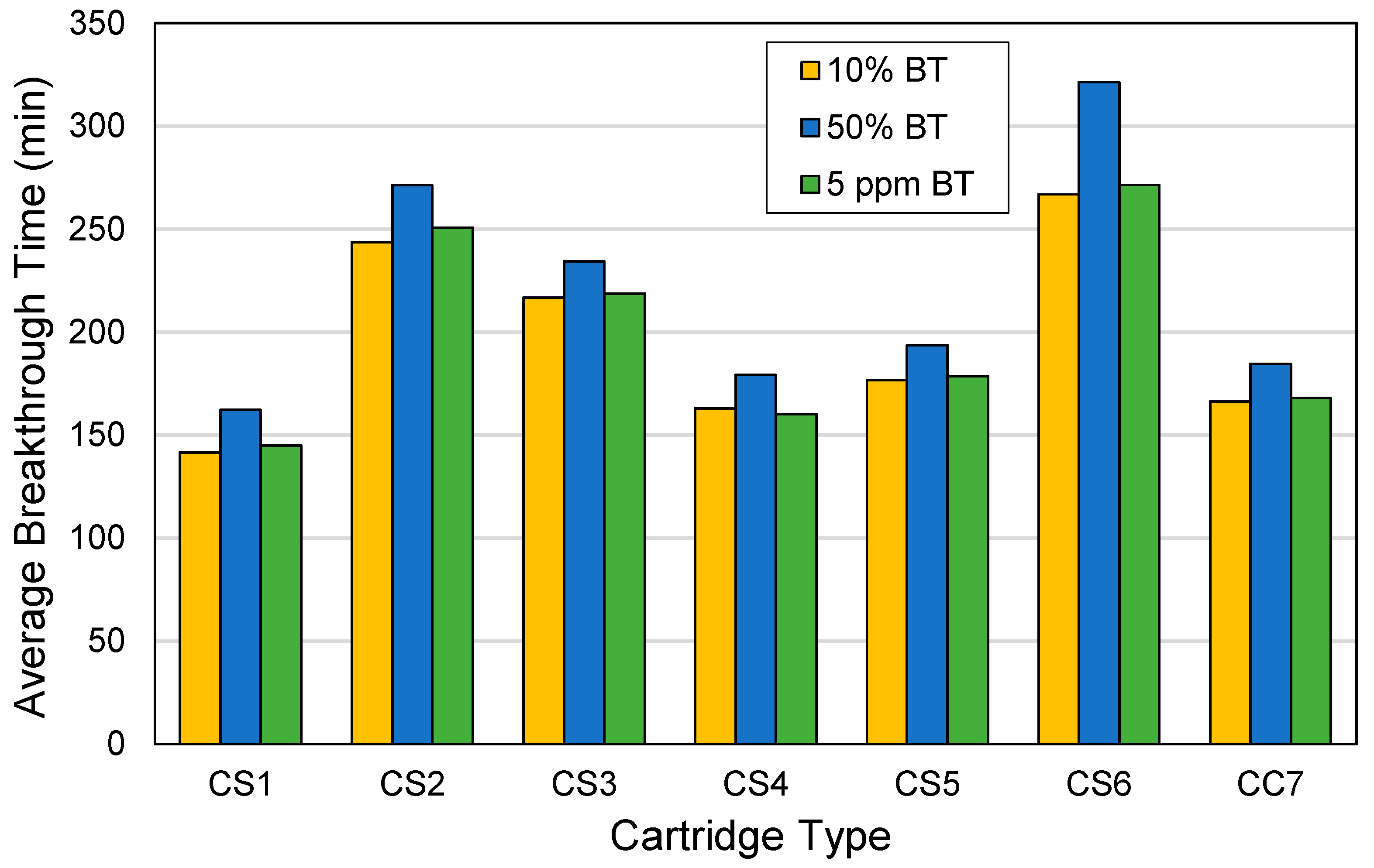
3.2. Breakthrough Characteristics by Cartridge Type
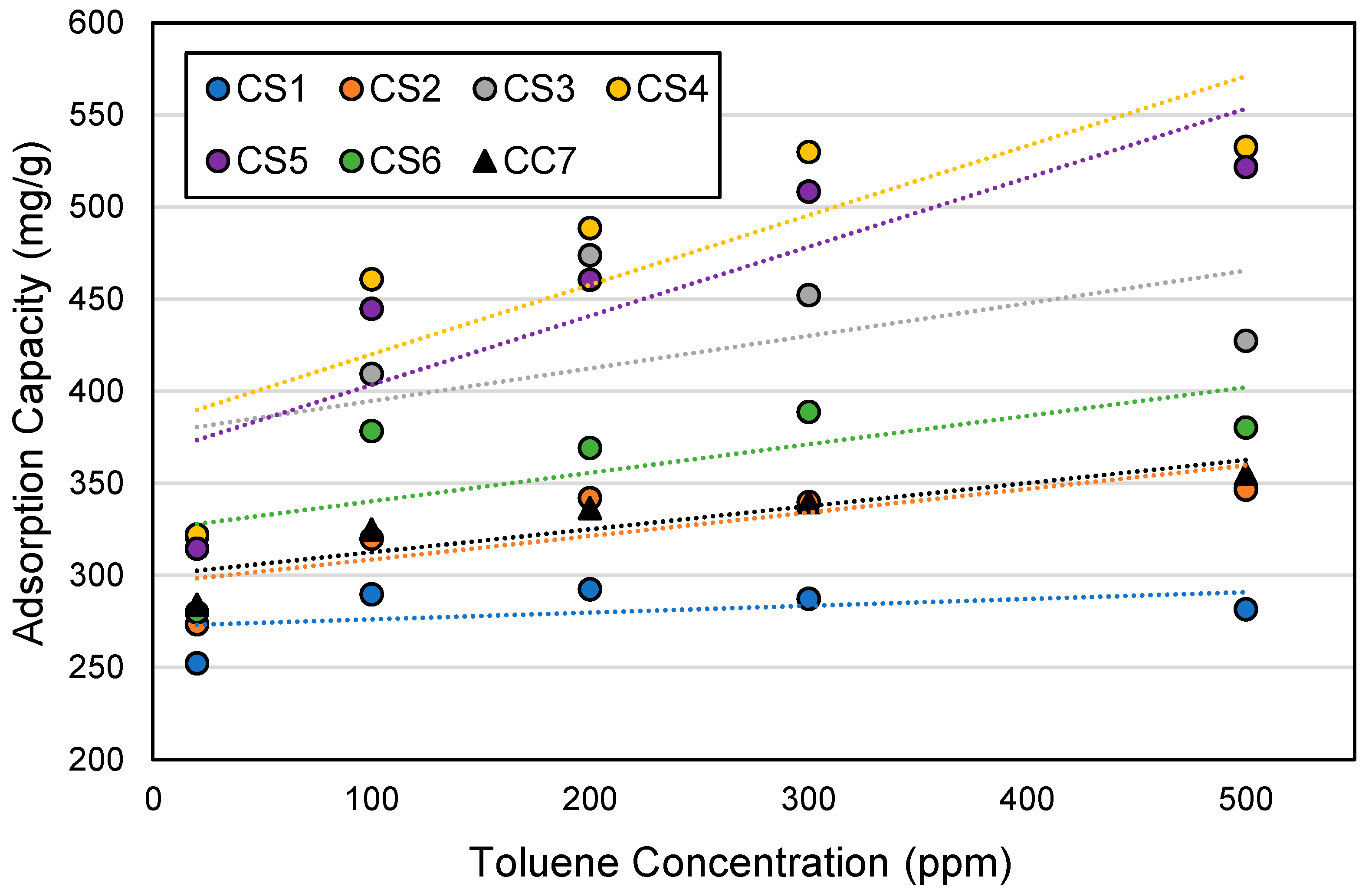
3.3. Effects of Toluene Concentration on Breakthrough Times
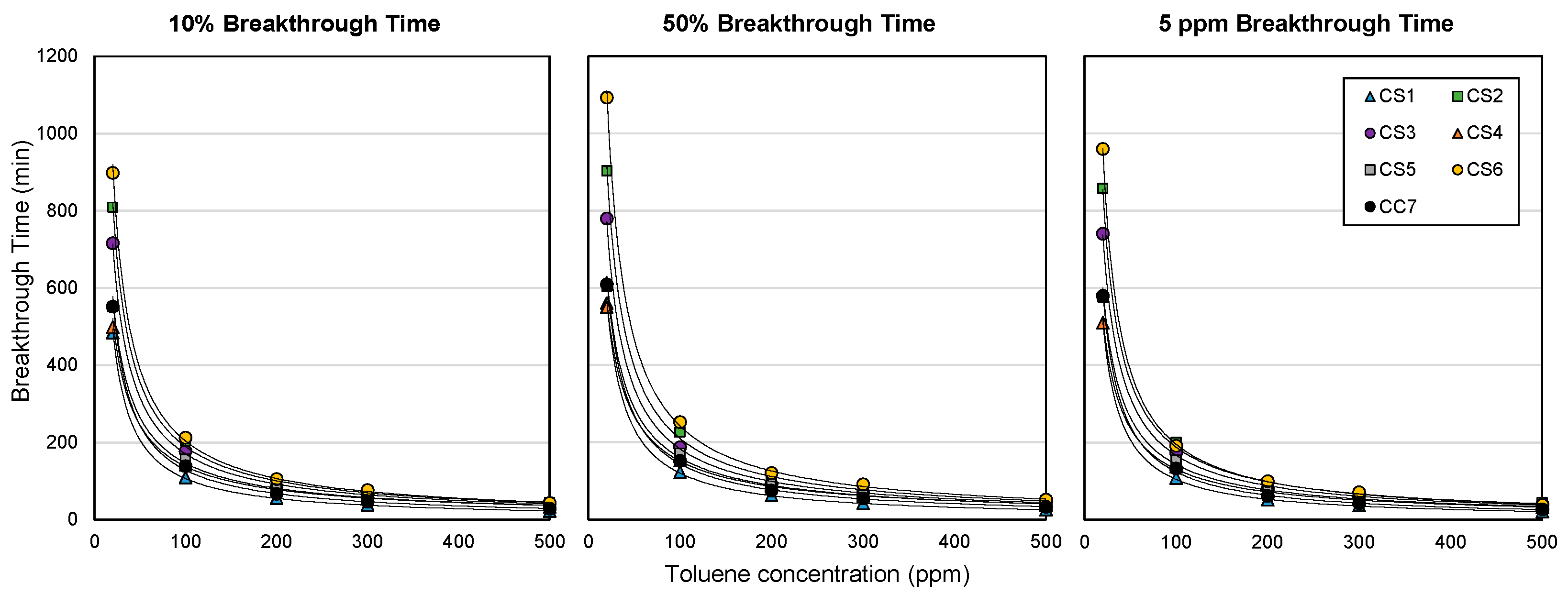
3.4. Breakthrough Times by Cartridge Type
3.5. Estimation of ACF Cartridge Service Lives
3.6. Mass Transfer Zone (MTZ) Length by Cartridge Type
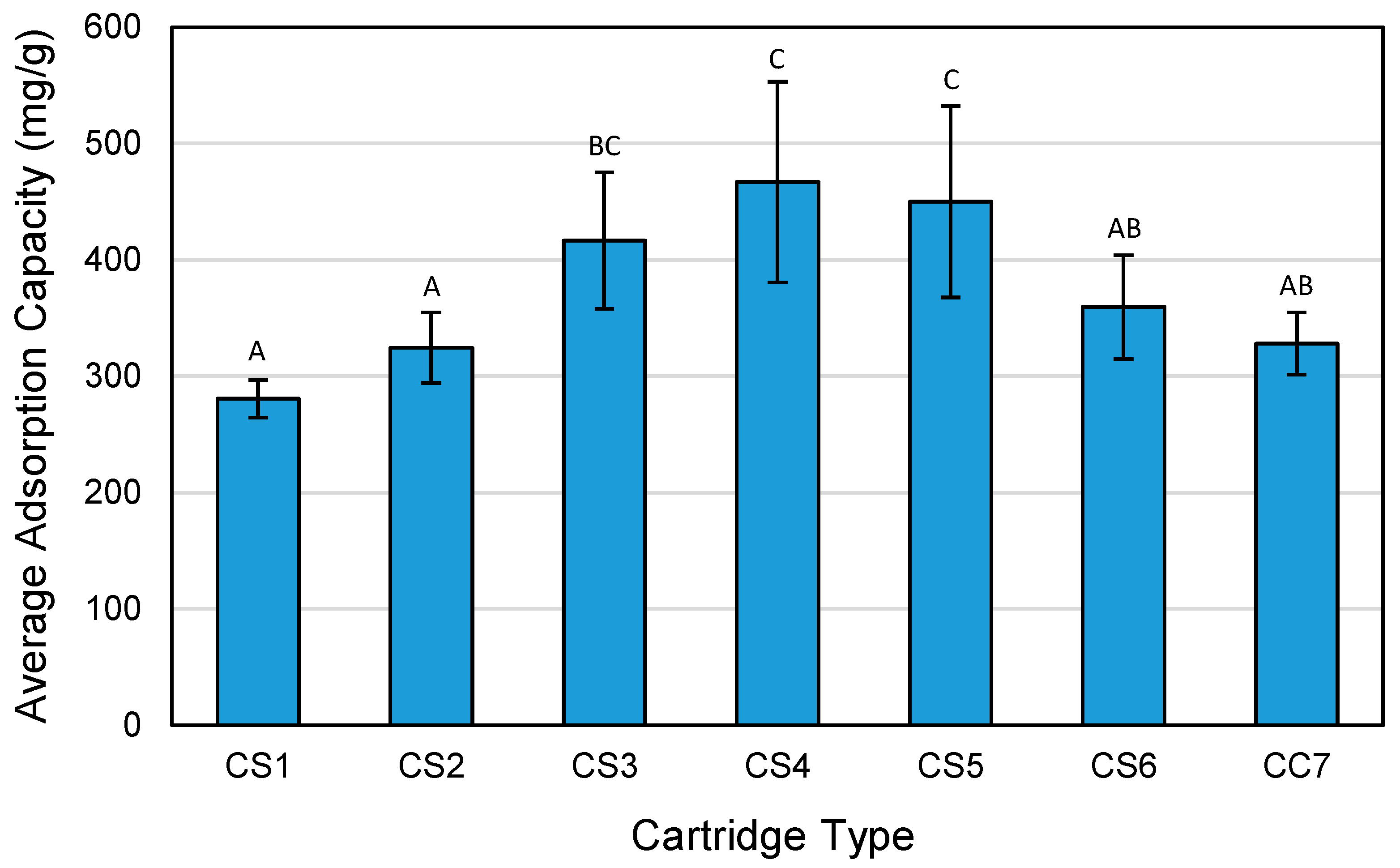
3.7. Adsorption Capacity by Cartridge Type
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Occupational Safety and Health Administration. Safety and Health Topics: Respiratory Protection; U.S. Department of Labor: Washington, DC, USA. Available online: https://www.osha.gov/SLTC/respiratoryprotection/ (accessed on 23 June 2021).
- Balanay, J.G.; Bartolucci, A.A.; Lungu, C.T. Adsorption characteristics of activated carbon fibers (ACFs) for toluene: Application in respiratory protection. J. Occup. Environ. Hyg. 2014, 11, 133–143. [Google Scholar] [CrossRef]
- Radonovich, L.J.; Cheng, J.; Shenal, B.V.; Hodgson, M.; Bender, B.S. Respirator tolerance in health care workers. JAMA 2009, 30, 36–38. [Google Scholar] [CrossRef] [Green Version]
- Baig, A.S.; Knapp, C.; Eagan, A.E.; Radonovich, L.J. Health care workers’ views about respirator use and features that should be included in the next generation of respirators. Am. J. Infect. Control 2010, 38, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Hooper, A.J.; Crawford, J.O.; Thomas, D. An evaluation of physiological demands and comfort between the use of conventional and lightweight self-contained breathing apparatus. Appl. Ergon. 2001, 32, 399–406. [Google Scholar] [CrossRef]
- Suzuki, M. Activated carbon fiber: Fundamentals and applications. Carbon 1994, 32, 577–586. [Google Scholar] [CrossRef]
- Lu, A.H.; Zheng, J.T. Study of microstructure of high-surface-area polyacrylonitrile activated carbon fibers. J. Colloid Interface Sci. 2001, 236, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.H.; Kang, F.; Zheng, Y.P.; Yang, J.B.; Liang, K.M. Adsorption of trace polar methy-ethyl-ketone and non-polar benzene vapors on viscose rayon-based activated carbon fibers. Carbon 2002, 40, 1363–1367. [Google Scholar] [CrossRef]
- Balanay, J.G.; Crawford, S.A.; Lungu, C.T. Comparison of toluene adsorption among granular activated carbon and different types of activated carbon fibers (ACFs). J. Occup. Environ. Hyg. 2011, 8, 573–579. [Google Scholar] [CrossRef]
- Balanay, J.G.; Lungu, C.T. Morphologic and surface characterization of different types of activated carbon fibres. Adsorpt. Sci. Technol. 2012, 30, 355–367. [Google Scholar] [CrossRef]
- Lu, A.H.; Zheng, J.T. Microstructures of PAN-ACF modified by catalytic benzene deposition. Carbon 2002, 40, 1353–1361. [Google Scholar] [CrossRef]
- Feng, W.; Kwon, S.; Borguet, E.; Vidic, R. Adsorption of hydrogen sulfide onto activated carbon fibers: Effect of pore structure and surface chemistry. Environ. Sci. Technol. 2005, 39, 9744–9749. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, D.; Sullivan, P.D.; Rood, M.J.; James, K.J. Equilibrium adsorption of organic vapors on phenol-, tire- and coal-derived activated carbons. J. Environ. Eng. 2004, 130, 231–241. [Google Scholar] [CrossRef]
- Sullivan, P.D.; Rood, M.J.; Dombrowski, K.D.; James, K.J. Capture and recovery of organic compounds using adsorption and electrothermal regeneration. J. Environ. Eng. 2004, 130, 258–267. [Google Scholar] [CrossRef]
- Fournel, L.; Mocho, P.; Fanlo, J.L.; Le Cloirec, P. External capillary condensation and adsorption of VOCs onto activated carbon fiber cloth and felt. Environ. Technol. 2005, 26, 1277–1287. [Google Scholar] [CrossRef]
- Mallouk, K.E.; Johnsen, D.L.; Rood, M.J. Capture and recovery of isobutane by electrothermal swing adsorption with post-desorption post-desorption liquefaction. Environ. Sci. Technol. 2010, 44, 7070–7075. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Yu, L.; Song, B.; Meng, F.; Tang, X. Alkali promoted the adsorption of toluene by adjusting the surface properties of lignin-derived carbon fibers. Environ. Sci. Pollut. Res. 2019, 26, 22284–22294. [Google Scholar] [CrossRef]
- Brasquet, C.; Le Cloirec, P. Adsorption onto activated carbon fibers: Application to water and air treatments. Carbon 1997, 35, 1307–1313. [Google Scholar] [CrossRef]
- Cheng, T.; Jiang, Y.; Zhang, Y.; Liu, S. Prediction of breakthrough curves for adsorption on activated carbon fibers in a fixed bed. Carbon 2004, 42, 3081–3085. [Google Scholar] [CrossRef]
- Das, D.; Gaur, V.; Verma, N. Removal of volatile organic compound by activated carbon fiber. Carbon 2004, 42, 2949–2963. [Google Scholar] [CrossRef]
- Hashisho, Z.; Rood, M. Microwave-swing adsorption to capture and recover vapors from air streams with activated carbon fiber cloth. Environ. Sci. Technol. 2005, 39, 6851–6859. [Google Scholar] [CrossRef] [PubMed]
- Lorimier, C.; Subrenat, A.; Le Coq, L.; Le Cloirec, P. Adsorption of toluene onto activated carbon fibre cloths and felts: Application to indoor air treatment. Environ. Technol. 2005, 26, 1217–1230. [Google Scholar] [CrossRef]
- Sidheswaran, M.A.; Destaillats, H.; Sullivan, D.P.; Cohn, S.; Fisk, W.J. Energy efficient indoor VOC air cleaning with activated carbon fiber (ACF) filters. Build. Environ. 2012, 47, 357–367. [Google Scholar] [CrossRef] [Green Version]
- Son, H.K.; Sivakumar, S.; Rood, M.J.; Kim, B.J. Electrothermal adsorption and desorption of volatile organic compounds on activated carbon fiber cloth. J. Hazard. Mater. 2016, 301, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Roegiers, J.; Denys, S. CFD-modelling of activated carbon fibers for indoor air purification. Chem. Eng. J. 2019, 365, 80–87. [Google Scholar] [CrossRef]
- Balanay, J.G.; Floyd, E.L.; Lungu, C.T. Breakthrough curves for toluene adsorption of different types of activated carbon fibers: Application in respiratory protection. Ann. Occup. Hyg. 2015, 59, 481–490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National Institute for Occupational Safety and Health. Determination of Inhalation Resistance Test, Air-purifying Respirators Standard Testing Procedure (STP); Procedure No. TEB-APR-STP-0007, Revision 2.3; U.S. Department of Health and Human Services: Pittsburgh, PA, USA, 2019. Available online: https://www.cdc.gov/niosh/npptl/stps/pdfs/TEB-APR-STP-0007-508.pdf (accessed on 19 June 2021).
- Balanay, J.G.; Lungu, C.T. Determination of pressure drop across activated carbon fiber respirator cartridges. J. Occup. Environ. Hyg. 2016, 13, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Agency for Toxic Substances and Disease Registry. Toxicological Profile for Toluene; U.S. Department of Health and Human Services: Atlanta, GA, USA, 2017. Available online: https://www.atsdr.cdc.gov/toxprofiles/tp56.pdf (accessed on 27 July 2021).
- National Institute for Occupational Safety and Health. Determination of Organic Vapor (Carbon Tetrachloride) Service Life Test, Air-purifying Respirators with Cartridges Standard Testing Procedure (STP); Procedure No. TEB-APR-STP-0046A, Revision 2.0; U.S. Department of Health and Human Services: Pittsburgh, PA, USA, 2006. Available online: https://www.cdc.gov/niosh/npptl/stps/pdfs/TEB-APR-STP-0046A-508.pdf (accessed on 19 June 2021).
- Amid, H.; Mazé, B.; Flickinger, M.C.; Pourdeyhimi, B. Dynamic adsorption of ammonia: Apparatus, testing conditions, and adsorption capacities. Meas. Sci. Technol. 2017, 28, 055901. [Google Scholar] [CrossRef]
- Gregg, S.J.; Sing, K.S.W. Adsorption, Surface Area and Porosity, 2nd ed.; Academic Press: London, UK, 1982; ISBN 0-12-300956-1. [Google Scholar]
- Foster, K.L.; Fuerman, R.G.; Economy, J.; Larson, S.M.; Rood, M.J. Adsorption characteristics of trace volatile organic compounds in gas streams onto activated carbon fibres. Chem. Mater. 1992, 4, 1068–1073. [Google Scholar] [CrossRef]
- Bradley, R.H.; Rand, B. On the physical adsorption of vapors by microporous carbons. J. Colloid Interface Sci. 1995, 169, 168–176. [Google Scholar] [CrossRef]
- Fuertes, A.B.; Marbán, G.; Nevskaia, D.M. Adsorption of volatile organic compounds by means of activated carbon fibre-based monoliths. Carbon 2003, 41, 87–96. [Google Scholar] [CrossRef]
- Cohen, H.J.; Garrison, R.P. Development of a field method for evaluating the service life of organic vapor cartridges: Results of laboratory testing using carbon tetrachloride. Am. Ind. Hyg. Assoc. J. 1989, 50, 486–495. [Google Scholar] [CrossRef]
- Janvier, F.; Tuduri, L.; Cossement, D.; Drolet, D.; Lara, J. Micropore characterization of activated carbons of respirator cartridges with argon, carbon dioxide, and organic vapors of different vapor pressures. Carbon 2015, 94, 781–791. [Google Scholar] [CrossRef]
- Janvier, F.; Tuduri, L.; Cossement, D.; Drolet, D.; Lara, J. Systematic evaluation of the adsorption of organic vapors onto a miniaturized cartridge device using breakthrough tests in parallel experiment with a full size respirator cartridge. Adsorpt. Sci. Technol. 2016, 34, 287–306. [Google Scholar] [CrossRef] [Green Version]
- Wood, G.O. Estimating service lives of organic vapor cartridges. Am. Ind. Hyg. Assoc. J. 1994, 55, 11–15. [Google Scholar] [CrossRef]
- Yoon, Y.H.; Nelson, J.H.; Lara, J. Respirator cartridge service-life: Exposure to mixtures. Am. Ind. Hyg. Assoc. J. 1996, 57, 809–819. [Google Scholar] [CrossRef]
- Wood, G. Correlating and extrapolating air purifying respirator cartridge breakthrough times: A review. J. Int. Soc. Respir. Prot. 2015, 32, 23–36. [Google Scholar]
- Lopez, J.M.; Navarro, M.V.; Garcia, T.; Murillo, R.; Mastral, A.M.; Varela-Gandia, F.J.; Lozano-Castello, D.; Bueno-Lopez, A.; Cazorla-Amoros, D. Screening of different zeolites and silicoaluminophosphates for the retention of propene under cold start conditions. Microporous Mesoporous Mater. 2010, 130, 239–247. [Google Scholar] [CrossRef]
- Chebbi, M.; Azambre, B.; Cantrel, L.; Huvé, M.; Albiol, T. Influence of structural, textural and chemical parameters of silver zeolites on the retention of methyl iodide. Microporous Mesoporous Mater. 2017, 244, 137–150. [Google Scholar] [CrossRef]
- Tanada, S.; Kawasaki, N.; Nakamura, T.; Araki, T.; Tachibana, Y. Characteristics of nonafluorobutyl methyl ether (NFE) adsorption onto activated carbon fibers and different-size-activated carbon particles. J. Colloid Interface Sci. 2000, 228, 220–225. [Google Scholar] [CrossRef]
- Zhang, S.; Shao, T.; Kose, H.S.; Karanfil, T. Adsorption of aromatic compounds by carbonaceous adsorbents: A comparative study on granular activated carbon, activated carbon fiber, and carbon nanotubes. Environ. Sci. Technol. 2010, 44, 6377–6383. [Google Scholar] [CrossRef]


| Cartridge Type | ACF Composition a by % Weight | Number of ACF Layers | Average ACF Mass (g) b |
|---|---|---|---|
| CS1 | 100% AF1 | 11 | 4.97 ± 0.06 |
| CS2 | 100% AF2 | 9 | 7.76 ± 0.64 |
| CS3 | 100% AF3 | 8 | 5.31 ± 0.46 |
| CS4 | 100% AF4 | 10 | 3.96 ± 0.20 |
| CS5 | 100% AF5 | 8 | 4.43 ± 0.16 |
| CS6 | 100% AF6 | 5 | 8.01 ± 0.31 |
| CC7 | 71% AF1/29% AC1 | 8 (AF1)/3 (AC1) | 5.04 ± 0.06 |
| ACF Type | Weave Type | Thickness (cm) a | Density (g/cm3) a | BET Surface Area (m2/g) b | Micropore Area (m2/g) b | % Micropore by Area b | Micropore Volume (cm3/g) b | Pore Size (nm) b |
|---|---|---|---|---|---|---|---|---|
| AF1 | Non-woven | 0.23 | 0.043 | 1203.63 ± 2.61 | 1122.14 ± 1.66 | 93.23 ± 0.10 | 0.43 ± 0.00 | 1.66 ± 0.00 |
| AF2 | Non-woven | 0.29 | 0.069 | 1265.48 ± 34.76 | 1172.93 ± 35.85 | 92.68 ± 0.29 | 0.45 ± 0.01 | 1.58 ± 0.00 |
| AF3 | Non-woven | 0.29 | 0.057 | 1707.28 ± 19.45 | 1529.05 ± 14.38 | 89.56 ± 0.18 | 0.59 ± 0.01 | 1.59 ± 0.04 |
| AF4 | Non-woven | 0.24 | 0.043 | 2091.13 ± 9.28 | 1757.11 ± 5.11 | 84.03 ± 0.19 | 0.69 ± 0.00 | 1.62 ± 0.00 |
| AF5 | Non-woven | 0.30 | 0.041 | 2028.08 ± 12.65 | 1701.54 ± 9.49 | 83.90 ± 0.06 | 0.67 ± 0.00 | 1.69 ± 0.00 |
| AF6 | Non-woven | 0.50 | 0.070 | 1474.60 ± 26.56 | 1350.12 ± 33.55 | 91.55 ± 0.62 | 0.51 ± 0.01 | 1.67 ± 0.00 |
| AC1 | Woven | 0.11 | 0.101 | 1903.45 ± 0.56 | 1637.87 ± 0.15 | 86.05 ± 0.03 | 0.64 ± 0.00 | 1.59 ± 0.00 |
| Toluene Concentration (ppm) | Minimum Service Life Requirement (Minutes) a | 5-ppm Breakthrough Times (Minutes) b |
|---|---|---|
| 500 | 81 | 21–38 |
| 300 | 116 | 36–70 |
| 200 | 154 | 51–99 |
| 100 | 250 | 107–191 |
| 20 | 770 | 510–960 |
| MTZ Properties | Cartridge Type | C0 = 20 ppm | C0 = 100 ppm | C0 = 200 ppm | C0 = 200 ppm | C0 = 500 ppm |
|---|---|---|---|---|---|---|
| Length of mass transfer zone, LT (cm) | CS1 | 0.42 | 0.31 | 1.15 | 0.30 | 0.30 |
| CS2 | 0.34 | 0.29 | 0.30 | 0.33 | 0.21 | |
| CS3 | 0.25 | 0.19 | 0.20 | 0.22 | 0.23 | |
| CS4 | 0.63 | 0.27 | 0.35 | 0.77 | 0.40 | |
| CS5 | 0.28 | 0.81 | 0.85 | 0.25 | 1.11 | |
| CS6 | 0.89 | 0.61 | 0.38 | 0.50 | 0.45 | |
| CC7 | 0.32 | 0.33 | 0.37 | 0.42 | 0.46 | |
| LT/L | CS1 | 0.17 | 0.12 | 0.45 | 0.12 | 0.12 |
| CS2 | 0.14 | 0.12 | 0.12 | 0.13 | 0.08 | |
| CS3 | 0.10 | 0.07 | 0.08 | 0.09 | 0.09 | |
| CS4 | 0.25 | 0.10 | 0.14 | 0.30 | 0.16 | |
| CS5 | 0.11 | 0.32 | 0.34 | 0.10 | 0.44 | |
| CS6 | 0.35 | 0.24 | 0.15 | 0.20 | 0.18 | |
| CC7 | 0.13 | 0.13 | 0.15 | 0.17 | 0.18 | |
| Total equilibrium time, tf (minute) | CS1 | 561 | 122 | 95 | 42 | 25 |
| CS2 | 903 | 225 | 102 | 78 | 49 | |
| CS3 | 779 | 187 | 91 | 70 | 45 | |
| CS4 | 648 | 153 | 94 | 80.5 | 38 | |
| CS5 | 604 | 225 | 119 | 62 | 58.5 | |
| CS6 | 1315 | 252 | 120 | 91 | 51 | |
| CC7 | 609 | 152 | 75 | 54 | 33 | |
| 5% breakthrough time, tr (minutes) | CS1 | 468 | 107 | 52 | 37 | 22 |
| CS2 | 781 | 199 | 90 | 68 | 45 | |
| CS3 | 701 | 173 | 84 | 64 | 41 | |
| CS4 | 488 | 137 | 81 | 56 | 32 | |
| CS5 | 537 | 153 | 79 | 56 | 33 | |
| CS6 | 852 | 191 | 102 | 73 | 42 | |
| CC7 | 532 | 132 | 64 | 45 | 27 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balanay, J.A.G.; Oh, J. Adsorption Characteristics of Activated Carbon Fibers in Respirator Cartridges for Toluene. Int. J. Environ. Res. Public Health 2021, 18, 8505. https://doi.org/10.3390/ijerph18168505
Balanay JAG, Oh J. Adsorption Characteristics of Activated Carbon Fibers in Respirator Cartridges for Toluene. International Journal of Environmental Research and Public Health. 2021; 18(16):8505. https://doi.org/10.3390/ijerph18168505
Chicago/Turabian StyleBalanay, Jo Anne G., and Jonghwa Oh. 2021. "Adsorption Characteristics of Activated Carbon Fibers in Respirator Cartridges for Toluene" International Journal of Environmental Research and Public Health 18, no. 16: 8505. https://doi.org/10.3390/ijerph18168505
APA StyleBalanay, J. A. G., & Oh, J. (2021). Adsorption Characteristics of Activated Carbon Fibers in Respirator Cartridges for Toluene. International Journal of Environmental Research and Public Health, 18(16), 8505. https://doi.org/10.3390/ijerph18168505







