Selenium Status, Its Interaction with Selected Essential and Toxic Elements, and a Possible Sex-Dependent Response In Utero, in a South African Birth Cohort
Abstract
:1. Introduction
2. Materials and Methods
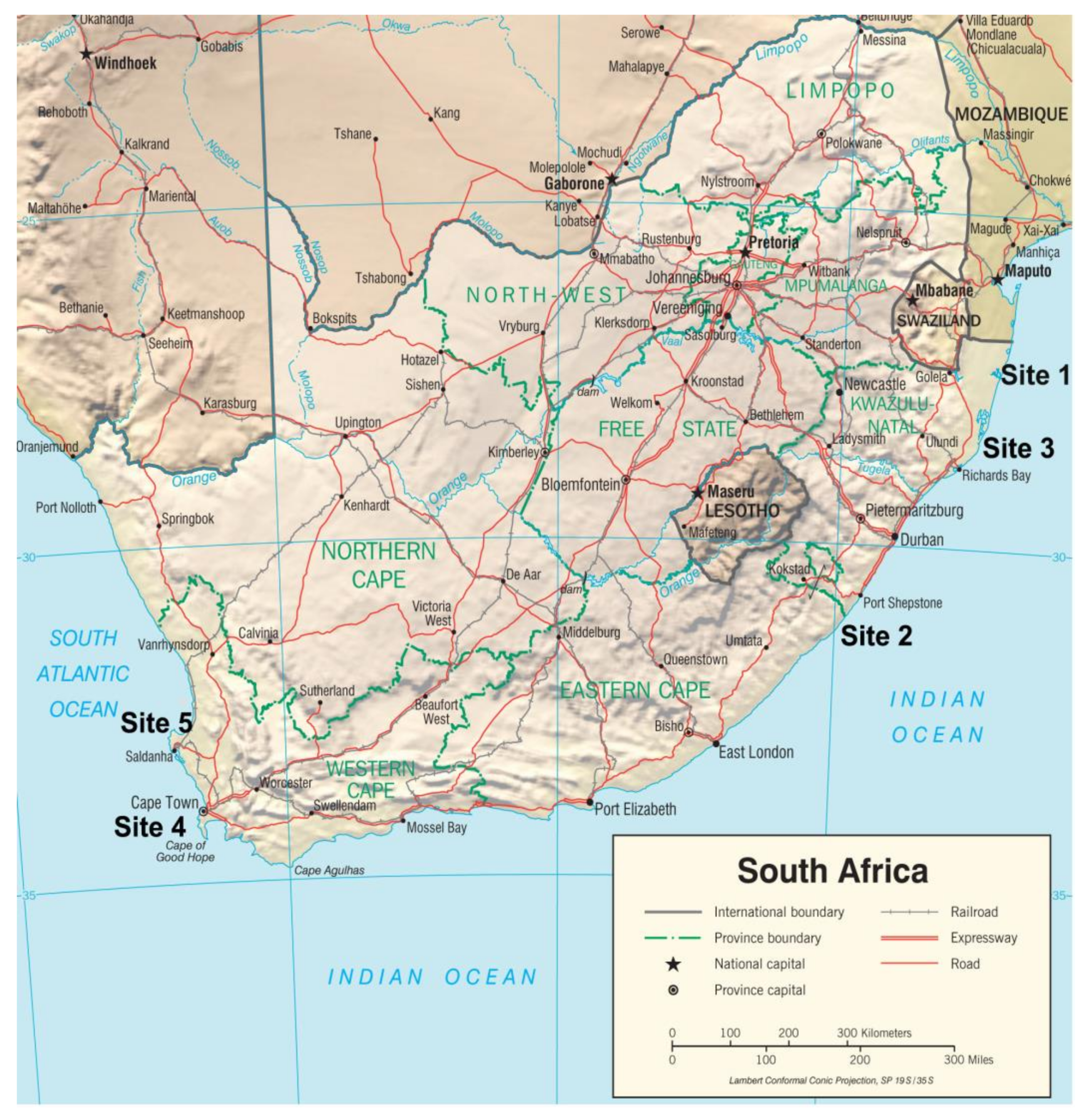
2.1. Study Sites and Participants
2.2. Sample Collection and Analytical Procedures
2.2.1. Serum Analyses
2.2.2. Whole Blood Analysis
2.3. Covariates
2.4. Statistical Analyses
2.5. Ethical Considerations
3. Results
3.1. Characteristics of the Study Population
3.2. Obstetrics and Birth Outcomes
3.3. Serum Se Levels in Mothers and Infant Anthropometry Measures at Birth
3.4. Concentrations of Se and Selected Essential and Toxic Elements at Delivery
3.5. Spearman’s Rank Correlation Association between Se and Measured Essential and Toxic Elements
3.6. Maternal Serum Se and Sex-Specific Association with Essential and Toxic Elements at Delivery
3.7. Univariate and Multi-Variable Regression Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Selenium in Drinking Water, Background Document for Development of WHO Guidelines for Drinking-Water Quality; WHO: Geneva, Switzerland, 2011. [Google Scholar]
- Kabata-Pendias, A. Geochemistry of selenium. J. Environ. Pathol. Toxicol. Oncol. 1998, 17, 173–177. [Google Scholar]
- Hurst, R.; Siyame, E.W.; Young, S.D.; Chilimba, A.D.; Joy, E.J.; Black, C.R.; Ander, E.L.; Watts, M.J.; Chilima, B.; Gondwe, J.; et al. Soil-type influences human selenium status and underlies widespread selenium deficiency risks in Malawi. Sci. Rep. 2013, 3, 1425. [Google Scholar] [CrossRef] [Green Version]
- IOM, Institute of Medicine (US) Panel on Dietary Antioxidants and Related Compounds. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids; National Academies Press: Washington, DC, USA, 2000. [Google Scholar]
- EFSA. Panel on Dietetic Products, Nutrition and Allergies (NDA). Allergies, Scientific Opinion on Dietary Reference Values for selenium. EFSA J. 2014, 12, 3846. [Google Scholar] [CrossRef]
- Fairweather-Tait, S.J.; Bao, Y.; Broadley, M.R.; Collings, R.; Ford, D.; Hesketh, J.E.; Hurst, R. Selenium in human health and disease. Antioxid. Redox. Signal 2011, 14, 1337–1383. [Google Scholar] [CrossRef]
- Hoffmann, P.R.; Berry, M.J. The influence of selenium on immune responses. Mol. Nutr. Food Res. 2008, 52, 1273–1280. [Google Scholar] [CrossRef] [PubMed]
- Hurst, R.; Armah, C.N.; Dainty, J.R.; Hart, D.J.; Teucher, B.; Goldson, A.J.; Broadley, M.R.; Motley, A.K.; Fairweather-Tait, S.J. Establishing optimal selenium status: Results of a randomized, double-blind, placebo-controlled trial. Am. J. Clin. Nutr. 2010, 91, 923–931. [Google Scholar] [CrossRef] [Green Version]
- Kryukov, G.V.; Castellano, S.; Novoselov, S.V.; Lobanov, A.V.; Zehtab, O.; Guigó, R.; Gladyshev, V.N. Characterization of mammalian selenoproteomes. Science 2003, 300, 1439–1443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, K.M.; Arthur, J.R. Selenium, selenoproteins and human health: A review. Public Health Nutr. 2001, 4, 593–599. [Google Scholar] [CrossRef] [Green Version]
- Köhrl, J.; Brigelius-Flohé, R.; Böck, A.; Gärtner, R.; Meyer, O.; Flohé, L. Selenium in biology: Facts and medical perspectives. Biol. Chem. 2000, 381, 849–864. [Google Scholar] [CrossRef] [Green Version]
- Arthur, J.R.; McKenzie, R.C.; Beckett, G.J. Selenium in the immune system. J. Nutr 2003, 133 (Suppl. 1), 1457S–1459S. [Google Scholar] [CrossRef]
- Barrington, J.W.; Taylor, M.; Smith, S.; Bowen-Simpkins, P. Selenium and recurrent miscarriage. J. Obstet. Gynaecol. 1997, 17, 199–200. [Google Scholar]
- Combs, G.F.; Gray, W.P. Chemopreventive agents: Selenium. Pharmacol. Ther. 1998, 79, 179–192. [Google Scholar] [CrossRef]
- Hawkes, W.C.; Hornbostel, L. Effects of dietary selenium on mood in healthy men living in a metabolic research unit. Biol. Psychiatry 1996, 39, 121–128. [Google Scholar] [CrossRef]
- Nève, J. Selenium as a risk factor for cardiovascular diseases. J. Cardiovasc. Risk. 1996, 3, 42–47. [Google Scholar] [CrossRef]
- Rudge, C.V.; Röllin, H.B.; Nogueira, C.M.; Thomassen, Y.; Rudge, M.C.; Odland, J. The placenta as a barrier for toxic and essential elements in paired maternal and cord blood samples of South African delivering women. J. Environ. Monit. 2009, 11, 1322–1330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakamoto, M.; Chan, H.M.; Domingo, J.L.; Koriyama, C.; Murata, K. Placental transfer and levels of mercury, selenium, vitamin E, and docosahexaenoic acid in maternal and umbilical cord blood. Environ. Internat. 2018, 111, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.; Garcia-Fuentes, E.; Callejon-Leblic, B.; Garcia-Barrera, T.; Gomez-Ariza, J.L.; Rayman, M.P.; Velasco, I. Selenium, selenoproteins and selenometabolites in mothers and babies at the time of birth. Br. J. Nutr. 2017, 117, 1304–1311. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Myers, R.; Wei, T.; Bind, E.; Kassim, P.; Wang, G.; Ji, Y.; Hong, X.; Caruso, D.; Bartell, T.; et al. Placental transfer and concentrations of cadmium, mercury, lead, and selenium in mothers, newborns, and young children. J. Expo. Sci. Environ. Epidemiol. 2014, 24, 537–544. [Google Scholar] [CrossRef] [Green Version]
- Nandakumaran, M.; Dashti, H.M.; Surname, F. Maternal-fetal transport kinetics of copper, selenium, magnesium and iron in perfused human placental lobule: In vitro study. Mol. Cell Biochem. 2002, 231, 9–14. [Google Scholar] [CrossRef]
- Combs, G.F. Food system-based approaches to improving micronutrient nutrition: The case for selenium. Biofactors 2000, 12, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Stuss, M.; Michalska-Kasiczak, M.; Sewerynek, E. The role of selenium in thyroid gland pathophysiology. Endokrynol. Pol. 2017, 68, 440–465. [Google Scholar] [CrossRef]
- Zimmermann, M.B.; Köhrle, J. The impact of iron and selenium deficiencies on iodine and thyroid metabolism: Biochemistry and relevance to public health. Thyroid 2002, 12, 867–878. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Xiao, T.; Zheng, B. Medical geology of arsenic, selenium and thallium in China. Sci. Total. Environ. 2012, 421–422, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Keshan, Disease Research Group. Epidemiologic studies on the etiologic relationship of selenium and Keshan Disease. Chin. Med. J. 1979, 92, 477–482. [Google Scholar]
- Johnson, R.A.; Baker, S.S.; Fallon, J.T.; Maynard, E.P.; Ruskin, J.N.; Wen, Z.; Ge, K.; Cohen, H.J. An accidental case of cardiomyopathy and selenium deficiency. N. Engl. J. Med. 1981, 304, 1210–1212. [Google Scholar] [CrossRef]
- Duntas, L.H.; Benvenga, S. Selenium: An element for life. Endocrine 2015, 48, 756–775. [Google Scholar] [CrossRef]
- Rayman, M.P. Selenium and human health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef]
- Al-Kunani, A.S.; Knight, R.; Haswell, S.J.; Thompson, J.W.; Lindow, S.W. The selenium status of women with a history of recurrent miscarriage. Br. J. Obstet. Gynaecol. 2001, 108, 1094–1097. [Google Scholar]
- Liu, P.J.; Yao, A.; Ma, L.; Chen, X.Y.; Yu, S.L.; Liu, Y.; Hou, Y.X. Associations of Serum Selenium Levels in the First Trimester of Pregnancy with the Risk of Gestational Diabetes Mellitus and Preterm Birth: A Preliminary Cohort Study. Biol. Trace Elem. Res. 2021, 199, 527–534. [Google Scholar] [CrossRef]
- Rayman, M.P.; Searle, E.; Kelly, L.; Johnsen, S.; Bodman-Smith, K.; Bath, S.C.; Mao, J.; Redman, C.W. Effect of selenium on markers of risk of pre-eclampsia in UK pregnant women: A randomised, controlled pilot trial. Br. J. Nutr. 2014, 112, 99–111. [Google Scholar] [CrossRef]
- Makwe, C.C.; Nwabua, F.I.; Anorlu, R.I. Selenium status and infant birth weight among HIV-positive and HIV-negative pregnant women in Lagos, Nigeria. Nig. Q. J. Hosp. Med. 2015, 25, 209–215. [Google Scholar]
- Alexander, E.K.; Pearce, E.N.; Brent, G.A.; Brown, R.S.; Chen, H.; Dosiou, C.; Grobman, W.A.; Laurberg, P.; Lazarus, J.H.; Mandel, S.J.; et al. Guidelines of the American Thyroid Association for the Diagnosis and Management of Thyroid Disease during Pregnancy and the Postpartum. Thyroid 2017, 27, 315–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nuttall, K.L. Evaluating selenium poisoning. Ann. Clin. Lab. Sci. 2006, 36, 409–420. [Google Scholar]
- Nehru, L.B.; Bansal, M.P. Effect of selenium supplementation on the glutathione redox system in the kidney of mice after chronic cadmium exposures. J. Appl. Toxicol. 1997, 17, 81–84. [Google Scholar] [CrossRef]
- Ponomarenko, O.; La Porte, P.F.; Singh, S.P.; Langan, G.; Fleming, D.E.B.; Spallholz, J.E.; Alauddin, M.; Ahsan, H.; Ahmed, S.; Gailer, J.; et al. Selenium-mediated arsenic excretion in mammals: A synchrotron-based study of whole-body distribution and tissue-specific chemistry. Metallomics 2017, 9, 1585–1595. [Google Scholar] [CrossRef] [PubMed]
- Wan, N.; Xu, Z.; Liu, T.; Min, Y.; Li, S. Ameliorative Effects of Selenium on Cadmium-Induced Injury in the Chicken Ovary: Mechanisms of Oxidative Stress and Endoplasmic Reticulum Stress in Cadmium-Induced Apoptosis. Biol. Trace Elem. Res. 2018, 184, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Kuraś, R.; Janasik, B.; Wąsowicz, W.; Stanisławska, M. Revision of reciprocal action of mercury and selenium. Int. J. Occup. Med. Environ. Health 2018, 31, 575–592. [Google Scholar]
- Channa, K.; Odland, J.; Kootbodien, T.; Theodorou, P.; Naik, I.; Sandanger, T.M.; Röllin, H.B. Differences in prenatal exposure to mercury in South African communities residing along the Indian Ocean. Sci. Total Environ. 2013, 463–464, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Röllin, H.B.; Channa, K.; Olutola, B.G.; Odland, J. Evaluation of in utero exposure to arsenic in South Africa. Sci. Total Environ. 2017, 575, 338–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Röllin, H.B.; Kootbodien, T.; Channa, K.; Odland, J. Prenatal Exposure to Cadmium, Placental Permeability and Birth Outcomes in Coastal Populations of South Africa. PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Röllin, H.B.; Kootbodien, T.; Theodorou, P.; Odland, J. Prenatal exposure to manganese in South African coastal communities. Environ. Sci. Process. Impacts 2014, 16, 1903–1912. [Google Scholar] [CrossRef] [Green Version]
- Röllin, H.B.; Olutola, B.; Channa, K.; Odland, J. Reduction of in utero lead exposures in South African populations: Positive impact of unleaded petrol. PLoS ONE 2017, 12. [Google Scholar] [CrossRef] [Green Version]
- Kassu, A.; Yabutani, T.; Mulu, A.; Tessema, B.; Ota, F. Serum zinc, copper, selenium, calcium, and magnesium levels in pregnant and non-pregnant women in Gondar, Northwest Ethiopia. Biol. Trace Elem. Res. 2008, 122, 97–106. [Google Scholar] [CrossRef]
- Navarro, M.; López, H.; Pérez, V.; López, M.C. Serum selenium levels during normal pregnancy in healthy Spanish women. Sci. Total Environ. 1996, 186, 237–242. [Google Scholar] [CrossRef]
- Al-Saleh, E.; Nandakumaran, M.; Al-Shammari, M.; Al-Harouny, A. Maternal-fetal status of copper, iron, molybdenum, selenium and zinc in patients with gestational diabetes. J. Matern. Fetal Neonatal. Med. 2004, 16, 15–21. [Google Scholar] [CrossRef]
- Hansen, S.; Nieboer, E.; Sandanger, T.M.; Wilsgaard, T.; Thomassen, Y.; Veyhe, A.S.; Odland, J.O. Changes in maternal blood concentrations of selected essential and toxic elements during and after pregnancy. J. Environ. Monit. 2011, 13, 2143–2152. [Google Scholar] [CrossRef] [PubMed]
- Choi, R.; Sun, J.; Yoo, H.; Kim, S.; Cho, Y.Y.; Kim, H.J.; Kim, S.W.; Chung, J.H.; Oh, S.Y.; Lee, S.Y. A Prospective Study of Serum Trace Elements in Healthy Korean Pregnant Women. Nutrients 2016, 8, 749. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Zhang, Y.; Piao, J.; Mao, D.; Li, Y.; Li, W.; Yang, L.; Yang, X. Reference Values of 14 Serum Trace Elements for Pregnant Chinese Women: A Cross-Sectional Study in the China Nutrition and Health Survey 2010–2012. Nutrients 2017, 9, 309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Ryssen, J.B.J. Geographical distribution of the selenium status of herbivores in South Africa. S. Afr. J. Anim. Sci. 2001, 31, 1–7. [Google Scholar] [CrossRef]
- Pieczyńska, J.; Grajeta, H. The role of selenium in human conception and pregnancy. J. Trace Elem. Med. Biol. 2015, 29, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, M.; Shibata, E.; Morokuma, S.; Tanaka, R.; Senju, A.; Araki, S.; Sanefuji, M.; Koriyama, C.; Yamamoto, M.; Ishihara, Y.; et al. The association between whole blood concentrations of heavy metals in pregnant women and premature births: The Japan Environment and Children’s Study (JECS). Environ. Res. 2018, 166, 562–569. [Google Scholar] [CrossRef] [PubMed]
- Chiudzu, G.; Choko, A.; Maluwa, A.; Huber, S.; Odland, J. Maternal serum concentration of selenium, copper and zinc during pregnancy are associated with risk of spontaneous preterm birth: A case control study from Malawi. medRxiv 2020. medRxiv:2020.04.14.20064717. [Google Scholar] [CrossRef]
- Braun, M.V.I. Use of dietary supplements, and awareness and knowledge of the recommended fruit and vegetable intakes and consumption of health food store customers in the Cape Town city bowl. S. Afr. J. Clin. Nutri. 2008, 21, 323–330. [Google Scholar] [CrossRef]
- Adegboye, A.R.; Ojo, O.; Begum, G. The Use of Dietary Supplements Among African and Caribbean Women Living in the UK: A Cross-Sectional Study. Nutrients 2020, 12, 847. [Google Scholar] [CrossRef] [Green Version]
- Laclaustra, M.; Stranges, S.; Navas-Acien, A.; Ordovas, J.M.; Guallar, E. Serum selenium and serum lipids in US adults: National Health and Nutrition Examination Survey (NHANES) 2003–2004. Atherosclerosis 2010, 210, 643–648. [Google Scholar] [CrossRef] [Green Version]
- Bogden, J.D.; Kemp, F.W.; Chen, X.; Stagnaro-Green, A.; Stein, T.P.; Scholl, T.O. Low-normal serum selenium early in human pregnancy predicts lower birth weight. Nutr. Res. 2006, 26, 497–502. [Google Scholar] [CrossRef]
- Cengiz, B.; Söylemez, F.; Oztürk, E.; Cavdar, A.O. Serum zinc, selenium, copper, and lead levels in women with second-trimester induced abortion resulting from neural tube defects: A preliminary study. Biol. Trace Elem. Res. 2004, 97, 225–235. [Google Scholar] [CrossRef]
- Izquierdo Alvarez, S.; Castañón, S.G.; Ruata, M.L.; Aragüés, E.F.; Terraz, P.B.; Irazabal, Y.G.; González, E.G.; Rodríguez, B.G. Updating of normal levels of copper, zinc and selenium in serum of pregnant women. J. Trace Elem. Med. Biol. 2007, 21 (Suppl. 1), 49–52. [Google Scholar] [CrossRef] [PubMed]
- Flora, S.J.S.; Singh, S.; Tandon, S.K. Role of Selenium in Protection against Lead Intoxication. Acta Pharmacol. Toxicol. 1983, 53, 28–32. [Google Scholar] [CrossRef]
- Zwolak, I. The Role of Selenium in Arsenic and Cadmium Toxicity: An Updated Review of Scientific Literature. Biol. Trace Elem. Res. 2020, 193, 44–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viezeliene, D.; Jansen, E.; Rodovicius, H.; Kasauskas, A.; Ivanov, L. Protective effect of selenium on aluminium-induced oxidative stress in mouse liver in vivo. Environ. Toxicol. Pharmacol. 2011, 31, 302–306. [Google Scholar] [CrossRef] [PubMed]
- Gabory, A.; Roseboom, T.J.; Moore, T.; Moore, L.G.; Junien, C. Placental contribution to the origins of sexual dimorphism in health and diseases: Sex chromosomes and epigenetics. Biol. Sex Differ. 2013, 4, 5. [Google Scholar] [CrossRef] [Green Version]
- Rosenfeld, C.S. Sex-Specific Placental Responses in Fetal Development. Endocrinology 2015, 156, 3422–3434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caillie-Bertrand, M.V.; Degenhart, H.J.; Fernandes, J. Influence of Age on the Selenium Status in Belgium and The Netherlands. Pediatr. Res. 1986, 20, 574–576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galan, P.; Viteri, F.E.; Bertrais, S.; Czernichow, S.; Faure, H.; Arnaud, J.; Ruffieux, D.; Chenal, S.; Arnault, N.; Favier, A.; et al. Serum concentrations of beta-carotene, vitamins C and E, zinc and selenium are influenced by sex, age, diet, smoking status, alcohol consumption and corpulence in a general French adult population. Eur. J. Clin. Nutri. 2005, 59, 1181–1190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Characteristic | Total (n = 650) | Indian Ocean (n = 350) | Atlantic Ocean (n = 300) | p-Value |
|---|---|---|---|---|
| Marital status (%, n) | <0.001 | |||
| Married | 22.5 (146) | 11.7 (41) | 35.0 (105) | |
| Divorced/single/widow | 58.6 (381) | 71.7 (251) | 43.3 (130) | |
| Living together | 17.2 (112) | 14.3 (50) | 20.7 (62) | |
| Missing | 1.7 (11) | 2.3 (8) | 1.0 (3) | |
| Education (%, n) | <0.001 | |||
| None/Primary school | 8.3 (54) | 14.3 (50) | 1.3 (4) | |
| Secondary school | 62.0 (403) | 44.0 (154) | 83 (249) | |
| Tertiary | 25.9 (168) | 35.4 (124) | 14.7 (44) | |
| Missing | 3.9 (25) | 6.3 (22) | 1.0 (3) | |
| Race/Ethnicity (%, n) | <0.001 | |||
| African/Black | 76.6 (498) | 96.6 (338) | 53.3 (160) | |
| Other | 21.1 (137) | 1.1 (4) | 44.3 (133) | |
| Missing | 2.3 (15) | 2.3 (8) | 2.3 (7) | |
| Employment (%, n) | <0.001 | |||
| Employed | 23.5 (153) | 13.7 (48) | 35.0 (105) | |
| Unemployed | 74.3 (483) | 84.0 (294) | 63.0 (189) | |
| Missing | 2.2 (14) | 2.3 (8) | 2.0 (6) | |
| Housing type (%, n) | <0.001 | |||
| Formal housing | 60.9 (396) | 81.4 (285) | 37.0 (111) | |
| Flat | 4.9 (32) | 3.4 (12) | 6.7 (20) | |
| Backyard dwelling | 13.5 (88) | 8.3 (29) | 19.7 (59) | |
| Informal house (shack) | 3.4 (22) | 4.6 (16) | 2.0 (6) | |
| Missing | 17.2 (112) | 2.3 (8) | 34.7 (104) | |
| Water source (%, n) | <0.001 | |||
| Indoor tap | 25.9 (168) | 10.3 (36) | 44.0 (132) | |
| Outdoor tap | 46.9 (305) | 68.6 (240) | 21.7 (65) | |
| Others | 9.4 (61) | 17.4 (61) | 0 (0) | |
| Missing | 17.9 (116) | 3.7 (13) | 34.3 (103) | |
| Gestational age (%, n) | <0.001 | |||
| Pre-term (24–37 weeks) | 31.7 (206) | 44.6 (156) | 16.7 (50) | |
| Full-term (38–44 weeks) | 53.5 (348) | 43.1 (151) | 65.7 (197) | |
| Missing | 14.8 (96) | 12.3 (43) | 17.7 (53) | |
| Cooking fuel (%, n) | <0.001 | |||
| Electricity | 73.7 (479) | 55.7 (195) | 94.7 (284) | |
| Paraffin | 5.2 (34) | 7.4 (26) | 2.7 (8) | |
| Gas/wood | 19.5 (127) | 35.1 (123) | 1.3 (4) | |
| Missing | 1.5 (10) | 1.7 (6) | 1.3 (4) | |
| Heating (%, n) | <0.001 | |||
| Electricity | 44.8 (291) | 38.6 (135) | 52.0 (156) | |
| Paraffin | 12.0 (78) | 4.9 (17) | 20.3 (61) | |
| Gas/wood/coal | 13.4 (87) | 22.6 (79) | 2.7 (8) | |
| None | 23.7 (154) | 31.1 (109) | 15.0 (45) | |
| Missing | 6.2 (40) | 2.9 (10) | 10.0 (30) | |
| Prescribed vitamin supplements during pregnancy (%, n) | <0.001 | |||
| No supplements | 19.9 (129) | 33.4 (117) | 4.0 (12) | |
| Supplements | 43.7 (284) | 27.4 (96) | 62.7 (188) | |
| Missing | 36.5 (237) | 39.1 (137) | 33.3 (100) | |
| Meat consumption before pregnancy (%, n) | 0.004 | |||
| Seldom/At least once a week | 60.5 (395) | 66.3 (232) | 53.7 (161) | |
| Almost everyday | 32.9 (214) | 28.6 (100) | 38.0 (114) | |
| Missing | 6.6 (43) | 5.1 (18) | 8.3 (25) | |
| Meat consumption during pregnancy (%, n) | <0.001 | |||
| Seldom/At least once a week | 58.5 (380) | 66.0 (231) | 49.7 (149) | |
| Almost everyday | 30.6 (199) | 27.1 (95) | 34.7 (104) | |
| Missing | 10.9 (71) | 6.9 (24) | 15.7 (47) | |
| Fresh fish consumption before pregnancy | 0.001 | |||
| Seldom/At least once a week | 66.8 (434) | 62.6 (219) | 71.7 (215) | |
| Almost everyday | 10.6 (69) | 9.1 (32) | 12.3 (37) | |
| Missing | 22.6 (147) | 28.3 (99) | 16.0 (48) | |
| Fresh fish consumption during pregnancy (%, n) | <0.001 | |||
| Seldom/At least once a week | 65.4 (425) | 61.4 (215) | 70.0 (210) | |
| Almost everyday | 10.6 (69) | 8.6 (30) | 13.0 (39) | |
| Missing | 24.0 (156) | 30.0 (105) | 17.0 (51) | |
| Fruit consumption before pregnancy (%, n) | <0.001 | |||
| Seldom/At least once a week | 23.9 (155) | 36.3(127) | 9.3 (28) | |
| Almost everyday | 65.1 (423) | 48.6(170) | 84.3 (253) | |
| Missing | 11.1 (72) | 15.1(53) | 6.3 (19) | |
| Fruit consumption during pregnancy (%, n) | <0.001 | |||
| Seldom/At least once a week | 23.4 (152) | 36.6 (128) | 8.0 (24) | |
| Almost everyday | 65.5 (426) | 48.3 (169) | 85.7 (257) | |
| Missing | 11.1 (72) | 15.1 (53) | 6.3 (19) | |
| Consumed dairy products before pregnancy (%, n) | <0.001 | |||
| Seldom/At least once a week | 28.6 (186) | 42.6 (149) | 12.3 (37) | |
| Almost everyday | 57.7 (375) | 41.7 (146) | 76.3 (229) | |
| Missing | 13.7 (89) | 15.7 (55) | 11.3 (34) | |
| Consumed dairy products during pregnancy (%, n) | <0.001 | |||
| Seldom/At least once a week | 27.9 (181) | 41.7 (146) | 11.7 (35) | |
| Almost everyday | 57.5 (374) | 41.1 (144) | 76.7 (230) | |
| Missing | 14.6 (95) | 17.1 (60) | 11.7 (35) |
| Characteristic | Total (n = 650) | Indian Ocean (n = 350) | Atlantic Ocean (n = 300) | p-Value |
|---|---|---|---|---|
| Maternal age (y) | 24.0 | 23.0 | 26.0 | <0.001 |
| Maternal weight (kg) | 71.6 | 71.0 | 72.0 | 0.716 |
| Maternal height (cm) | 159.0 | 160.0 | 158.0 | 0.117 |
| Maternal blood pressure (BP, mmHg) | ||||
| BP Systolic | - | - | 120.0 | |
| BP Diastolic | - | - | 70.0 | |
| Gestational age (weeks) | 38.2 | 37.0 | 39.0 | <0.001 |
| Birth weight (g) | 3100.0 | 3100.0 | 3100.0 | 0.295 |
| Birth length (cm) | 50.0 | 50.0 | 50.0 | 1.000 |
| Head circumference (cm) | 35.0 | 35.0 | 34.0 | <0.001 |
| Placenta weight (g) | - | - | 600.0 | |
| Apgar score 1 min (%, n) | <0.001 | |||
| 0–3 | 1.4 (9) | 1.1 (4) | 1.7 (5) | |
| 4–6 | 5.2 (34) | 1.7 (6) | 9.3 (28) | |
| 7–10 | 85.9 (558) | 87.4 (306) | 84.0 (252) | |
| Missing | 7.5 (49) | 9.7 (34) | 5.0 (15) | |
| Apgar score 5 min | 0.044 | |||
| 0–3 | 0.3 (2) | 0.3 (1) | 0.3 (1) | |
| 4–6 | 0.9 (6) | 1.1 (4) | 0.7 (2) | |
| 7–10 | 91.4 (594) | 88.6 (310) | 94.7 (284) | |
| Missing | 7.4 (48) | 10.0 (35) | 4.3 (13) | |
| Sex (%, n) | 0.596 | |||
| Male | 49.9 (324) | 48.0 (168) | 52.0 (156) | |
| Female | 44.6 (290) | 46.3 (162) | 42.7 (128) | |
| Missing | 5.5 (36) | 5.7 (20) | 5.3 (16) | |
| Parity (%, n) | 0.005 | |||
| 0 | 42.0 (273) | 47.7 (167) | 35.3 (106) | |
| 1+ | 54.5 (354) | 49.4 (173) | 60.3 (181) | |
| Missing | 3.5 (23) | 2.9 (10) | 4.3 (13) |
| Characteristic | Total (n = 554) | Pre-Term (24–37 Weeks) (n = 206) | Full-Term (38–44 Weeks) (n = 348) |
|---|---|---|---|
| Birth outcome | β | β | β |
| Birth length | −0.030 (0.499) | −0.097 (0.187) | −0.016 (0.780) |
| Birth weight | 0.005 (0.920) | −0.062 (0.402) | 0.010 (0.855) |
| Apgar score 1 min | 0.025 (0.576) | 0.075 (0.309) | 0.002 (0.970) |
| Apgar score 5 min | −0.052 (0.248) | −0.059 (0.419) | −0.040 (0.484) |
| Head circumference | −0.164 (< 0.001) *** | −0.234 (0.001) *** | −0.132 (0.020) * |
| Parity | 0.126 (0.005) ** | 0.122 (0.096) | 0.125 (0.027) * |
| Selenium Concentrations | Total (n = 635) | Indian Ocean (n = 345) | Atlantic Ocean (n = 290) | p-Value |
|---|---|---|---|---|
| Se | <0.001 | |||
| Mean (SD) | 69.0 (23.7) | 63.2 (19.5) | 76.0 (26.3) | |
| Geometric mean (GM) | 65.9 | 60.8 | 72.6 | |
| Range | 10–328.2 | 26.1–182.5 | 10.0–328.2 | |
| 95% Conf. Interval | 64.4–67.5 | 59.0–62.6 | 70.2–75.2 | |
| Median | 66.2 | 60.5 | 75.0 | |
| Range | 64.5–68.2 | 26.1–182.5 | 10–328.2 |
| Element | N* | Mean (SD) | Range | GM | 95% CI | Median | 95% CI |
|---|---|---|---|---|---|---|---|
| Essential elements-Se, Zn Cu, Mn levels | |||||||
| Se serum | 635 | 69.0 (23.7) | 10.0–328.2 | 65.9 | 64.4–67.5 | 66.2 | 64.49–68.16 |
| Zn serum | 637 | 503.1 (148.7) | 127.7–1810.0 | 483.8 | 473.4–494.4 | 486.1 | 477.00–497.16 |
| Cu serum | 639 | 2482.2 (531.0) | 204.8–4453.0 | 2423.3 | 2380.5–2467.0 | 2417.0 | 2371.40–2468.87 |
| Mn blood | 636 | 16.3 (6.6) | 2.4–43.9 | 15.1 | 14.6–15.6 | 15.3 | 14.86–15.83 |
| Toxic elements-Pb Hg, As, Cd, Al levels | |||||||
| Pb blood | 640 | 17.7 (18.5) | 0.4–316.9 | 13.2 | 12.4–14.0 | 14.0 | 13.02–15.00 |
| Hg blood | 638 | 1.2 (1.6) | 0.2–24.2 | 0.8 | 0.8–0.9 | 0.7 | 0.67–0.79 |
| As blood | 641 | 0.9 (0.7) | 0.07–6.2 | 0.6 | 0.6–0.7 | 0.7 | 0.67–0.74 |
| Al serum | 616 | 14.9 (13.5) | 0.2–60.1 | 9.1 | 8.3–10.0 | 9.7 | 8.94–10.80 |
| Cd blood | 641 | 0.4 (0.5) | 0.03–4.9 | 0.3 | 0.2–0.3 | 0.3 | 0.24–0.30 |
| Element | Total Rho (p-Value) | Indian Ocean Rho (p-Value) | Atlantic Ocean Rho (p-Value) |
|---|---|---|---|
| Se and essential element Zn, Cu, Mn levels-total and by region | |||
| Zn | 0.164 ( < 0.001) *** | 0.362 ( < 0.001) *** | 0.269 ( < 0.001) *** |
| Cu | 0.273 ( < 0.001) *** | 0.298 ( < 0.001) *** | 0.338 ( < 0.001) *** |
| Mn | −0.053 (0.189) | −0.053 (0.332) | 0.046 (0.449) |
| Se and toxic element Pb, Hg, As, Cd, Al levels-total and by region | |||
| Pb | −0.163 ( < 0.001) *** | −0.029 (0.594) | 0.010 (0.880) |
| Hg | 0.205 ( < 0.001) *** | 0.067 (0.216) | 0.124 (0.047) * |
| As | −0.145 ( < 0.001) *** | 0.069 (0.202) | 0.055 (0.379) |
| Al | 0.122 (0.003) ** | −0.041 (0.451) | 0.171 (0.006) ** |
| Cd | 0.094 (0.020) * | 0.132 (0.015) * | (0.215) |
| Element | Total Cohort Rho (p-Value) | Male Cohort Rho (p-Value) | Female Cohort Rho (p-Value) |
|---|---|---|---|
| Selected essential elements | |||
| Zn serum | 0.164 (<0.001) *** | 0.202 (<0.001) *** | 0.114 (0.057) |
| Cu serum | 0.273 (<0.001) *** | 0.236 (<0.001) *** | 0.323 (<0.001) *** |
| Mn blood | −0.053 (0.189) | −0.096 (0.091) | 0.005 (0.935) |
| Selected toxic elements | |||
| Pb blood | −0.163 (<0.001) *** | −0.185 (0.001) *** | −0.118 (0.053) * |
| Hg blood | 0.205 (<0.001) *** | 0.243 (<0.001) *** | 0.189 (0.002) ** |
| As blood | −0.145 (<0.001) *** | −0.092 (0.110) | −0.179 (0.003) ** |
| Al serum | 0.122 (0.003) ** | 0.159 (0.006) ** | 0.065 (0.286) |
| Cd blood | 0.094 (0.020) * | 0.105 (0.068) | 0.073 (0.232) |
| Univariate | Multi-Variable | |||||
|---|---|---|---|---|---|---|
| Characteristic | Coefficient | p-Value | 95% CI | Coefficient | p-Value | 95% CI* |
| Head circumference | −1.205 | 0.007 | −2.083 to −0.327 | - | - | - |
| Apgar score 1 min | −0.442 | 0.570 | −1.969 to 1.085 | - | - | - |
| Apgar score 5 min | −1.495 | 0.206 | −3.813 to 0.823 | - | - | - |
| Birthweight | −0.002 | 0.217 | −0.006 to 0.001 | - | - | - |
| Birth length | −0.250 | 0.346 | −0.769 to 0.270 | - | - | |
| Parity | ||||||
| 0 | 1.0 | |||||
| 1 | 4.993 | 0.006 | 1.412 to 8.574 | - | - | - |
| Region | ||||||
| Indian | 1.0 | |||||
| Atlantic | 14.460 | <0.001 | 11.287 to 17.633 | - | - | - |
| Gestational age | 0.178 | 0.707 | −0.752 to 1.108 | - | - | - |
| Maternal age | 0.328 | 0.022 | 0.048 to 0.608 | 0.367 | 0.004 | 0.121 to 0.612 |
| Maternal weight | 0.062 | 0.325 | −0.062 to 0.186 | - | - | - |
| Maternal height | −0.086 | 0.373 | −0.274 to 0.103 | - | - | - |
| Zn | 0.018 | 0.005 | 0.005−0.030 | 0.022 | <0.001 | 0.111 to 0.033 |
| Cu | 0.010 | <0.001 | 0.007 to 0.013 | 0.008 | <0.001 | 0.005 to 0.011 |
| Mn | −0.262 | 0.060 | −0.534 to 0.011 | - | - | - |
| Pb | −0.115 | 0.018 | −0.211 to −0.020 | - | - | - |
| Hg | 3.264 | <0.001 | 2.251 to 4.277 | - | - | - |
| As | −2.118 | 0.092 | −4.585 to 0.350 | - | - | - |
| Al | 0.368 | <0.001 | 0.242 to 0.494 | - | - | - |
| Cd | 5.007 | 0.006 | 1.451 to 8.564 | - | - | - |
| Location | ||||||
| Rural | 1.0 | |||||
| Urban | 12.130 | <0.001 | 8.456 to 15.804 | - | - | - |
| Race | ||||||
| African/Black | 1.0 | 1.0 | ||||
| Other | 11.730 | <0.001 | 7.925 to 15.535 | 13.505 | <0.001 | 9.735 to 17.275 |
| Smoked during pregnancy | ||||||
| No | 1.0 | |||||
| Yes | 6.420 | 0.001 | 2.731 to 10.109 | - | - | - |
| Consumed dairy products before pregnancy | ||||||
| Seldom/At least once a week | 1.0 | |||||
| Almost everyday | 6.402 | 0.001 | 2.567 to 10.238 | - | - | - |
| Consumed dairy products during pregnancy | ||||||
| Seldom/At least once a week | 1.0 | |||||
| Almost everyday | 6.800 | <0.001 | 3.000 to 10.603 | - | - | - |
| Consumed fruits before pregnancy | ||||||
| Seldom/At least once a week | 1.0 | |||||
| Almost everyday | 7.630 | 0.001 | 3.026 to 12.235 | - | - | - |
| Consumed fruits during pregnancy | ||||||
| Seldom/At least once a week | 1.0 | |||||
| Almost everyday | 7.540 | 0.001 | 3.043 to 12.037 | - | - | - |
| Use of supplements | ||||||
| No supplement use | 1.0 | |||||
| Supplement use | 6.070 | 0.024 | 0.796 to 11.344 | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Röllin, H.B.; Channa, K.; Olutola, B.; Odland, J.Ø. Selenium Status, Its Interaction with Selected Essential and Toxic Elements, and a Possible Sex-Dependent Response In Utero, in a South African Birth Cohort. Int. J. Environ. Res. Public Health 2021, 18, 8344. https://doi.org/10.3390/ijerph18168344
Röllin HB, Channa K, Olutola B, Odland JØ. Selenium Status, Its Interaction with Selected Essential and Toxic Elements, and a Possible Sex-Dependent Response In Utero, in a South African Birth Cohort. International Journal of Environmental Research and Public Health. 2021; 18(16):8344. https://doi.org/10.3390/ijerph18168344
Chicago/Turabian StyleRöllin, Halina B., Kalavati Channa, Bukola Olutola, and Jon Øyvind Odland. 2021. "Selenium Status, Its Interaction with Selected Essential and Toxic Elements, and a Possible Sex-Dependent Response In Utero, in a South African Birth Cohort" International Journal of Environmental Research and Public Health 18, no. 16: 8344. https://doi.org/10.3390/ijerph18168344
APA StyleRöllin, H. B., Channa, K., Olutola, B., & Odland, J. Ø. (2021). Selenium Status, Its Interaction with Selected Essential and Toxic Elements, and a Possible Sex-Dependent Response In Utero, in a South African Birth Cohort. International Journal of Environmental Research and Public Health, 18(16), 8344. https://doi.org/10.3390/ijerph18168344







