Population-Based Data Reveal Factors Associated with Organised and Non-Organised Colorectal Cancer Screening: An Important Step towards Improving Coverage
Abstract
:1. Introduction
2. Methods
2.1. Flanders and Its Organised CRC Screening Programme
2.2. Study Population and Data Sources
2.3. Main Outcomes
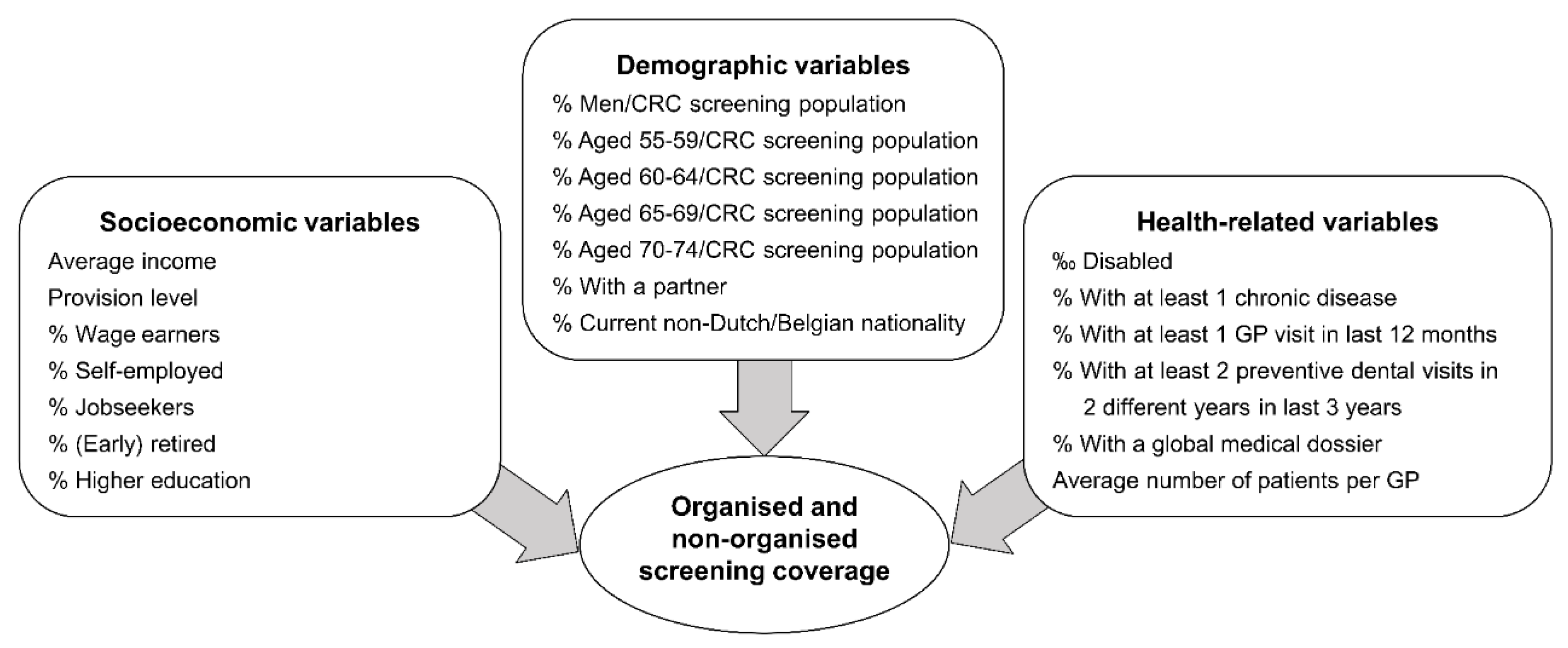
2.4. Determinants Considered
Variable Explanation
2.5. Covariates
2.6. Statistical Analysis
2.6.1. Missing Data
2.6.2. Sample Size
2.6.3. Main Analysis
2.7. Ethics
3. Results
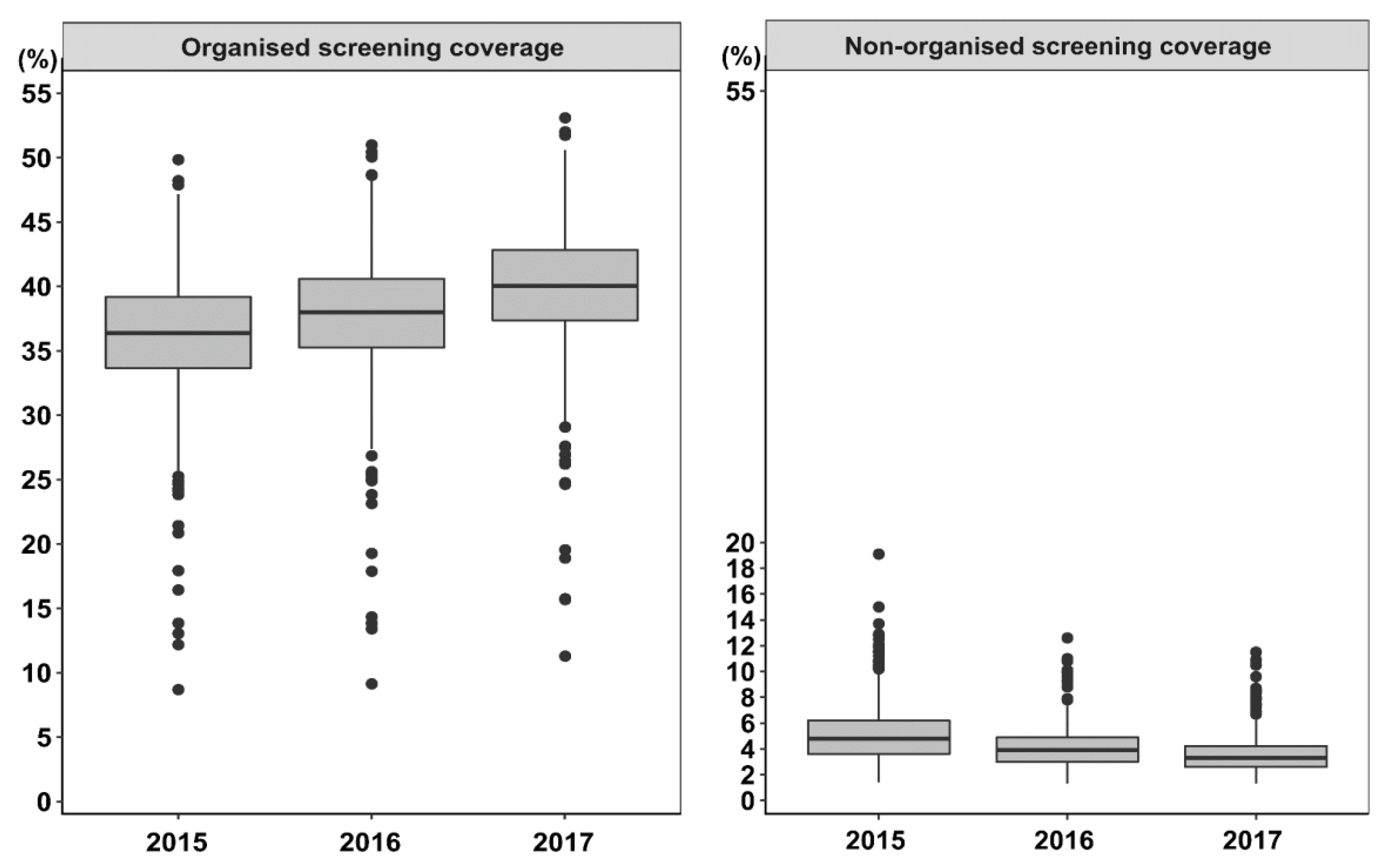
3.1. Municipal Characteristics
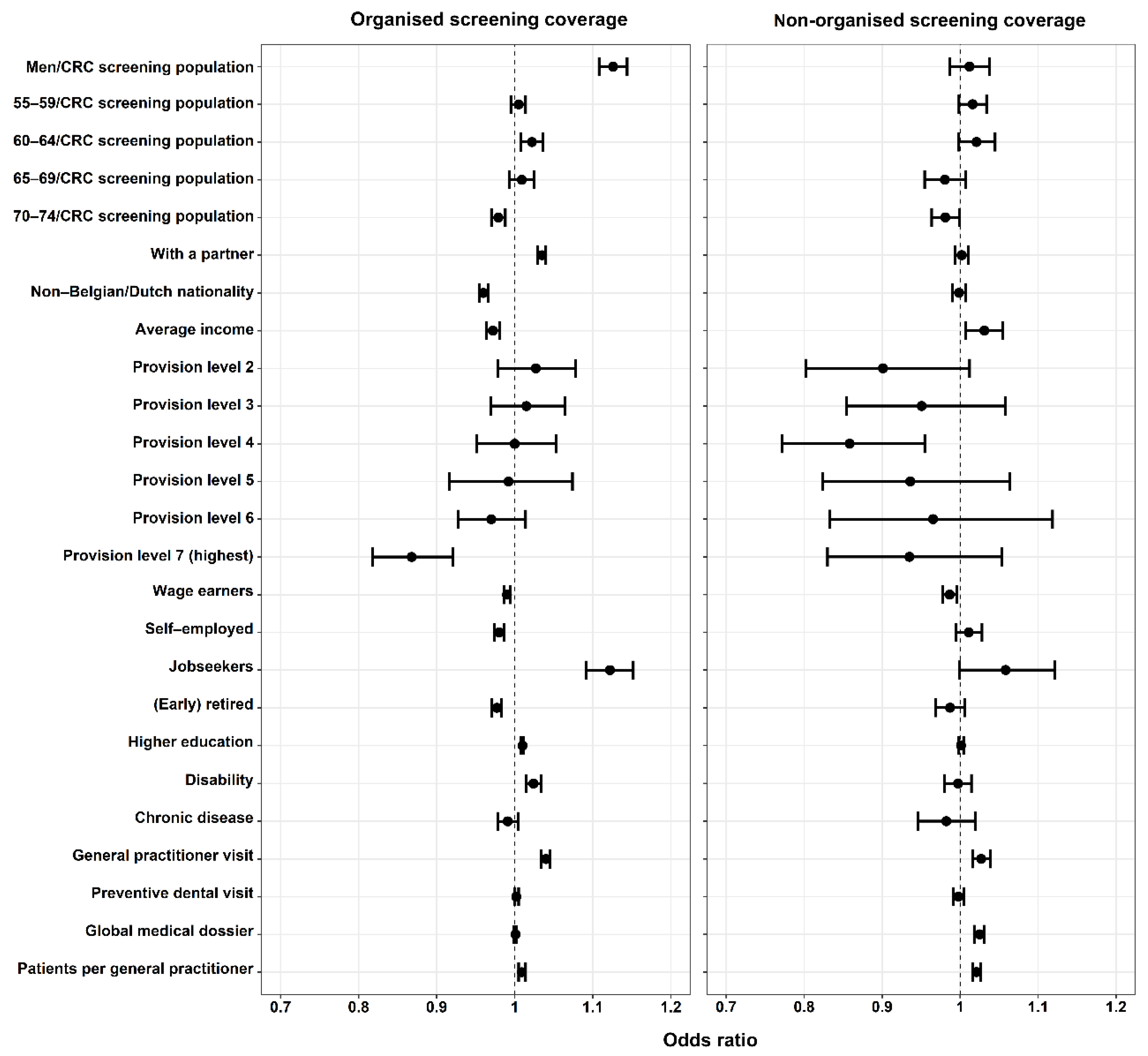
3.2. Factors Associated with Organised and Non-Organised Screening Coverage
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Belgian Cancer Registry. Requesting Specific Data 2020. Available online: https://kankerregister.org/Requesting%20specific%20data (accessed on 30 October 2020).
- STATBEL. Structure of the Population 2020. Available online: https://statbel.fgov.be/en/themes/population/structure-population (accessed on 16 February 2021).
- Publications Office of the European Union. European Guidelines for Quality Assurance in Colorectal Cancer Screening and Diagnosis, 1st ed.; Segnan, N., Patnick, J., von Karsa, L., Eds.; Publications Office of the European Union: Luxembourg, Luxembourg, 2010. [Google Scholar]
- Ponti, A.; Anttila, A.; Ronco, G.; Senore, C.; Basu, P.; Segnan, N.; Tomatis, M.; Žakelj, M.P.; Dillner, J.; Fernan, M.; et al. Cancer Screening in the European Union. Report on the implementation of Council Recommendation on Cancer Screening. Brussels: European Commission 2017. Available online: https://ec.europa.eu/health/sites/health/files/major_chronic_diseases/docs/2017_cancerscreening_2ndreportimplementation_en.pdf (accessed on 16 February 2021).
- Center for Cancer Detection. Monitoring Report of the Flemish Colorectal Cancer Screening Programme 2020. Available online: https://www.bevolkingsonderzoek.be/sites/default/files/atoms/files/Jaarrapport%202020.pdf (accessed on 1 December 2020).
- Decker, K.M.; Demers, A.A.; Nugent, Z.; Biswanger, N.; Singh, H. Reducing income-related inequities in colorectal cancer screening: Lessons learned from a retrospective analysis of organised programme and non-programme screening delivery in Winnipeg, Manitoba. BMJ Open 2016, 6, e009470. [Google Scholar] [CrossRef] [Green Version]
- Hoeck, S.; van de Veerdonk, W.; De Brabander, I.; Kellen, E. Does the Flemish colorectal cancer screening programme reach equity in FIT uptake? Eur. J. Public Health 2019, 29, 1108–1114. [Google Scholar] [CrossRef]
- De Maesschalck, F.; Van Hecke, E. Equipment Level of the Flemish Municipalities 2018. Available online: https://www.west-vlaanderen.be/sites/default/files/2018-12/Uitrustingsgraad-van-de-vlaamse-gemeenten-typologie-.pdf (accessed on 17 August 2020).
- Sauer, B.; Brookhart, M.; Roy, J.; VanderWeele, T.J. Covariate Selection. In Developing a Protocol for Observational Comparative Effectiveness Research: A User’s Guide; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2013; pp. 93–108. [Google Scholar]
- Peduzzi, P.; Concato, J.; Kemper, E.; Holford, T.R.; Feinstein, A.R. A simulation study of the number of events per variable in logistic regression analysis. J. Clin. Epidemiol. 1996, 49, 1373–1379. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. BMJ 2007, 335, 806–808. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, L.C.; von Wagner, C.; Wardle, J. Perceived Life Expectancy Is Associated with Colorectal Cancer Screening in England. Ann. Behav. Med. 2017, 51, 327–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoeck, S.; Hoste, J.; Vandeputte, L.; Dekker, N. Colorectal Cancer Screening 2017. Available online: https://www.domusmedica.be/sites/default/files/Richtlijn%20Dikkedarmkankerscreening.pdf (accessed on 6 November 2020).
- Fon Sing, M.; Leuraud, K.; Duport, N. Characteristics of French people using organised colorectal cancer screening. Analysis of the 2010 French Health, Healthcare and Insurance Survey. Prev. Med. 2013, 57, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Suh, M.; Choi, K.S.; Lee, H.Y.; Hahm, M.I.; Lee, Y.Y.; Jun, J.K.; Park, E.C. Socioeconomic Disparities in Colorectal Cancer Screening in Korea: A Nationwide Cross-Sectional Study. Medicine 2015, 94, e1368. [Google Scholar] [CrossRef]
- Turnbull, E.; Priaulx, J.; de Kok, I.; Lansdorp-Vogelaar, I.; Anttila, A.; Sarkeala, T.; Senore, C.; Segnan, N.; Csanádi, M.; Pitter, J.; et al. Results of a health systems approach to identify barriers to population-based cervical and colorectal cancer screening programmes in six European countries. Health Policy 2018, 122, 1206–1211. [Google Scholar] [CrossRef]
- Hoeck, S.; Van Roy, K.; Willems, S. Barriers and facilitators to participate in the colorectal cancer screening programme in Flanders (Belgium): A focus group study. Acta Clin. Belg. 2020, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Statistics Flanders. Population by Nationality 2019. Available online: https://www.statistiekvlaanderen.be/en/population-by-nationality-0 (accessed on 18 August 2020).
- Frederiksen, B.L.; Jorgensen, T.; Brasso, K.; Holten, I.; Osler, M. Socioeconomic position and participation in colorectal cancer screening. Br. J. Cancer 2010, 103, 1496–1501. [Google Scholar] [CrossRef]
- Wools, A.; Dapper, E.A.; de Leeuw, J.R. Colorectal cancer screening participation: A systematic review. Eur. J. Public Health 2016, 26, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Osborne, J.M.; Flight, I.; Wilson, C.J.; Chen, G.; Ratcliffe, J.; Young, G.P. The impact of sample type and procedural attributes on relative acceptability of different colorectal cancer screening regimens. Patient Prefer. Adherence 2018, 12, 1825–1836. [Google Scholar] [CrossRef] [Green Version]
- Artama, M.; Heinävaara, S.; Sarkeala, T.; Prättälä, R.; Pukkala, E.; Malila, N. Determinants of non-participation in a mass screening program for colorectal cancer in Finland. Acta Oncol. 2016, 55, 870–874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewis, M.A.; Rook, K.S. Social control in personal relationships: Impact on health behaviors and psychological distress. Health Psychol. 1999, 18, 63–71. [Google Scholar] [CrossRef] [PubMed]
- van Jaarsveld, C.H.; Miles, A.; Edwards, R.; Wardle, J. Marriage and cancer prevention: Does marital status and inviting both spouses together influence colorectal cancer screening participation? J. Med. Screen 2006, 13, 172–176. [Google Scholar] [CrossRef] [Green Version]
- Kellen, E.; Nuyens, C.; Molleman, C.; Hoeck, S. Uptake of cancer screening among adults with disabilities in Flanders (Belgium). J. Med. Screen 2020, 27, 48–51. [Google Scholar] [CrossRef]
- Shin, D.W.; Chang, D.; Jung, J.H.; Han, K.; Kim, S.Y.; Choi, K.S.; Lee, W.C.; Park, J.H.; Park, J.H. Disparities in the Participation Rate of Colorectal Cancer Screening by Fecal Occult Blood Test among People with Disabilities: A National Database Study in South Korea. Cancer Res. Treat 2020, 52, 60–73. [Google Scholar] [CrossRef] [Green Version]
- Iezzoni, L.I.; Kurtz, S.G.; Rao, S.R. Trends in colorectal cancer screening over time for persons with and without chronic disability. Disabil. Health J. 2016, 9, 498–509. [Google Scholar] [CrossRef] [Green Version]
- Portillo, I.; Arana-Arri, E.; Gutiérrez-Ibarluzea, I.; Bilbao, I.; Luis Hurtado, J.; Sarasqueta, C.; Idigoras, I.; Bujanda, L. Factors related to the participation and detection of lesions in colorectal cancer screening programme-based faecal immunochemical test. Eur. J. Public Health 2018, 28, 1143–1148. [Google Scholar] [CrossRef]
- Pornet, C.; Dejardin, O.; Morlais, F.; Bouvier, V.; Launoy, G. Socioeconomic determinants for compliance to colorectal cancer screening. A multilevel analysis. J. Epidemiol. Community Health 2010, 64, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; March, S.; Ireland, M.J.; Crawford-Williams, F.; Goodwin, B.; Hyde, M.K.; Chambers, S.K.; Aitken, J.F.; Dunn, J. Socio-demographic factors drive regional differences in participation in the National Bowel Cancer Screening Program—An ecological analysis. Aust. N. Z. J. Public Health 2018, 42, 92–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van de Veerdonk, W.; Hoeck, S.; Peeters, M.; van Hal, G.; Francart, J.; de Brabander, I. Occurrence and characteristics of faecal immunochemical screen-detected cancers vs non-screen-detected cancers: Results from a Flemish colorectal cancer screening programme. United Eur. Gastroenterol. J. 2020, 8, 185–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McAlister, A.L.; Perry, C.L.; Parcel, G.S. How individuals, environments, and health behaviors interact: Social cognitive theory. In Health Behavior and Health Education: Theory, Research and Practice, 4th ed.; Glanz, K., Rimer, B.K., Viswanath, K., Eds.; Jossey-Bass: San Francisco, CA, USA, 2008. [Google Scholar]
| Main Determinant of Assessment | Covariates for Adjustment in Multivariable Analyses |
|---|---|
| Men/CRC screening population | Year |
| Age groups/CRC screening population | Provision level, year |
| With a partner | Age groups/CRC screening population, year |
| Current non-Belgian/Dutch nationality | Provision level, year |
| Average income | With a partner, age groups/CRC screening population, current non-Belgian/Dutch nationality, chronic disease, disability, education level, provision level, men/CRC screening population, position in labour market, year |
| Provision level | Year |
| Position in labour market | Age groups/CRC screening population, current non-Belgian/Dutch nationality, disability, education level, provision level, men/CRC screening population, year |
| Education level § | GP visit, with a partner, age groups/CRC screening population, average income, current non-Belgian/Dutch nationality, chronic disease, disability, provision level, men/CRC screening population, global medical dossier, position in labour market, preventive dental visit, year |
| Disability | Provision level, year |
| Chronic disease | Age groups/CRC screening population, current non-Belgian/Dutch nationality, disability, education level, provision level, men/CRC screening population, year |
| GP visit | Age groups/CRC screening population, average income, chronic disease, disability, education level, men/CRC screening population, preventive dental visit, year |
| Preventive dental visit | With a partner, age groups/CRC screening population, current non-Belgian/Dutch nationality, chronic disease, education level, men/CRC screening population, global medical dossier, position in labour market, year |
| Global medical dossier | With a partner, age groups/CRC screening population, current non-Belgian/Dutch nationality, chronic disease, education level, men/CRC screening population, position in labour market, preventive dental visit, year |
| Average number of patients per GP | Provision level, year |
| Median (IQR), unless Stated Otherwise | |||
|---|---|---|---|
| 2015 (n = 303) † | 2016 (n = 303) † | 2017 (n = 304) † | |
| Demographic characteristics | |||
| % Men/CRC screening population | 50.1 (49.2–50.8) | 50.0 (49.2–50.8) | 50.0 (49.3–50.8) |
| Age groups | |||
| % 55–59/CRC screening population | 25.9 (24.9–26.9) | 25.6 (24.8–26.5) | 30.0 (29.2–31.3) |
| % 60–64/CRC screening population | 28.5 (27.8–29.5) | 28.5 (27.8–29.2) | 26.6 (25.9–29.2) |
| % 65–69/CRC screening population | 25.8 (24.9–26.6) | 25.1 (24.3–25.9) | 25.1 (24.3–25.9) |
| % 70–74/CRC screening population | 19.7 (18.8–20.6) | 20.6 (19.7–21.8) | 19.9 (19.0–21.0) |
| % With a partner | 52.7 (51.1–53.9) | 52.6 (51.1–53.8) | 52.6 (50.9–53.7) |
| % Current non-Belgian/Dutch nationality | 2.6 (1.90–4.20) | 3.0 (2.1–4.6) | 3.3 (2.3–4.8) |
| Socioeconomic characteristics | |||
| Average income (per 1000 EUR) ‡ | 19.2 (18.1–20.6) | 19.3 (18.1–20.9) | 19.9 (18.7–21.4) |
| Provision level (Number, percentage) | |||
| Level 1 (lowest) | 59 (19.5%) | 59 (19.5%) | 60 (19.7%) |
| Level 2 | 65 (21.5%) | 65 (21.5%) | 65 (21.4%) |
| Level 3 | 81 (26.7%) | 81 (26.7%) | 81 (26.6%) |
| Level 4 | 53 (17.5%) | 53 (17.5%) | 53 (17.4%) |
| Level 5 | 18 (5.9%) | 18 (5.9%) | 18 (5.9%) |
| Level 6 | 14 (4.6%) | 14 (4.6%) | 14 (4.6%) |
| Level 7 (highest) | 13 (4.3%) | 13 (4.3%) | 13 (4.3%) |
| Position in labour market | |||
| % Wage earners | 36.6 (34.8–37.9) | 36.6 (34.5–37.9) | 36.8 (34.8–38.1) |
| % Self-employed | 7.9 (6.9–9.2) | 8.0 (7.0–9.3) | 8.1 (7.1–9.5) |
| % Jobseekers | 1.8 (1.5–2.2) | 1.8 (1.7–2.1) | 1.6 (1.3–1.9) |
| % (Early)retired | 19.7 (18.5–20.9) | 19.9 (18.8–21.1) | 20.1 (19.0–21.2) |
| % Higher education | 44.4 (39.1–49.2) | 44.8 (39.8–49.7) | 45.5 (40.8–51.1) |
| Health-related characteristics | |||
| ‰ Disabled | 6.4 (5.0–7.9) | 6.5 (5.1–7.9) | 6.4 (5.0–7.8) |
| % With at least 1 chronic disease | 9.7 (8.8–10.6) | 10.4 (9.6–10.5) | 11.0 (10.1–12.1) |
| % With at least 1 GP visit in last 12 months | 84.2 (82.1–86.0) | 84.9 (82.7–86.6) | 84.4 (82.3–86.4) |
| % With at least 2 preventive dental visits in 2 different years in last 3 years | 34.7 (31.0–37.6) | 37.4 (33.5–40.6) | 40.1 (36.1–43.4) |
| % With a global medical dossier | 74.8 (69.0–80.6) | 78.4 (73.5–82.7) | 82.0 (77.0–85.3) |
| Average number of patients per GP (per 100 patients) ‡ | 14.1 (12.1–16.2) | 14.5 (12.6–16.8) | 14.7 (12.5–17.4) |
| Univariable Analyses | Multivariable Analyses | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p Value | OR | 95% CI | p Value | |
| Demographic characteristics | ||||||
| Men/CRC screening population (%) | 1.13 | 1.11–1.15 | <0.001 * | 1.13 | 1.11–1.14 | <0.001 * |
| Age categories | ||||||
| 55–59/CRC screening population (%) | 1.02 | 1.01–1.03 | <0.001 * | 1.005 | 0.995–1.014 | 0.37 |
| 60–64/CRC screening population (%) | 0.986 | 0.973–0.9996 | 0.044 * | 1.02 | 1.01–1.04 | 0.002 * |
| 65–69/CRC screening population (%) | 0.983 | 0.971–0.995 | 0.005 * | 1.01 | 0.99–1.03 | 0.27 |
| 70–74/CRC screening population (%) | 0.98 | 0.97–0.99 | <0.001 * | 0.98 | 0.97–0.99 | <0.001 * |
| With a partner (%) | 1.035 | 1.029–1.041 | <0.001 * | 1.035 | 1.029–1.040 | <0.001 * |
| Current non-Belgian/Dutch nationality (%) | 0.969 | 0.964–0.975 | <0.001 * | 0.962 | 0.957–0.967 | <0.001 * |
| Socioeconomic characteristics | ||||||
| Average income (per 1000 EUR) ‡ | 1.003 | 0.988–1.018 | 0.71 | 0.97 | 0.96–0.98 | <0.001 * |
| Provision level | ||||||
| Level 1 (lowest) | (ref) | (ref) | ||||
| Level 2 | 1.03 | 0.98–1.08 | 0.31 | 1.03 | 0.98–1.08 | 0.29 |
| Level 3 | 1.02 | 0.97–1.07 | 0.54 | 1.02 | 0.97–1.06 | 0.52 |
| Level 4 | 1.000 | 0.948–1.054 | 0.99 | 1.000 | 0.951–1.053 | 0.99 |
| Level 5 | 0.99 | 0.91–1.08 | 0.83 | 0.99 | 0.92–1.07 | 0.83 |
| Level 6 | 0.97 | 0.92–1.02 | 0.21 | 0.97 | 0.93–1.01 | 0.17 |
| Level 7 (highest) | 0.87 | 0.81–0.93 | <0.001 * | 0.87 | 0.82–0.92 | <0.001 * |
| Position in labour market | ||||||
| Wage earners (%) | 1.02 | 1.01–1.03 | <0.001 * | 0.990 | 0.986–0.994 | <0.001 * |
| Self-employed (%) | 1.003 | 0.990–1.016 | 0.67 | 0.98 | 0.97–0.99 | <0.001 * |
| Jobseekers (%) | 0.92 | 0.90–0.95 | <0.001 * | 1.12 | 1.09–1.15 | <0.001 * |
| (Early)retired (%) | 1.004 | 0.994–1.014 | 0.42 | 0.977 | 0.971–0.983 | <0.001 * |
| Higher education (%) | 1.007 | 1.003–1.010 | <0.001 * | 1.010 | 1.008–1.011 | <0.001 * |
| Health-related characteristics | ||||||
| Disability (‰) | 1.02 | 1.01–1.03 | <0.001 * | 1.024 | 1.015–1.034 | <0.001 * |
| Chronic disease (%) | 1.04 | 1.02–1.06 | <0.001 * | 0.991 | 0.978–1.004 | 0.18 |
| GP visit (%) | 1.043 | 1.040–1.047 | <0.001 * | 1.04 | 1.03–1.05 | <0.001 * |
| Preventive dental visit (%) | 1.017 | 1.014–1.021 | <0.001 * | 1.002 | 1.000–1.005 | 0.051 |
| Global medical dossier (%) | 1.019 | 1.017–1.021 | <0.001 * | 1.001 | 0.999–1.002 | 0.37 |
| Patients per GP (per 100 patients) ‡ | 1.010 | 1.005–1.015 | <0.001 * | 1.009 | 1.005–1.014 | <0.001 * |
| Univariable Analyses | Multivariable Analyses | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p Value | OR | 95% CI | p Value | |
| Demographic characteristics | ||||||
| Men/CRC screening population (%) | 1.01 | 0.98–1.04 | 0.61 | 1.01 | 0.99–1.04 | 0.36 |
| Age categories | ||||||
| 55–59/CRC screening population (%) | 0.97 | 0.96–0.99 | <0.001 * | 1.016 | 0.999–1.034 | 0.07 |
| 60–64/CRC screening population (%) | 1.07 | 1.05–1.10 | <0.001 * | 1.021 | 0.998–1.045 | 0.08 |
| 65–69/CRC screening population (%) | 1.05 | 1.03–1.07 | <0.001 * | 0.98 | 0.95–1.01 | 0.14 |
| 70–74/CRC screening population (%) | 0.97 | 0.96–0.99 | 0.002 * | 0.981 | 0.964–0.999 | 0.037 * |
| With a partner (%) | 1.002 | 0.992–1.012 | 0.68 | 1.002 | 0.994–1.011 | 0.64 |
| Current non-Belgian/Dutch nationality (%) | 0.997 | 0.989–1.005 | 0.51 | 0.998 | 0.990–1.007 | 0.71 |
| Socioeconomic characteristics | ||||||
| Average income (per 1000 EUR) ‡ | 1.001 | 0.985–1.016 | 0.95 | 1.03 | 1.01–1.06 | 0.010 * |
| Provision level | ||||||
| Level 1 (lowest) | (ref) | (ref) | ||||
| Level 2 | 0.90 | 0.80–1.02 | 0.09 | 0.90 | 0.80–1.01 | 0.08 |
| Level 3 | 0.95 | 0.85–1.06 | 0.38 | 0.95 | 0.85–1.06 | 0.35 |
| Level 4 | 0.86 | 0.77–0.96 | 0.008 * | 0.86 | 0.77–0.95 | 0.005 * |
| Level 5 | 0.94 | 0.82–1.07 | 0.35 | 0.94 | 0.82–1.06 | 0.31 |
| Level 6 | 0.97 | 0.83–1.13 | 0.66 | 0.97 | 0.83–1.12 | 0.63 |
| Level 7 (highest) | 0.94 | 0.82–1.07 | 0.32 | 0.94 | 0.83–1.05 | 0.27 |
| Position in labour market | ||||||
| Wage earners (%) | 1.002 | 0.993–1.010 | 0.74 | 0.987 | 0.977–0.996 | 0.005 * |
| Self-employed (%) | 1.01 | 0.99–1.02 | 0.53 | 1.011 | 0.995–1.028 | 0.17 |
| Jobseekers (%) | 1.03 | 0.99–1.08 | 0.16 | 1.058 | 0.999–1.122 | 0.054 |
| (Early)retired (%) | 0.987 | 0.976–0.997 | 0.012 * | 0.99 | 0.97–1.01 | 0.18 |
| Higher education (%) | 1.005 | 1.003–1.008 | <0.001 * | 1.001 | 0.998–1.004 | 0.53 |
| Health-related characteristics | ||||||
| Disability (‰) | 0.9996 | 0.9821–1.0174 | 0.96 | 0.997 | 0.980–1.015 | 0.76 |
| Chronic disease (%) | 0.96 | 0.94–0.98 | <0.001 * | 0.98 | 0.95–1.02 | 0.35 |
| GP visit (%) | 1.011 | 1.001–1.021 | 0.038 * | 1.03 | 1.02–1.04 | <0.001 * |
| Preventive dental visit (%) | 0.997 | 0.992–1.003 | 0.32 | 0.998 | 0.991–1.005 | 0.55 |
| Global medical dossier (%) | 1.004 | 1.000–1.008 | 0.033 * | 1.025 | 1.018–1.031 | <0.001 * |
| Patients per GP (per 100 patients) ‡ | 1.018 | 1.013–1.024 | <0.001 * | 1.021 | 1.016–1.026 | <0.001 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tran, T.N.; Van Hal, G.; Peeters, M.; Jidkova, S.; De Schutter, H.; Hoeck, S. Population-Based Data Reveal Factors Associated with Organised and Non-Organised Colorectal Cancer Screening: An Important Step towards Improving Coverage. Int. J. Environ. Res. Public Health 2021, 18, 8373. https://doi.org/10.3390/ijerph18168373
Tran TN, Van Hal G, Peeters M, Jidkova S, De Schutter H, Hoeck S. Population-Based Data Reveal Factors Associated with Organised and Non-Organised Colorectal Cancer Screening: An Important Step towards Improving Coverage. International Journal of Environmental Research and Public Health. 2021; 18(16):8373. https://doi.org/10.3390/ijerph18168373
Chicago/Turabian StyleTran, Thuy Ngan, Guido Van Hal, Marc Peeters, Svetlana Jidkova, Harlinde De Schutter, and Sarah Hoeck. 2021. "Population-Based Data Reveal Factors Associated with Organised and Non-Organised Colorectal Cancer Screening: An Important Step towards Improving Coverage" International Journal of Environmental Research and Public Health 18, no. 16: 8373. https://doi.org/10.3390/ijerph18168373
APA StyleTran, T. N., Van Hal, G., Peeters, M., Jidkova, S., De Schutter, H., & Hoeck, S. (2021). Population-Based Data Reveal Factors Associated with Organised and Non-Organised Colorectal Cancer Screening: An Important Step towards Improving Coverage. International Journal of Environmental Research and Public Health, 18(16), 8373. https://doi.org/10.3390/ijerph18168373








