COVID-19-Associated Mortality in US Veterans with and without SARS-CoV-2 Infection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Data Source
2.2. Cohort Definition
2.3. Study Variables Created for the COVID-19 Cohort
2.4. Statistical Analysis
3. Results
3.1. Cohort Characteristics
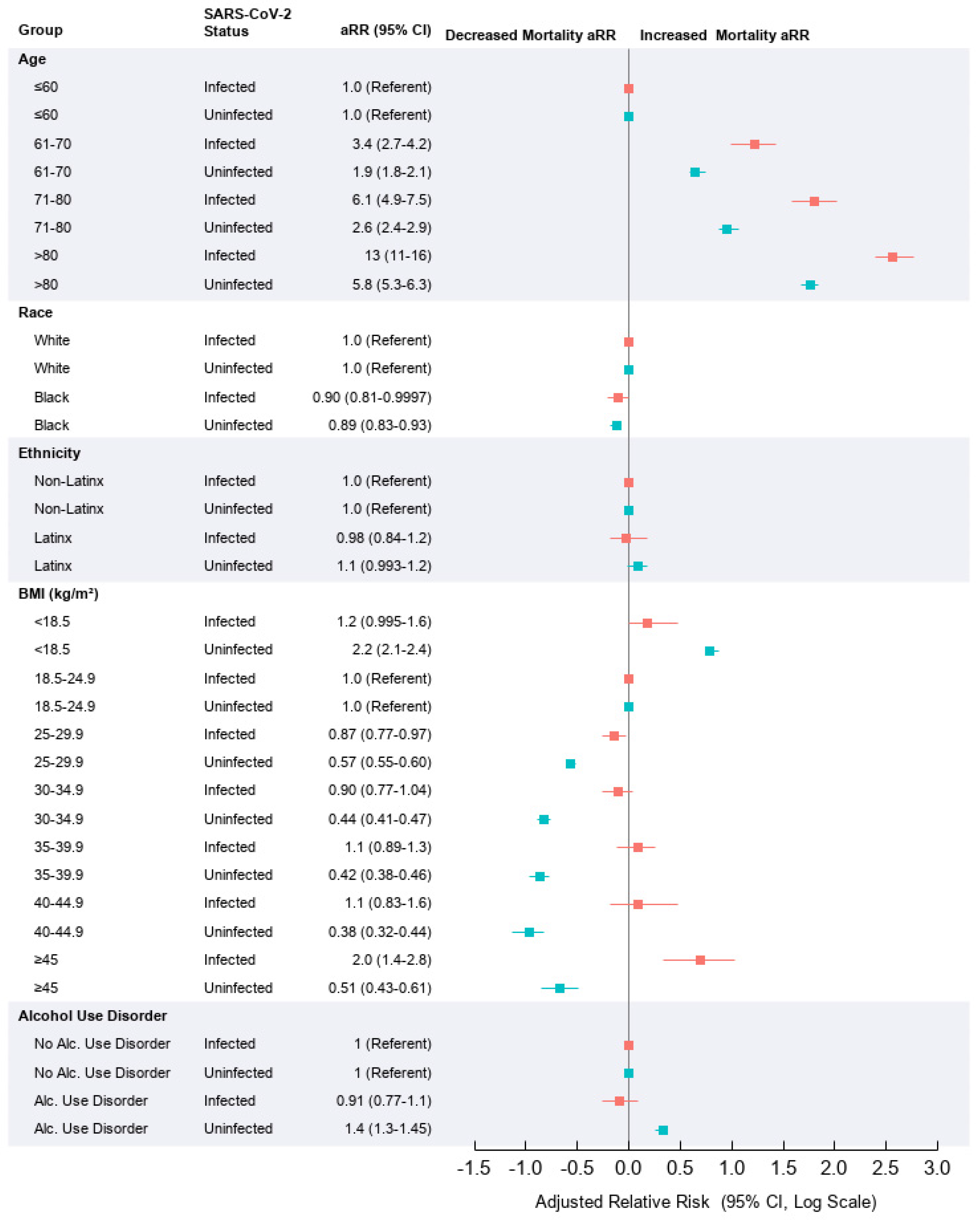
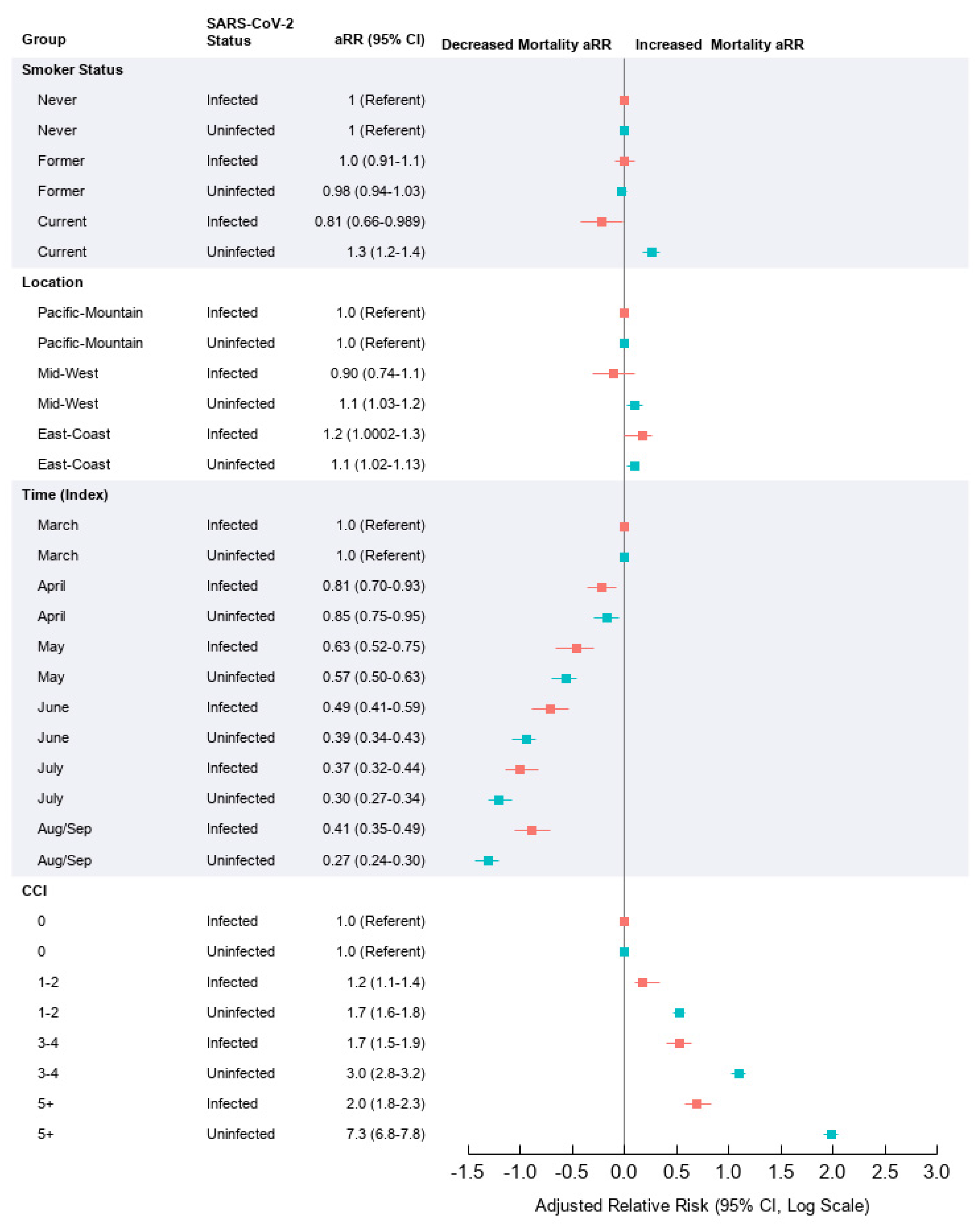
3.2. Risk Factors Affecting Mortality in SARS-CoV-2 Infected Relative to Uninfected
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wiersinga, W.J.; Rhodes, A.; Cheng, A.C.; Peacock, S.J.; Prescott, H.C. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review. JAMA 2020, 324, 782–793. [Google Scholar] [CrossRef]
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.-L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2020. [Google Scholar] [CrossRef]
- Woolf, S.H.; Chapman, D.A.; Lee, J.H. COVID-19 as the Leading Cause of Death in the United States. JAMA 2021, 325, 123–124. [Google Scholar] [CrossRef]
- Oran, D.P.; Topol, E.J. Prevalence of Asymptomatic SARS-CoV-2 Infection. Ann. Internal Med. 2020, 173, 362–367. [Google Scholar] [CrossRef]
- Ioannou, G.N.; Locke, E.; Green, P. Risk Factors for Hospitalization, Mechanical Ventilation, or Death Among 10 131 US Veterans With SARS-CoV-2 Infection. JAMA Netw. Open 2020, 3, e2022310. [Google Scholar] [CrossRef] [PubMed]
- Price-Haywood, E.G.; Burton, J.; Fort, D.; Seoane, L. Hospitalization and Mortality among Black Patients and White Patients with Covid-19. N. Engl. J. Med. 2020, 382, 2534–2543. [Google Scholar] [CrossRef] [PubMed]
- Karaca-Mandic, P.; Georgiou, A.; Sen, S. Assessment of COVID-19 Hospitalizations by Race/Ethnicity in 12 States. JAMA Intern. Med. 2021, 181, 131–134. [Google Scholar] [CrossRef] [PubMed]
- Petrilli, C.M.; Jones, S.A.; Yang, J. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: Prospective cohort study. BMJ. 2020, 369, m1966. [Google Scholar] [CrossRef] [PubMed]
- Richardson, S.; Hirsch, J.S.; Narasimhan, M. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA 2020, 323, 2052–2059. [Google Scholar] [CrossRef]
- Ioannou, G.N.; Liang, P.S.; Locke, E. Cirrhosis and SARS-CoV-2 infection in US Veterans: Risk of infection, hospitalization, ventilation and mortality. Hepatology 2020. [Google Scholar] [CrossRef]
- Williamson, E.J.; Walker, A.J.; Bhaskaran, K. OpenSAFELY: Factors associated with COVID-19 death in 17 million patients. Nature 2020. [Google Scholar] [CrossRef]
- Donnelly, J.P.; Wang, X.Q.; Iwashyna, T.J.; Prescott, H.C. Readmission and Death After Initial Hospital Discharge Among Patients With COVID-19 in a Large Multihospital System. JAMA 2021, 325, 304–306. [Google Scholar] [CrossRef]
- VA COVID-19 Shared Data Resource. VA. 17 May 2021. Available online: https://vhacdwdwhweb100.vha.med.va.gov/phenotype/index.php/COVID-19:Shared_Data_Resource#ORDCOVID_PreIndexMedications (accessed on 17 May 2021).
- VA Cooperative Studies Program Epidemiologic Analytics Resource. Race (CSPEAR). Centralized Interactive Phenomics Resource (CIPHER). 22 December 2020; (Internal to VA). Available online: https://vhacdwdwhweb100.vha.med.va.gov/phenotype/index.php/Race_(CSPEAR) (accessed on 29 April 2021).
- Dempster, A.P.; Laird, N.M.; Rubin, D.B. Maximum Likelihood from Incomplete Data Via the EM Algorithm. J. R. Stat. Soc. Series B 1977, 39, 1–22. [Google Scholar] [CrossRef]
- Blizzard, L.; Hosmer, W. Parameter Estimation and Goodness-of-Fit in Log Binomial Regression. Biom. J. 2006, 48, 5–22. [Google Scholar] [CrossRef] [PubMed]
- Le Cam, L. Asymptotic Methods in Statistical Decision Theory; Springer-Verlag: Berlin/Heidelberg, Germany, 1986. [Google Scholar]
- Hansen, B.E. Efficient shrinkage in parametric models. J. Econom. 2016, 190, 115–132. [Google Scholar] [CrossRef] [Green Version]
- Silvey, S.D. The Lagrangian Multiplier Test. Ann. Math. Stat. 1959, 30, 389–407. [Google Scholar] [CrossRef]
- Bickel, P.D.K. Mathematical Statistics: Basic Ideas and Selected Topics; CRC Press: Boca Raton, FL, USA, 1977. [Google Scholar]
- Hochberg, Y. A sharper Bonferroni procedure for multiple tests of significance. Biometrika 1988, 75, 800–802. [Google Scholar] [CrossRef]
- Efird, J.T. Goldilocks Rounding: Achieving Balance Between Accuracy and Parsimony in the Reporting of Relative Effect Estimates. Cancer Inform. 2021, 20, 1176935120985132. [Google Scholar] [CrossRef]
- Tartof, S.Y.; Qian, L.; Hong, V. Obesity and Mortality Among Patients Diagnosed With COVID-19: Results From an Integrated Health Care Organization. Ann. Intern. Med. 2020, 173, 773–781. [Google Scholar] [CrossRef]
- Finelli, L.; Gupta, V.; Petigara, T.; Yu, K.; Bauer, K.A.; Puzniak, L.A. Mortality Among US Patients Hospitalized With SARS-CoV-2 Infection in 2020. JAMA Netw. Open 2021, 4, e216556. [Google Scholar] [CrossRef]
- Centers for Disease Control. Tobacco-Related Mortality. Centers for Disease Control. Updated 28 April 2020. Available online: https://www.cdc.gov/tobacco/data_statistics/fact_sheets/health_effects/tobacco_related_mortality/index.htm (accessed on 13 April 2021).
- Farsalinos, K.; Niaura, R.; Le Houezec, J. Editorial: Nicotine and SARS-CoV-2: COVID-19 may be a disease of the nicotinic cholinergic system. Toxicol. Rep. 2020, 7, 658–663. [Google Scholar] [CrossRef]
- Millett, E.R.C.; De Stavola, B.L.; Quint, J.K.; Smeeth, L.; Thomas, S.L. Risk factors for hospital admission in the 28 days following a community-acquired pneumonia diagnosis in older adults, and their contribution to increasing hospitalisation rates over time: A cohort study. BMJ Open 2015, 5, e008737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vardavas, C.I.; Nikitara, K. COVID-19 and smoking: A systematic review of the evidence. Tob Induc. Dis. 2020, 18, 20. [Google Scholar] [CrossRef] [PubMed]
- Reddy, R.K.; Charles, W.N.; Sklavounos, A.; Dutt, A.; Seed, P.T.; Khajuria, A. The effect of smoking on COVID-19 severity: A systematic review and meta-analysis. J. Med. Virol. 2021, 93, 1045–1056. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.D.; Bravata, D.M.; Bent, S. Association of Social and Behavioral Risk Factors With Mortality Among US Veterans With COVID-19. JAMA Netw. Open. 2021, 4, e2113031. [Google Scholar] [CrossRef] [PubMed]
- US Department of Veterans Affairs. 2018 Survey of Veteran Enrollees’ Health and Use of Healthcare Data Findings Reportpdf iconexternal icon; US Department of Veterans Affairs: Washington, DC, USA, 2019. Available online: https://www.va.gov/HEALTHPOLICYPLANNING/SOE2018/2018EnrolleeDataFindingsReport_9January2019Final508Compliant.pdf (accessed on 31 July 2021).
- Hippisley-Cox, J.; Young, D.; Coupland, C. Risk of severe COVID-19 disease with ACE inhibitors and angiotensin receptor blockers: Cohort study including 8.3 million people. Heart 2020, 106, 1503–1511. [Google Scholar] [CrossRef] [PubMed]
- Simons, D.S.; Lion, S.; Brown, J.; Perski, O. The association of smoking status with SARS-CoV-2 infection, hospitalisation and mortality from COVID-19: A living rapid evidence review with Bayesian meta-analyses. Qeios 2021. [Google Scholar] [CrossRef]
- Paleiron, N.; Mayet, A.; Marbac, V. Impact of Tobacco Smoking on the risk of COVID-19. A large scale retrospective cohort study. Nicotine Tob Res. 2021. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, A.G.; Lin, M.; Wu, J. From nicotine to the cholinergic anti-inflammatory reflex–Can nicotine alleviate the dysregulated inflammation in COVID-19? J. Immunotoxicol. 2021, 18, 23–29. [Google Scholar] [CrossRef]
- Chen, L.; Long, X.; Xu, Q. Elevated serum levels of S100A8/A9 and HMGB1 at hospital admission are correlated with inferior clinical outcomes in COVID-19 patients. Cell. Mol. Immunol. 2020, 17, 992–994. [Google Scholar] [CrossRef]
- Hreggvidsdottir, H.S.; Östberg, T.; Wähämaa, H. The alarmin HMGB1 acts in synergy with endogenous and exogenous danger signals to promote inflammation. J. Leukoc. Biol. 2009, 86, 655–662. [Google Scholar] [CrossRef]
- Sitapara, R.A.; Gauthier, A.G.; Valdés-Ferrer, S.I. The α7 nicotinic acetylcholine receptor agonist, GTS-21, attenuates hyperoxia-induced acute inflammatory lung injury by alleviating the accumulation of HMGB1 in the airways and the circulation. Mol. Med. 2020, 26, 63. [Google Scholar] [CrossRef]
- Andersson, U.; Ottestad, W.; Tracey, K.J. Extracellular HMGB1: A therapeutic target in severe pulmonary inflammation including COVID-19? Mol Med. 2020, 26, 42. [Google Scholar] [CrossRef]
- NCT04583410. Efficacy of Nicotine in Preventing COVID-19 Infection (NICOVID-PREV). Available online: https://clinicaltrials.gov/ct2/show/NCT04583410 (accessed on 25 April 2021).
- Simet, S.M.; Sisson, J.H. Alcohol’s Effects on Lung Health and Immunity. Alcohol Res. 2015, 37, 199–208. [Google Scholar]
- Konig, M.F.; Powell, M.; Staedtke, V. Preventing cytokine storm syndrome in COVID-19 using α-1 adrenergic receptor antagonists. J. Clin. Investig. 2020, 130. [Google Scholar] [CrossRef] [PubMed]
- Golden, S.E.; Hooker, E.R.; Shull, S. Validity of Veterans Health Administration structured data to determine accurate smoking status. Health Inform. J. 2020, 26, 1507–1515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hepner, K.A.; Watkins, K.E.; Farmer, C.M.; Rubenstein, L.; Pedersen, E.R.; Pincus, H.A. Quality of care measures for the management of unhealthy alcohol use. J. Subst. Abuse Treat. May 2017, 76, 11–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grossbard, J.; Malte, C.A.; Lapham, G. Prevalence of Alcohol Misuse and Follow-Up Care in a National Sample of OEF/OIF VA Patients With and Without TBI. Psychiatr. Serv. 2017, 68, 48–55. [Google Scholar] [CrossRef] [Green Version]
- Teeters, J.B.; Lancaster, C.L.; Brown, D.G.; Back, S.E. Substance use disorders in military veterans: Prevalence and treatment challenges. Subst. Abuse Rehabil. 2017, 8, 69–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Characteristic | SARS-CoV-2 Status | |||||
|---|---|---|---|---|---|---|
| Infected | Uninfected | |||||
| Dead | Alive | p¥ | Dead | Alive | p¥ | |
| n (%) | n (%) | n (%) | n (%) | |||
| Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | |||
| N (%) | 1520 (7) | 21,257 (93) | --- | 8163 (3) | 310,226 (97) | --- |
| Age (year) | 76 (16) | 60 (24) | <0.0001 | 74 (14) | 66 (19) | <0.0001 |
| ≤30 | 0 (0) | 1176 (6) | 7 (<1) | 9388 (3) | ||
| 31–40 | 2 (<1) | 2684 (13) | 35 (<1) | 27,001 (9) | ||
| 41–50 | 21 (1) | 2637 (12) | 76 (1) | 27,236 (9) | ||
| 51–60 | 69 (5) | 4171 (20) | 475 (6) | 52,105 (17) | ||
| 61–70 | 304 (20) | 4852 (23) | 1989 (24) | 83,164 (27) | ||
| 71–80 | 537 (35) | 4261 (20) | 3060 (37) | 84,926 (27) | ||
| 81–90 | 389 (26) | 1162 (5) | 1739 (21) | 21,590 (7) | ||
| >90 | 198 (13) | 314 (1) | 782 (10) | 4816 (2) | ||
| Black Race ^ | 532 (35) | 7889 (37) | 0.10 | 1588 (19) | 73,198 (24) | <0.0001 |
| Latinx ^ | 148 (10) | 3709 (17) | <0.0001 | 662 (8) | 31,051 (10) | <0.0001 |
| BMI (kg/m2) | 27 (8) | 30 (8) | <0.0001 | 25 (8) | 29 (8) | <0.0001 |
| <18.5 | 68 (4) | 256 (1) | 864 (11) | 5281 (2) | ||
| 18.5–24.9 | 467 (31) | 3358 (16) | 3258 (40) | 65,125 (21) | ||
| 25–29.9 | 467 (31) | 7115 (33) | 2216 (27) | 106,208 (34) | ||
| 30–34.9 | 289 (19) | 6136 (29) | 1081 (13) | 79,500 (26) | ||
| 35–39.9 | 144 (9) | 2828 (13) | 466 (6) | 34,797 (11) | ||
| 40–44.9 | 51 (3) | 1062 (5) | 158 (2) | 12,643 (4) | ||
| ≥45 | 34 (2) | 502 (2) | 120 (1) | 6672 (2) | ||
| Alcohol Use Disorder ^ | 152 (10) | 3002 (14) | <0.0001 | 1540 (19) | 55,965 (18) | 0.056 |
| Smoker § | <0.0001 | <0.0001 | ||||
| Never | 604 (40) | 10,197 (48) | 1748 (21) | 67,205 (42) | ||
| Former | 811 (53) | 8548 (40) | 4012 (49) | 131,564 (42) | ||
| Current | 105 (7) | 2512 (12) | 2404 (29) | 111,457 (36) | ||
| Hospitalization^ | 1049 (69) | 6100 (29) | <0.0001 | 4553 (56) | 75,240 (24) | <0.0001 |
| LOS (d) | 9 (9) | 6 (10) | <0.0001 | 4 (6) | 2 (4) | <0.0001 |
| ≤7 ~ | 896 (59) | 18,654 (88) | 6976 (85) | 6487 (2) | ||
| >7–14 | 369 (24) | 1191 (6) | 828 (10) | 5391 (2) | ||
| >14 | 255 (17) | 1412 (7) | 359 (4) | 5458 (2) | ||
| Mechanical Ventilation ^ | 557 (37) | 544 (3) | <0.0001 | 963 (12) | 5458 (2) | <0.0001 |
| Location (USA) | <0.0001 | <0.0001 | ||||
| Pacific-Mountain | 205 (13) | 4053 (19) | 1626 (20) | 72,970 (24) | ||
| Mid-West | 233 (15) | 4440 (21) | 1787 (22) | 62,805 (20) | ||
| East-Coast | 1082 (71) | 12,764 (60) | 4750 (58) | 174,451 (56) | ||
| Time (Index, 1 March–10 September) | <0.0001 | <0.0001 | ||||
| March | 242 (16) | 1621 (8) | 287 (4) | 5,558 (2) | ||
| April | 421 (28) | 2722 (13) | 1182 (14) | 19,534 (6) | ||
| May | 179 (12) | 1669 (8) | 1425 (17) | 34,482 (11) | ||
| June | 195 (13) | 3819 (18) | 1580 (19) | 61,545 (20) | ||
| July | 297 (20) | 7882 (37) | 1702 (21) | 91,403 (29) | ||
| August | 174 (11) | 3361 (16) | 1546 (19) | 83,980 (27) | ||
| September | 12 (1) | 183 (1) | 441 (5) | 13,724 (4) | ||
| Charlson Comorbidity Index | <0.0001 | <0.0001 | ||||
| 0 | 374 (25) | 11,280 (53) | 1036 (13) | 138,715 (45) | ||
| 1–2 | 541 (36) | 7023 (33) | 2171 (27) | 107,614 (35) | ||
| 3–4 | 373 (25) | 2019 (10) | 1979 (24) | 41,228 (13) | ||
| 5+ | 230 (15) | 935 (4) | 2977 (36) | 22,669 (7) | ||
| Comorbidity ^ | ||||||
| Asthma | 59 (4) | 1254 (6) | <0.0001 | 348 (4) | 19,852 (6) | <0.0001 |
| Atherosclerosis | 857 (54) | 5462 (26) | <0.0001 | 5233 (64) | 109,317 (35) | <0.0001 |
| Cancer | 375 (25) | 2599 (12) | <0.0001 | 4017 (49) | 62,782 (20) | <0.0001 |
| Chronic Kidney Disease | 584 (38) | 3208 (15) | <0.0001 | 3440 (42) | 55,028 (18) | <0.0001 |
| Chronic Liver Disease | 66 (4) | 500 (2) | <0.0001 | 937 (11) | 11,357 (4) | <0.0001 |
| Congestive Heart Failure | 425 (28) | 2375 (11) | <0.0001 | 3551 (44) | 48,881 (16) | <0.0001 |
| Chronic Obstructive Pulmonary Disease | 448 (29) | 2986 (14) | <0.0001 | 3822 (47) | 70,300 (23) | <0.0001 |
| Diabetes (Type II) | 771 (51) | 7098 (33) | <0.0001 | 3755 (46) | 105,024 (34) | <0.0001 |
| Hyperlipidemia | 990 (65) | 11,617 (55) | <0.0001 | 5546 (68) | 182,297 (59) | <0.0001 |
| Hypertension | 1170 (77) | 12,160 (57) | <0.0001 | 6425 (79) | 195,340 (63) | <0.0001 |
| Mental Illness | 661 (43) | 10,031 (47) | 0.0052 | 3936 (48) | 158,433 (51) | <0.0001 |
| Sleep Disorder | 378 (25) | 6053 (28) | 0.0025 | 2037 (25) | 90,685 (29) | <0.0001 |
| Substance Abuse | 289 (19) | 4771 (22) | 0.0019 | 2977 (36) | 97,902 (32) | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suzuki, A.; Efird, J.T.; Redding, T.S., IV; Thompson, A.D., Jr.; Press, A.M.; Williams, C.D.; Hostler, C.J.; Hunt, C.M. COVID-19-Associated Mortality in US Veterans with and without SARS-CoV-2 Infection. Int. J. Environ. Res. Public Health 2021, 18, 8486. https://doi.org/10.3390/ijerph18168486
Suzuki A, Efird JT, Redding TS IV, Thompson AD Jr., Press AM, Williams CD, Hostler CJ, Hunt CM. COVID-19-Associated Mortality in US Veterans with and without SARS-CoV-2 Infection. International Journal of Environmental Research and Public Health. 2021; 18(16):8486. https://doi.org/10.3390/ijerph18168486
Chicago/Turabian StyleSuzuki, Ayako, Jimmy T. Efird, Thomas S. Redding, IV, Andrew D. Thompson, Jr., Ashlyn M. Press, Christina D. Williams, Christopher J. Hostler, and Christine M. Hunt. 2021. "COVID-19-Associated Mortality in US Veterans with and without SARS-CoV-2 Infection" International Journal of Environmental Research and Public Health 18, no. 16: 8486. https://doi.org/10.3390/ijerph18168486
APA StyleSuzuki, A., Efird, J. T., Redding, T. S., IV, Thompson, A. D., Jr., Press, A. M., Williams, C. D., Hostler, C. J., & Hunt, C. M. (2021). COVID-19-Associated Mortality in US Veterans with and without SARS-CoV-2 Infection. International Journal of Environmental Research and Public Health, 18(16), 8486. https://doi.org/10.3390/ijerph18168486







