Diurnal Cortisol Rhythm in Female Flight Attendants
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Design
2.2. Sample Collection and Analysis
2.3. Statistical Analysis
3. Results
3.1. Characteristic of the Study Group and the Control Group
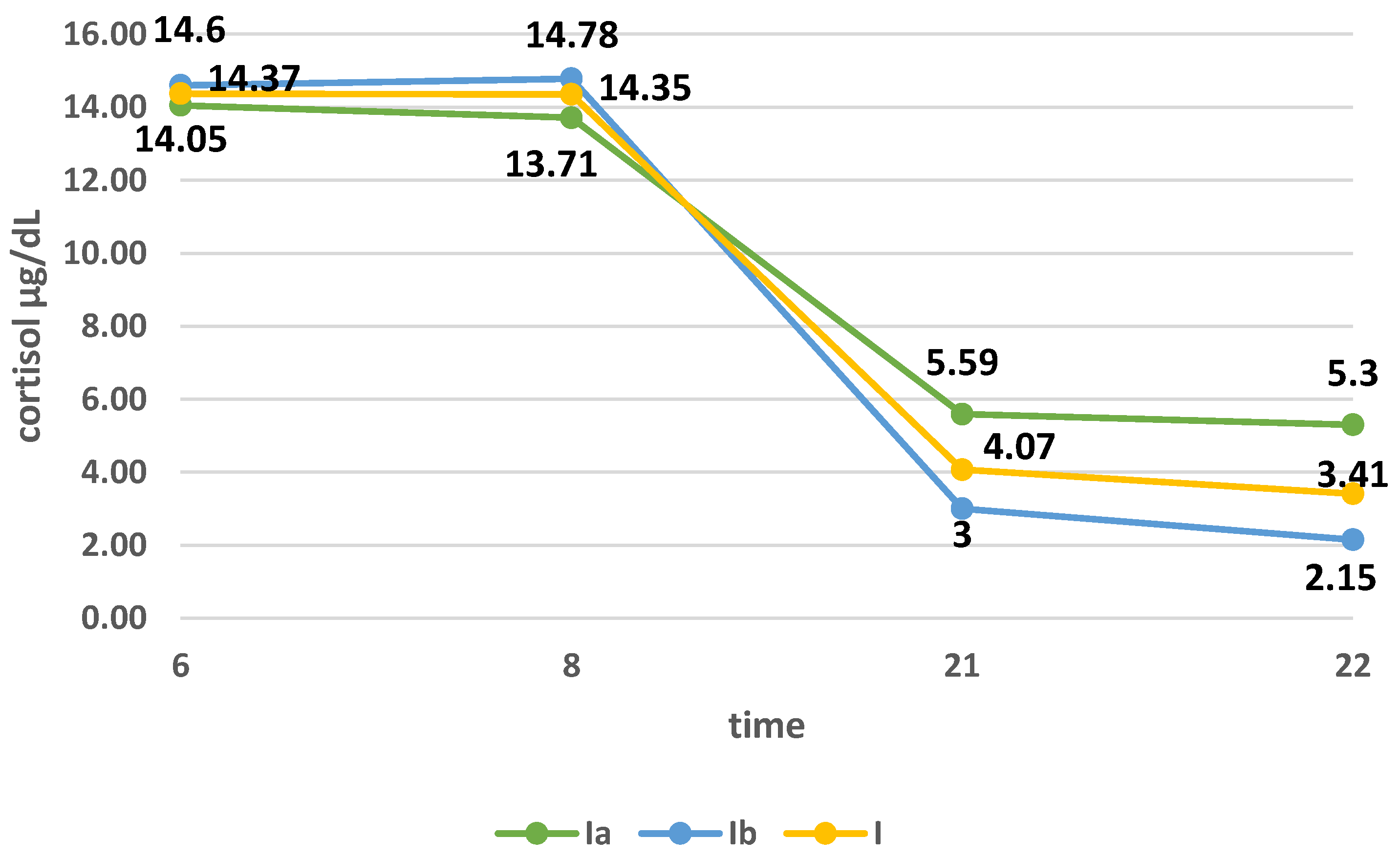
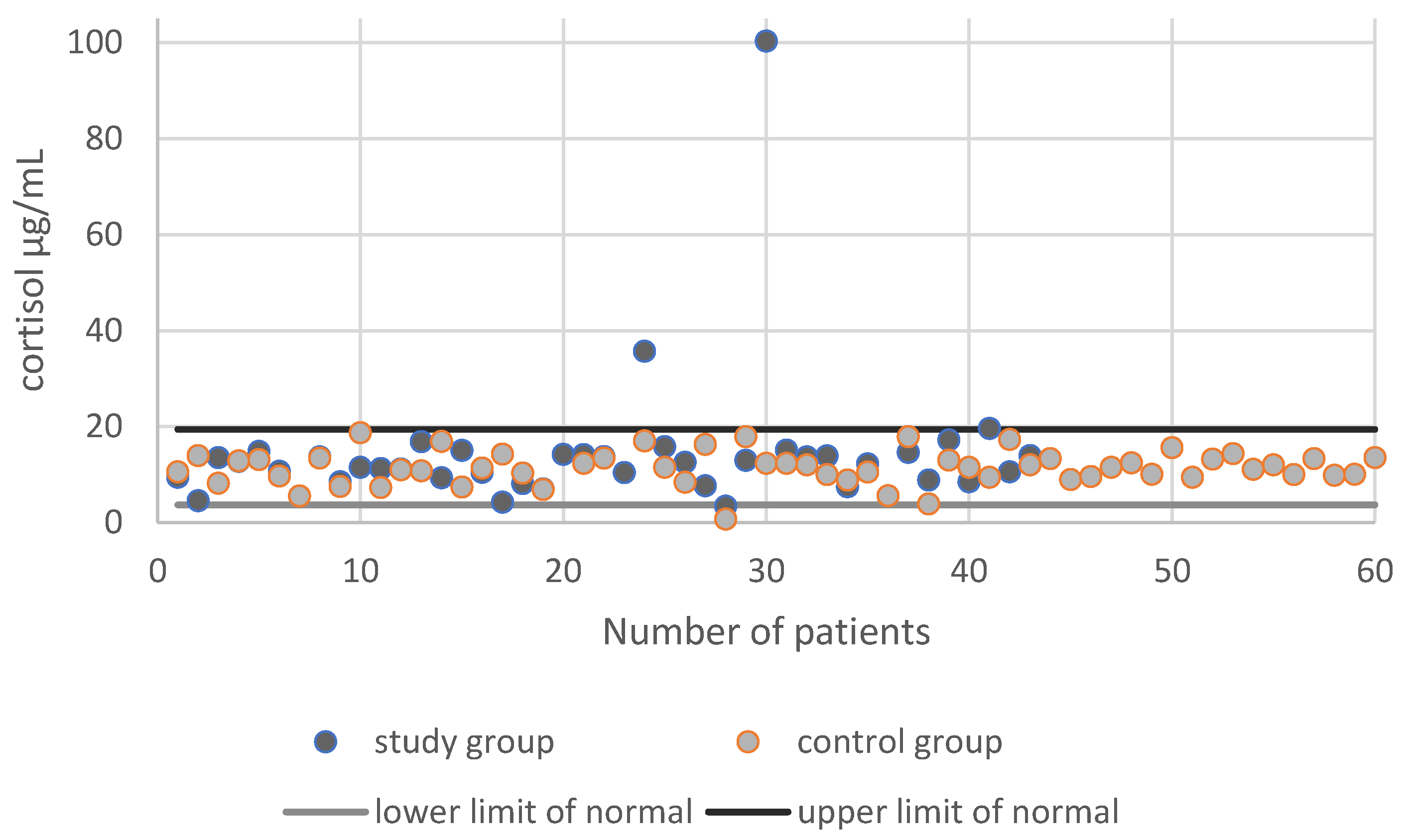
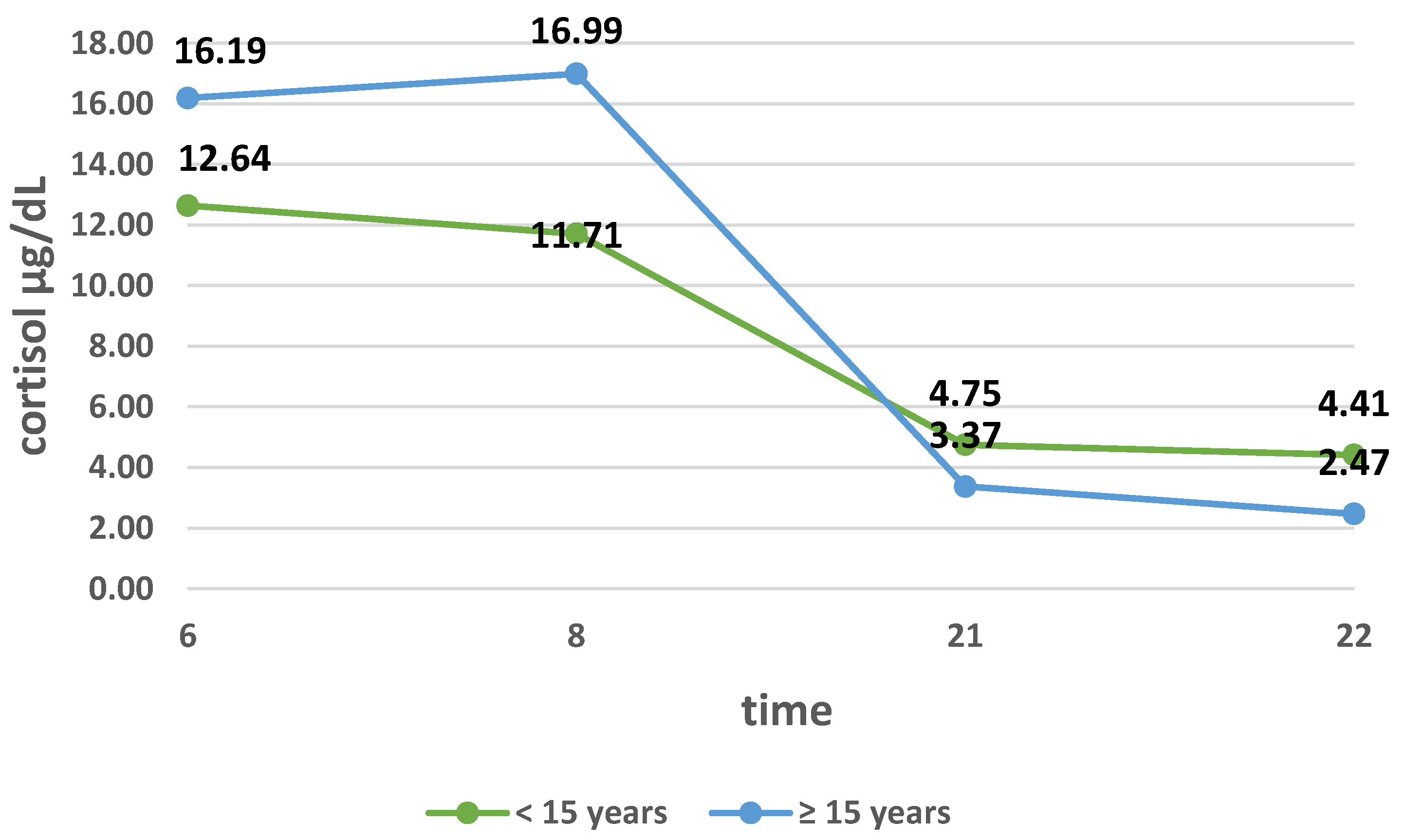
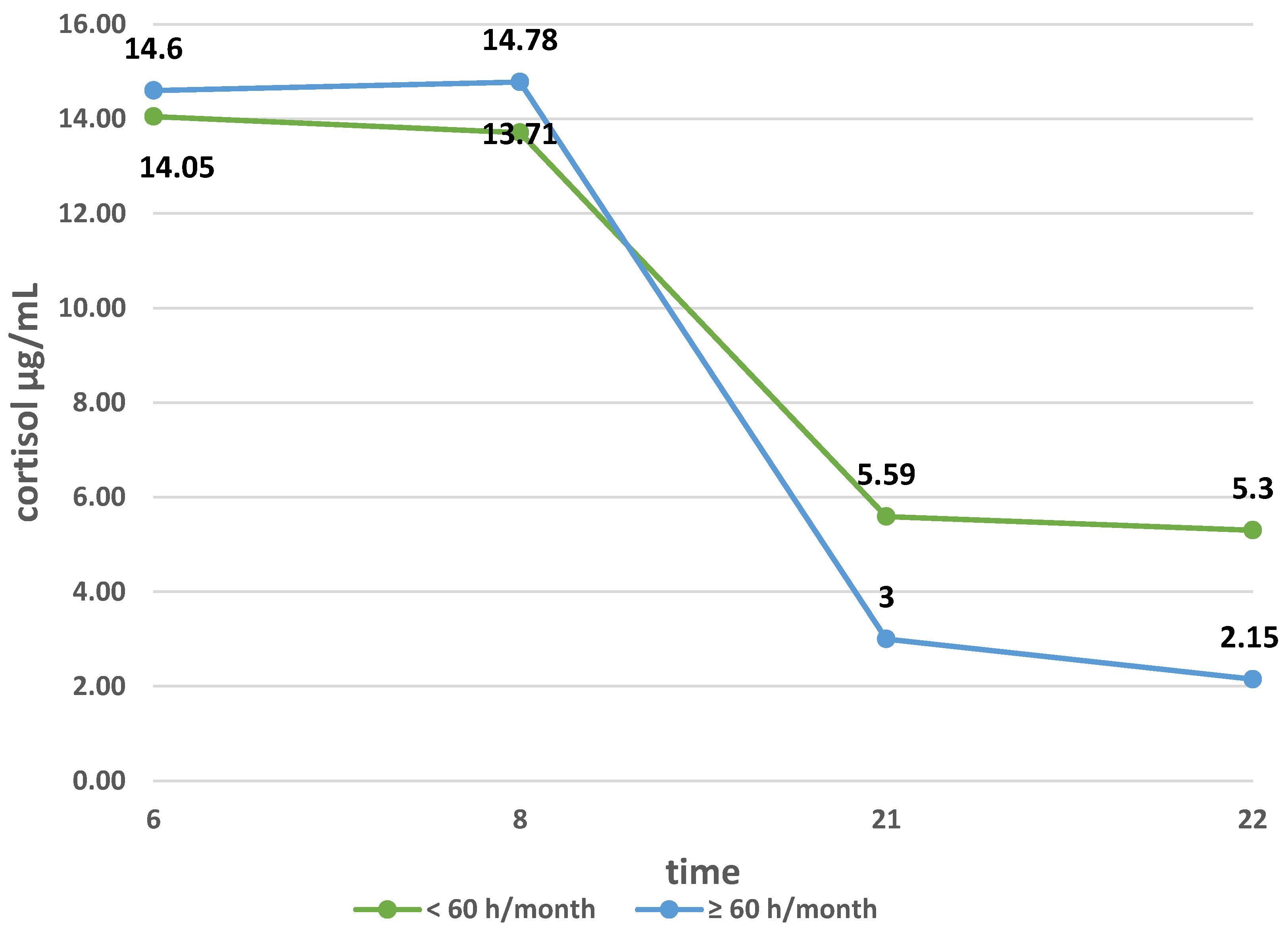
3.2. Cortisol Secretion
3.3. Testosterone Concentration
3.4. Progesterone Concentration
3.5. 17-Hydroxyprogesterone Concentration
4. Discussion
5. Conclusions
- Due to the character of work, female flight attendants do not have hypersecretion of cortisol.
- Frequency of flying and length of work affect the dysregulation of the HPA axis.
- Greater work experience (longer work experience, more frequent flying) reduces the stress of flying.
- Further research on a larger cohort of flight attendants is necessary to assess metabolic and hemodynamic parameters that may affect the cortisol profile.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Statement of Ethics
References
- Eckel-Mahan, K.; Sassone-Corsi, P. Metabolism and the circadian clock converge. Psychol. Rev. 2013, 93, 107–135. [Google Scholar] [CrossRef] [PubMed]
- Richards, J.; Gumz, M.L. Mechanism of the circafian clock in physiology. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 304, R1053–R1064. [Google Scholar] [CrossRef] [PubMed]
- Costa, G. Shift work and health: Current problems and preventive actions. Saf. Health Work 2010, 1, 112–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gamble, K.L.; Berry, R.; Frank, S.J.; Young, M.E. Circadian clock control of endocrine factors. Nat. Rev. Endocrinol. 2014, 10, 466–475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohd Azmi, N.A.S.; Juliana, N.; Azmani, S.; Effendy, N.M.; Abu, I.; Teng, N.M.F.; Das, S. Cortisol on circadian rhythm and its effect on cardiovascular system. Int. J. Environ. Res. Public Health 2021, 18, 676. [Google Scholar] [CrossRef] [PubMed]
- Herman, J.P.; McKlveen, J.M.; Ghosal, S.; Kopp, B.; Wulsin, A.; Makinson, R.; Scheimann, J.; Myers, B. Regulation of the hypothalamic-pituitary-adrenocortical stress response. Compr. Physiol. 2016, 6, 603–621. [Google Scholar] [PubMed] [Green Version]
- Roscoe, A.H. Stress and workload in pilots. Aviat. Space Environ. Med. 1978, 49, 630–633. [Google Scholar] [PubMed]
- Stephens, M.A.C.; Wand, G. Stress and the HPA Axis. Alcohol. Res. 2012, 34, 468–483. [Google Scholar] [PubMed]
- Joseph, J.J.; Golden, S.H. Cortisol dysregulation: The bidirectional link between stress, depression and type 2 diabetes mellitus. Ann. N. Y. Acad. Sci. 2017, 1391, 20–34. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, L.A.; Deddens, J.A.; Grajewski, B.A.; Whelan, E.A.; Hurrel, J.J. Job stress among female flight attendatns. J. Occup. Environ. Med. 2003, 45, 703–714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicolaides, N.C.; Charmandari, E.; Kino, T.; Chrousos, G.P. Stress-related and circadian secretion and target tissue actions of glucocorticoids: Impact of health. Front. Endocrionol. 2017, 8, 70. [Google Scholar] [CrossRef] [PubMed]
- Diez, J.J.; Vigo, D.E.; Lloret, S.P.; Rigters, S.; Role, N.; Cardinali, D.P.; Chada, D.P. Sleep habits, alertness, cortisol levels, and cardiac autonomic activity in short-distance bus drivers: Differences between morning and afternoon shifts. J. Occup. Environ. Med. 2011, 53, 806–811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bostock, S.; Steptoe, A. Influences of early shift work on the diurnal cortisol rhythm, mood and sleep: Within-subject variation in male airline pilots. Psychoneuroendocrinology 2013, 38, 533–541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schiffer, L.; Barnard, L.; Baranowski, E.S.; Gilligan, L.C.; Taylor, A.E.; Arlt, W.; Shackleton, C.H.L.; Storbeck, K.H. Human steroid biosynthesis, metabolism and excretion are differentially reflected by serum and urine steroid metabolomes: A comprehensive review. J. Steroid Biochem. Mol. Biol. 2019, 194, 105439. [Google Scholar] [CrossRef] [PubMed]
- Oyola, M.G.; Handa, R.J. Hypothalamic-pituitary-adrenal and hypothalamic-pituitary-gonadal axes: Sex differences in regulation of stress responsivity. Stress 2017, 20, 476–494. [Google Scholar] [CrossRef] [PubMed]
- Ukraintseva, Y.V.; Liaukovich, K.M.; Polishchuk, A.A.; Martynova, O.V.; Belov, D.A.; Simenel, E.S.; e Cruz, M.M.; Nizhnik, A.N. Slow-wave sleep and androgens: Selective slow-wave sleep suppression affects testosterone and 17α-hydroxyprogesterone secretion. Sleep Med. 2018, 48, 117–126. [Google Scholar] [CrossRef]

| Variable | Control Group (II) (n = 60) | Study Group (I) (n = 43) | Group Ia (n = 17) | Group Ib (n = 26) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Min | Max | M ± SD | Min | Max | M ± SD | p | Min | Max | M ± SD | Min | Max | M ± SD | p | |
| Age (years) | 23 | 46 | 34.0 ± 4.09 | 25 | 43 | 34.7 ± 4.41 | 0.203 | 26 | 43 | 34.1 ± 4.26 | 25 | 40 | 35.2 ± 4.53 | 0.219 |
| BMI (kg/m2) | 17.5 | 37 | 22.7 ± 3.78 | 17.5 | 29.0 | 22.4 ± 2.71 | 0.995 | 17.5 | 28 | 21.7 ± 2.67 | 19 | 29 | 22.8 ± 2.71 | 0.28 |
| Seniority (years) | 6 | 25 | 14.3 ± 5.06 | 6 | 18 | 13.0 ± 4.23 | 6 | 25 | 15.2 ± 5.44 | 0.084 | ||||
| Time of work in air (h/month) | 47 | 85 | 64.6 ± 7.89 | 47 | 70 | 59.5 ± 6.80 | 55 | 85 | 67.9 ± 6.81 | 0.001 | ||||
| Control Group (II) (n = 60) | Study Group (I) (n = 43) | Group Ia (n = 17) | Group Ib (n = 26) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Min | Max | M ± SD | Min | Max | M ± SD | p | Min | Max | M ± SD | Min | Max | M ± SD | p | |
| Estradiol (pg/mL) | 10 | 303 | 47 ± 39 | 9 | 139 | 48.8 ± 26.9 | 0.397 | 9 | 107 | 44.6 ± 23.4 | 19 | 139 | 51.6 ± 29.1 | 0.504 |
| SHBG (nmol/L) | 24.6 | 175.7 | 72 ± 36.1 | 38.1 | 164.9 | 71.35 ± 25.8 | 0.491 | 38.1 | 122.2 | 65.87 ± 23.7 | 41.3 | 164.9 | 74.64 ± 27 | 0.154 |
| Androstenedione (ng/mL) | 1 | 6 | 2.89 ± 1.11 | 1.4 | 9.4 | 2.97 ± 1.83 | 0.553 | 1.4 | 4.8 | 2.71 ± 1.34 | 1.7 | 9.4 | 3.16 ± 2.16 | 0.591 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radowicka, M.; Pietrzak, B.; Wielgoś, M. Diurnal Cortisol Rhythm in Female Flight Attendants. Int. J. Environ. Res. Public Health 2021, 18, 8395. https://doi.org/10.3390/ijerph18168395
Radowicka M, Pietrzak B, Wielgoś M. Diurnal Cortisol Rhythm in Female Flight Attendants. International Journal of Environmental Research and Public Health. 2021; 18(16):8395. https://doi.org/10.3390/ijerph18168395
Chicago/Turabian StyleRadowicka, Małgorzata, Bronisława Pietrzak, and Mirosław Wielgoś. 2021. "Diurnal Cortisol Rhythm in Female Flight Attendants" International Journal of Environmental Research and Public Health 18, no. 16: 8395. https://doi.org/10.3390/ijerph18168395
APA StyleRadowicka, M., Pietrzak, B., & Wielgoś, M. (2021). Diurnal Cortisol Rhythm in Female Flight Attendants. International Journal of Environmental Research and Public Health, 18(16), 8395. https://doi.org/10.3390/ijerph18168395





