Effectiveness of Colorectal Cancer Screening Promotion Using E-Media Decision Aids: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Protocol Registration
2.2. Database Used
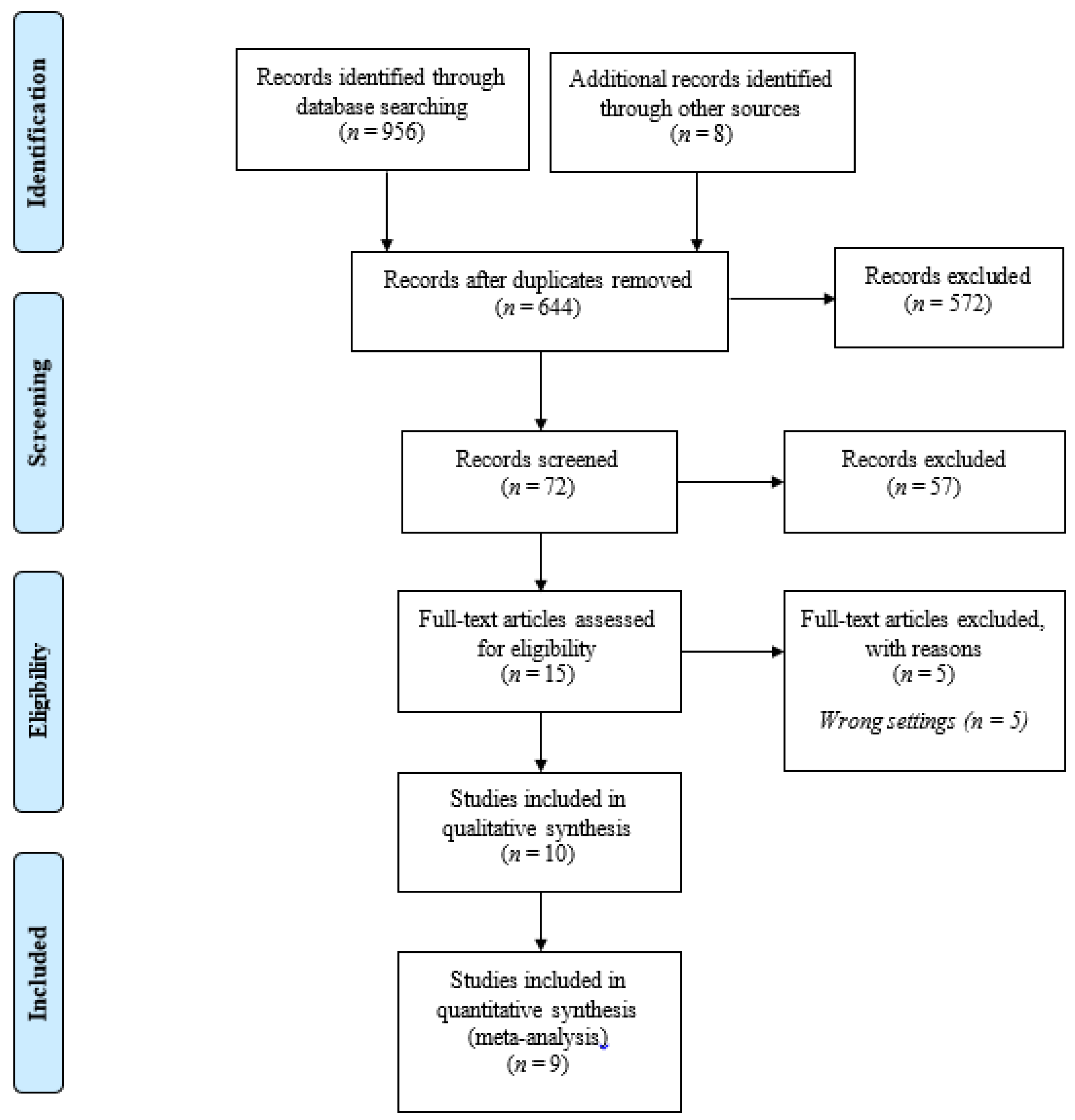
2.3. Systematic Review Process
2.4. Identification
2.5. Screening
2.6. Eligibility
2.7. Data Extraction
2.8. Risk of Bias Assessment
2.9. Data Analysis
3. Results
3.1. Characteristic of Studies
3.2. E-Media Decision Aid Intervention Type, Content and Duration Length
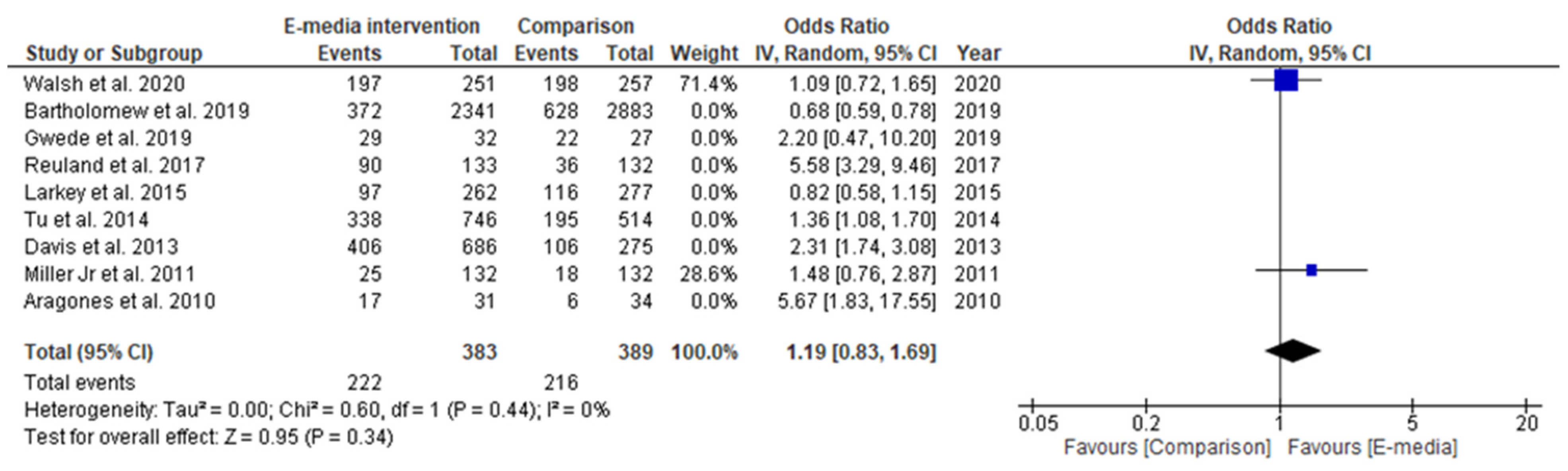
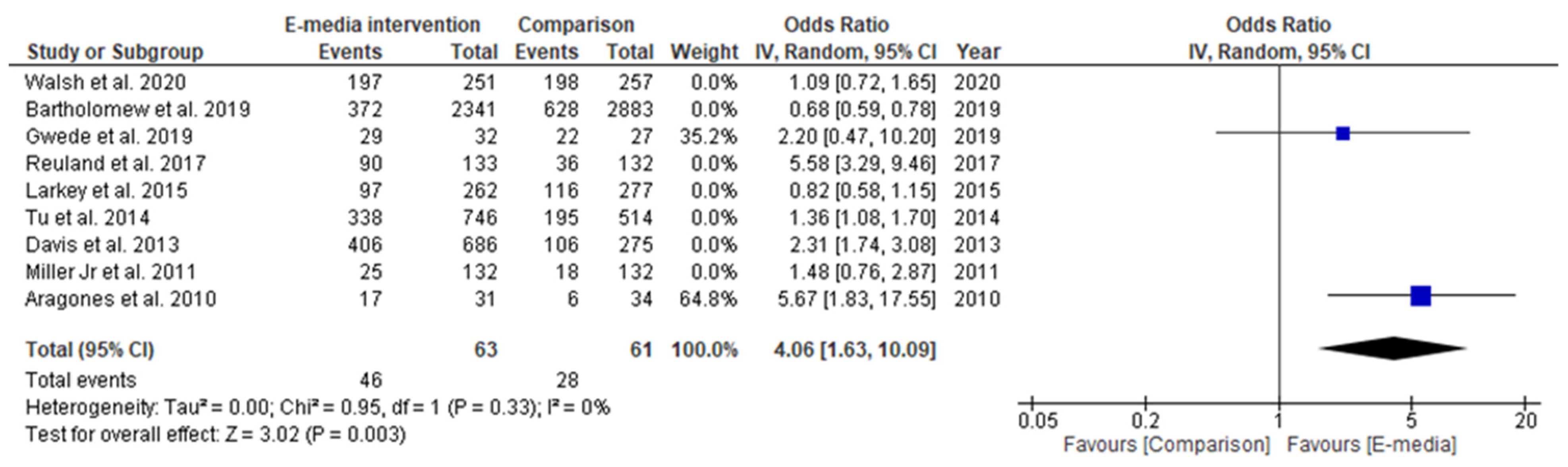
3.3. Effectiveness of CRC-Screening Promotion Using E-Media
3.3.1. Studies Included in Qualitative Synthesis
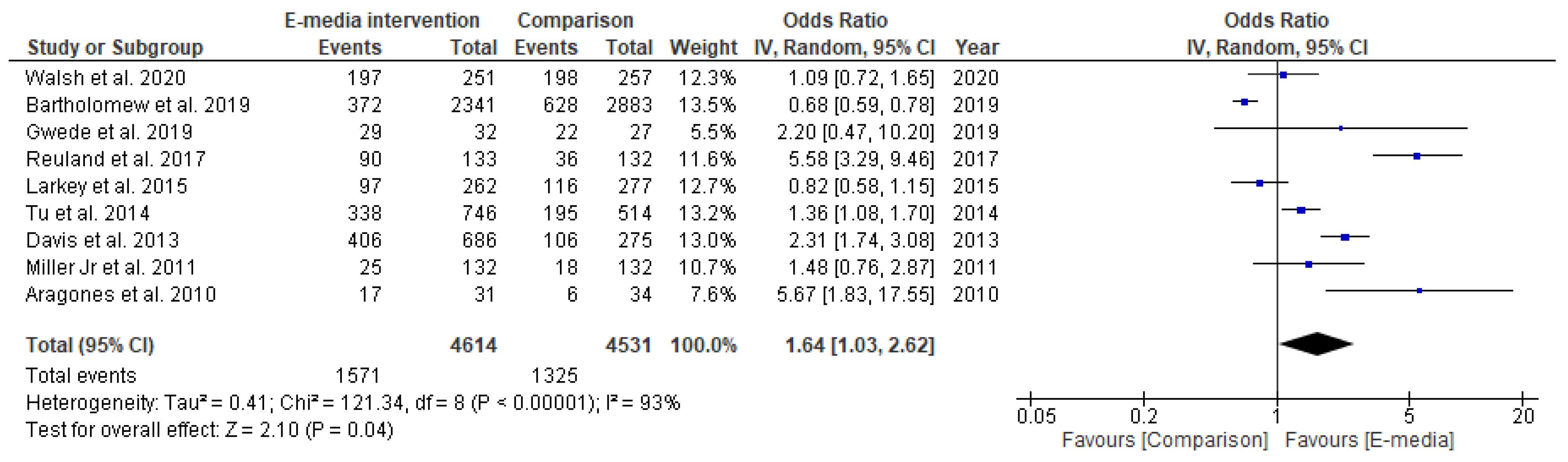
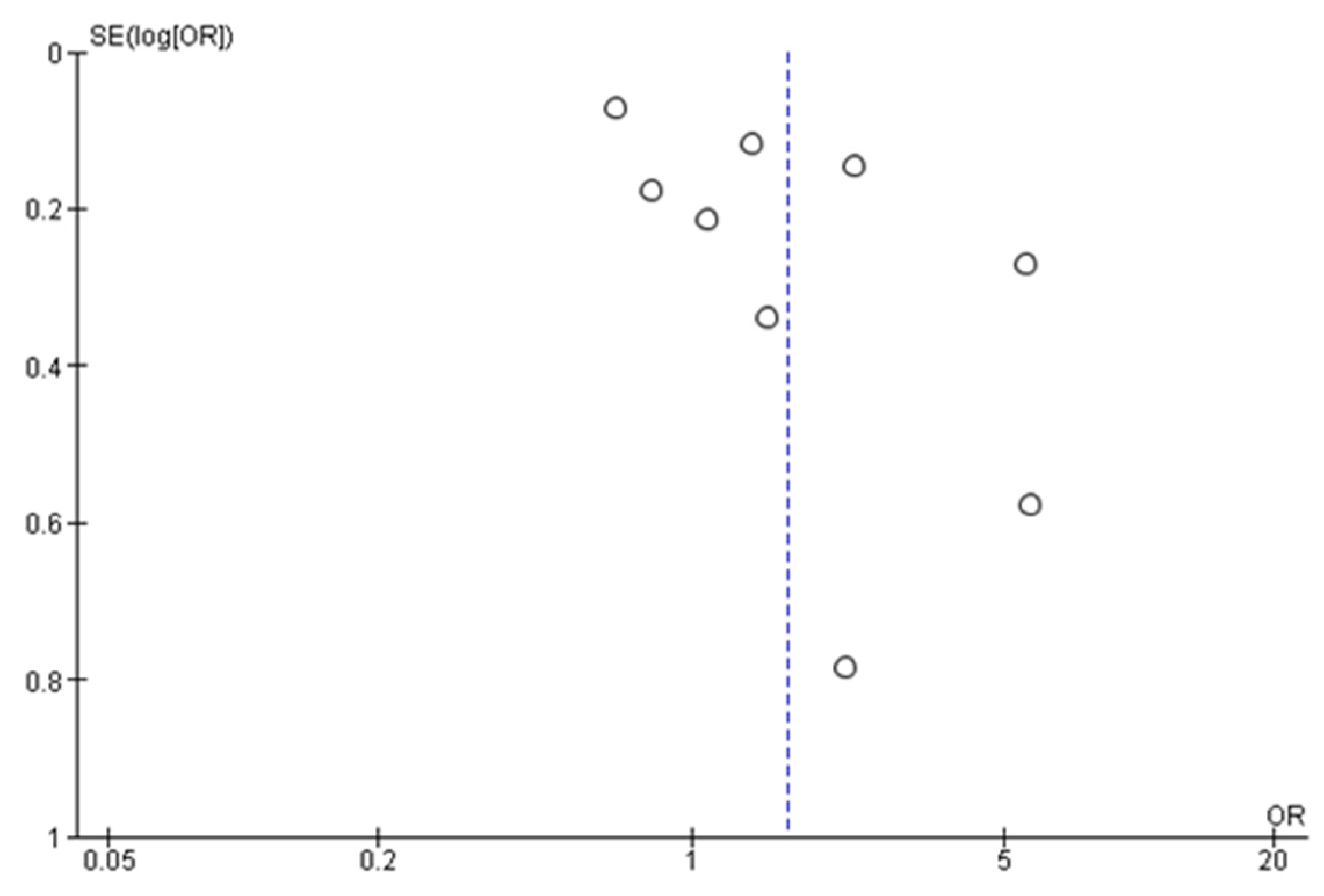
3.3.2. Studies Included in Quantitative Synthesis
3.3.3. Secondary Outcome
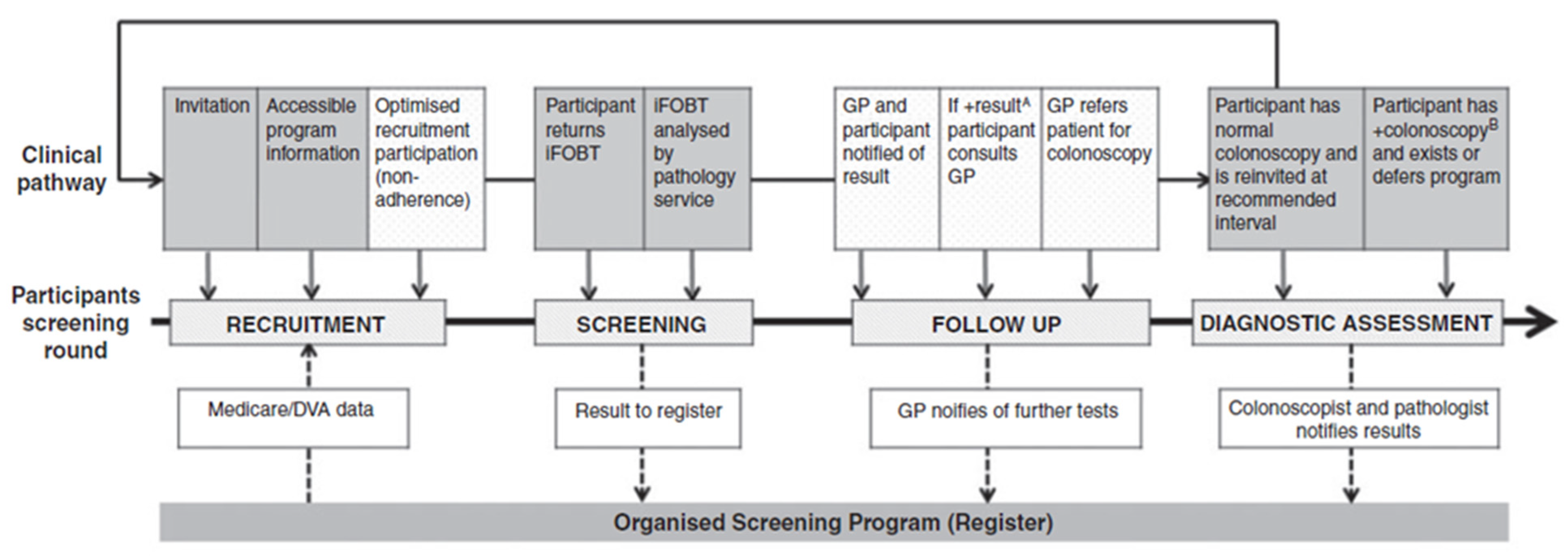
4. Discussion
Limitation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Global Cancer Observatory. Estimated Age-Standardized Incidence Rates (World) in 2020, Worldwide, All Ages. Available online: https://gco.iarc.fr/today/online-analysis-multi-bars?v=2020&mode=cancer&mode_population=countries&population=900&populations=900&key=asr&sex=1&cancer=39&type=0&statistic=5&prevalence=0&population_group=0&ages_group%5B%5D=0&ages_group%5B%5D=17&nb_items=10&group_cancer=1&include_nmsc=1&include_nmsc_other=1&type_multiple=%257B%2522inc%2522%253Atrue%252C%2522mort%2522%253Afalse%252C%2522prev%2522%253Afalse%257D&orientation=horizontal&type_sort=0&type_nb_items=%257B%2522top%2522%253Atrue%252C%2522bottom%2522%253Afalse%257D (accessed on 15 December 2020).
- Wong, M.C.; Huang, J.; Lok, V.; Wang, J.; Fung, F.; Ding, H.; Zheng, Z.-J. Differences in incidence and mortality trends of colorectal cancer, worldwide, based on sex, age, and anatomic location. Clin. Gastroenterol. Hepatol. 2021, 19, 955–966.e61. [Google Scholar] [CrossRef] [PubMed]
- Keum, N.; Giovannucci, E. Global burden of colorectal cancer: Emerging trends, risk factors and prevention strategies. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 713–732. [Google Scholar] [CrossRef] [PubMed]
- Azizah, A.M.; Hashimah, B.; Nirmal, K.; Siti Zubaidah, A.R.; Puteri, N.A.; Nabihah, A.; Sukumaran, R.; Balqis, B.; Nadia, S.M.R.; Sharifah, S.S.S.; et al. Malaysia National Cancer Registry Report (MNCRR) 2012–2016; Ministry of Health Malaysia: Putrajaya, Malaysia, 2019. [Google Scholar]
- Douaiher, J.; Ravipati, A.; Grams, B.; Chowdhury, S.; Alatise, O.; Are, C. Colorectal cancer—global burden, trends, and geographical variations. J. Surg. Oncol. 2017, 115, 619–630. [Google Scholar] [CrossRef] [PubMed]
- Walter, F.M.; Emery, J.D.; Mendonca, S.; Hall, N.; Morris, H.C.; Mills, K.; Dobson, C.; Bankhead, C.; Johnson, M.; Abel, G.A. Symptoms and patient factors associated with longer time to diagnosis for colorectal cancer: Results from a prospective cohort study. Br. J. Cancer 2016, 115, 533–541. [Google Scholar] [CrossRef] [Green Version]
- Wolf, A.M.; Fontham, E.T.; Church, T.R.; Flowers, C.R.; Guerra, C.E.; LaMonte, S.J.; Etzioni, R.; McKenna, M.T.; Oeffinger, K.C.; Shih, Y.C.T. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society. CA Cancer J. Clin. 2018, 68, 250–281. [Google Scholar] [CrossRef]
- Schreuders, E.H.; Ruco, A.; Rabeneck, L.; Schoen, R.E.; Sung, J.J.; Young, G.P.; Kuipers, E.J. Colorectal cancer screening: A global overview of existing programmes. Gut 2015, 64, 1637–1649. [Google Scholar] [CrossRef]
- Honein-AbouHaidar, G.N.; Kastner, M.; Vuong, V.; Perrier, L.; Daly, C.; Rabeneck, L.; Straus, S.; Baxter, N.N. Systematic review and meta-study synthesis of qualitative studies evaluating facilitators and barriers to participation in colorectal cancer screening. Cancer Epidemiol. Prev. Biomark. 2016, 25, 907–917. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Li, N.; Ren, J.; Feng, X.; Lyu, Z.; Wei, L.; Li, X.; Guo, L.; Zheng, Z.; Zou, S. Participation and yield of a population-based colorectal cancer screening programme in China. Gut 2019, 68, 1450–1457. [Google Scholar] [CrossRef]
- Norwati, D.; Harmy, M.; Norhayati, M.; Amry, A. Colorectal cancer screening practices of primary care providers: Results of a national survey in Malaysia. Asian Pac. J. Cancer Prev. 2014, 15, 2901. [Google Scholar] [CrossRef] [Green Version]
- May, F.P.; Almario, C.V.; Ponce, N.; Spiegel, B.M. Racial minorities are more likely than whites to report lack of provider recommendation for colon cancer screening. Am. J. Gastroenterol. 2015, 110, 1388–1394. [Google Scholar] [CrossRef]
- Ooi, C.Y.; Hanafi, N.S.; Liew, S.M. Knowledge and practice of colorectal cancer screening in an urban setting: Cross-sectional survey of primary care physicians in government clinics in Malaysia. Singap. Med. J. 2019, 60, 596. [Google Scholar] [CrossRef]
- Gandomani, H.S.; Aghajani, M.; Mohammadian-Hafshejani, A.; Tarazoj, A.A.; Pouyesh, V.; Salehiniya, H. Colorectal cancer in the world: Incidence, mortality and risk factors. Biomed. Res. Ther. 2017, 4, 1656–1675. [Google Scholar] [CrossRef] [Green Version]
- Abu Hassan, M.R.; Nik Mustapha, N.R.; Ahmad, F.; Soelar, S.A.; Mohd Suan, M.A.; Ismail, I.; Syahireen Mohammed, S.R.N.; Ali, S.M.; Chan, H.-K. National Cancer Patient Registry—Colorectal Cancer: Report for the Northern Region of Malaysia (2008–2014); Ministry of Health Malaysia, Clinical Research Centre: Alor Setar, Kedah, Malaysia, 2017. [Google Scholar]
- Gough, A.; Hunter, R.F.; Ajao, O.; Jurek, A.; McKeown, G.; Hong, J.; Barrett, E.; Ferguson, M.; McElwee, G.; McCarthy, M. Tweet for behavior change: Using social media for the dissemination of public health messages. JMIR Public Health Surveill. 2017, 3, e14. [Google Scholar] [CrossRef]
- Rowsell, A.; Muller, I.; Murray, E.; Little, P.; Byrne, C.D.; Ganahl, K.; Müller, G.; Gibney, S.; Lyles, C.R.; Lucas, A. Views of people with high and low levels of health literacy about a digital intervention to promote physical activity for diabetes: A qualitative study in five countries. J. Med. Internet Res. 2015, 17, e230. [Google Scholar] [CrossRef] [PubMed]
- Chou, W.-y.S.; Prestin, A.; Lyons, C.; Wen, K.-Y. Web 2.0 for health promotion: Reviewing the current evidence. Am. J. Public Health 2013, 103, e9–e18. [Google Scholar] [CrossRef]
- Hoffman, A.S.; Volk, R.J.; Saarimaki, A.; Stirling, C.; Li, L.C.; Härter, M.; Kamath, G.R.; Llewellyn-Thomas, H. Delivering patient decision aids on the Internet: Definitions, theories, current evidence, and emerging research areas. BMC Med. Inform. Decis. Mak. 2013, 13, S13. [Google Scholar] [CrossRef]
- O’Mara, B. Social media, digital video and health promotion in a culturally and linguistically diverse Australia. Health Promot. Int. 2013, 28, 466–476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lupton, D. Health promotion in the digital era: A critical commentary. Health Promot. Int. 2014, 30, 174–183. [Google Scholar] [CrossRef] [Green Version]
- Resources, P. MEDLINE®: Description of the Database. Available online: https://www.nlm.nih.gov/bsd/medline.html (accessed on 15 December 2020).
- Uzzi, B.; K.S.o.M.N.U. Web of Science. Available online: https://clarivate-com.ezplib.ukm.my/webofsciencegroup (accessed on 15 December 2020).
- Cochrane Library. About the Cochrane Library. Available online: https://www.cochranelibrary.com/about/about-cochrane-library (accessed on 15 December 2020).
- Fu, H.; Wang, M.; Li, P.; Jiang, S.; Hu, W.; Guo, X.; Cao, M. Tracing knowledge development trajectories of the internet of things domain: A main path analysis. IEEE Trans. Ind. Informatics 2019, 15, 6531–6540. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holden, C.A.; Frank, O.; Caruso, J.; Turnbull, D.; Reed, R.L.; Miller, C.L.; Olver, I. From participation to diagnostic assessment: A systematic scoping review of the role of the primary healthcare sector in the National Bowel Cancer Screening Program. Aust. J. Prim. Health 2020, 26, 191–206. [Google Scholar] [CrossRef]
- Sterne, J.A.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [Green Version]
- RevMan. Review Manager (RevMan) [Computer Program]. Available online: https://training.cochrane.org/online-learning/core-software-cochrane-reviews/revman/revman-5-download (accessed on 20 December 2020).
- Walsh, J.; Potter, M.; Salazar, R.; Ozer, E.; Gildengorin, G.; Dass, N.; Green, L. PreView: A randomized trial of a multi-site intervention in diverse primary care to increase rates of age-appropriate cancer screening. J. Gen. Intern. Med. 2020, 35, 449–456. [Google Scholar] [CrossRef] [Green Version]
- Gwede, C.K.; Sutton, S.K.; Chavarria, E.A.; Gutierrez, L.; Abdulla, R.; Christy, S.M.; Lopez, D.; Sanchez, J.; Meade, C.D. A culturally and linguistically salient pilot intervention to promote colorectal cancer screening among Latinos receiving care in a Federally Qualified Health Center. Health Educ. Res. 2019, 34, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Reuland, D.S.; Brenner, A.T.; Hoffman, R.; McWilliams, A.; Rhyne, R.L.; Getrich, C.; Tapp, H.; Weaver, M.A.; Callan, D.; Cubillos, L. Effect of combined patient decision aid and patient navigation vs usual care for colorectal cancer screening in a vulnerable patient population: A randomized clinical trial. JAMA Intern. Med. 2017, 177, 967–974. [Google Scholar] [CrossRef] [PubMed]
- Tu, S.-P.; Chun, A.; Yasui, Y.; Kuniyuki, A.; Yip, M.-P.; Taylor, V.; Bastani, R. Adaptation of an evidence-based intervention to promote colorectal cancer screening: A quasi-experimental study. Implement. Sci. 2014, 9, 85. [Google Scholar] [CrossRef]
- Larkey, L.K.; McClain, D.; Roe, D.J.; Hector, R.D.; Lopez, A.M.; Sillanpaa, B.; Gonzalez, J. Randomized controlled trial of storytelling compared to a personal risk tool intervention on colorectal cancer screening in low-income patients. Am. J. Health Prom. 2015, 30, e59–e70. [Google Scholar] [CrossRef] [PubMed]
- Davis, T.; Arnold, C.; Rademaker, A.; Bennett, C.; Bailey, S.; Platt, D.; Reynolds, C.; Liu, D.; Carias, E.; Bass, P., III; et al. Improving colon cancer screening in community clinics. Cancer 2013, 119, 3879–3886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, D.P., Jr.; Spangler, J.G.; Case, L.D.; Goff, D.C., Jr.; Singh, S.; Pignone, M.P. Effectiveness of a web-based colorectal cancer screening patient decision aid: A randomized controlled trial in a mixed-literacy population. Am. J. Prev. Med. 2011, 40, 608–615. [Google Scholar] [CrossRef] [Green Version]
- Aragones, A.; Schwartz, M.D.; Shah, N.R.; Gany, F.M. A randomized controlled trial of a multilevel intervention to increase colorectal cancer screening among Latino immigrants in a primary care facility. J. Gen. Intern. Med. 2010, 25, 564–567. [Google Scholar] [CrossRef] [Green Version]
- Bartholomew, K.; Zhou, L.; Crengle, S.; Buswell, E.; Buckley, A.; Sandiford, P. A targeted promotional DVD fails to improve Māori and Pacific participation rates in the New Zealand bowel screening pilot: Results from a pseudo-randomised controlled trial. BMC Publ. Health 2019, 19, 1245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Besharati, F.; Karimi-Shahanjarini, A.; Hazavehei, S.M.M.; Bashirian, S.; Bagheri, F.; Faradmal, J. Development of a colorectal cancer screening intervention for Iranian adults: Appling intervention mapping. Asian Pac. J. Cancer Prev. 2017, 18, 2193. [Google Scholar] [PubMed]
- Glanz, K.; Bishop, D.B. The role of behavioral science theory in development and implementation of public health interventions. Annu. Rev. Public Health 2010, 31, 399–418. [Google Scholar] [CrossRef] [Green Version]
- Skinner, C.S.; Tiro, J.; Champion, V.L. Background on the health belief model. Health Behav. Theory Res. Pract. 2015, 75. [Google Scholar]
- Green, E.C.; Murphy, E.M.; Gryboski, K. The health belief model. Wiley Encycl. Health Psychol. 2020, 211–214. [Google Scholar] [CrossRef]
- Hoffman, A.S.; Lowenstein, L.M.; Kamath, G.R.; Housten, A.J.; Leal, V.B.; Linder, S.K.; Jibaja-Weiss, M.L.; Raju, G.S.; Volk, R.J. An entertainment-education colorectal cancer screening decision aid for African American patients: A randomized controlled trial. Cancer 2017, 123, 1401–1408. [Google Scholar] [CrossRef] [Green Version]
- Volk, R.J.; Linder, S.K.; Lopez-Olivo, M.A.; Kamath, G.R.; Reuland, D.S.; Saraykar, S.S.; Leal, V.B.; Pignone, M.P. Patient decision aids for colorectal cancer screening: A systematic review and meta-analysis. Am. J. Prev. Med. 2016, 51, 779–791. [Google Scholar] [CrossRef] [Green Version]
- Cutrona, S.L.; Roblin, D.W.; Wagner, J.L.; Gaglio, B.; Williams, A.E.; Stone, R.T.; Field, T.S.; Mazor, K.M. Adult willingness to use email and social media for peer-to-peer cancer screening communication: Quantitative interview study. JMIR Res. Protoc. 2013, 2, e52. [Google Scholar] [CrossRef] [Green Version]
- Merino, P.; Bustamante, E.; Campillo-Artero, C.; Bartual, E.; Tuero, G.; Marí, J. Patient safety certification in a Department of Intensive Care Medicine: Our experience with standard UNE 179003:2013. Med. Intensiv. 2014, 38, 297–304. [Google Scholar] [CrossRef]
- Davis, F.D. Perceived usefulness, perceived ease of use, and user acceptance of information technology. MIS Q. 1989, 13, 319–340. [Google Scholar] [CrossRef] [Green Version]
- Mukewar, S.; Mani, P.; Wu, X.; Lopez, R.; Shen, B. YouTube® and inflammatory bowel disease. J. Crohns Colitis 2013, 7, 392–402. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.; Sussman, D.A.; Doubeni, C.A.; Anderson, D.S.; Day, L.; Deshpande, A.R.; Joseph Elmunzer, B.; Laiyemo, A.O.; Mendez, J.; Somsouk, M. Challenges and possible solutions to colorectal cancer screening for the underserved. J. Natl. Cancer Inst. 2014, 106, dju032. [Google Scholar] [CrossRef] [Green Version]
- Von Wagner, C.; Baio, G.; Raine, R.; Snowball, J.; Morris, S.; Atkin, W.; Obichere, A.; Handley, G.; Logan, R.F.; Rainbow, S. Inequalities in participation in an organized national colorectal cancer screening programme: Results from the first 2.6 million invitations in England. Int. J. Epidemiol. 2011, 40, 712–718. [Google Scholar] [CrossRef] [Green Version]
- Escoffery, C.; Fernandez, M.E.; Vernon, S.W.; Liang, S.; Maxwell, A.E.; Allen, J.D.; Dwyer, A.; Hannon, P.A.; Kohn, M.; DeGroff, A. Patient navigation in a colorectal cancer screening program. J Public Health Manag Pract. 2015, 21, 433. [Google Scholar] [CrossRef] [Green Version]
- Kemper, K.E.; Glaze, B.L.; Eastman, C.L.; Waldron, R.C.; Hoover, S.; Flagg, T.R.; Tangka, F.K.; Subramanian, S. Effectiveness and cost of multilayered colorectal cancer screening promotion interventions at federally qualified health centers in Washington State. Cancer 2018, 124, 4121–4129. [Google Scholar] [CrossRef]
- Roland, K.B.; Milliken, E.L.; Rohan, E.A.; DeGroff, A.; White, S.; Melillo, S.; Rorie, W.E.; Signes, C.C.; Young, P.A. Use of community health workers and patient navigators to improve cancer outcomes among patients served by federally qualified health centers: A systematic literature review. Health Equity 2017, 1, 61–76. [Google Scholar] [CrossRef] [PubMed]
- Rohan, E.A.; Slotman, B.; DeGroff, A.; Morrissey, K.G.; Murillo, J.; Schroy, P. Refining the patient navigation role in a colorectal cancer screening program: Results from an intervention study. J. Natl. Compr. Cancer Netw. 2016, 14, 1371–1378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahin, A.N.; Sahin, A.S.; Schwenter, F.; Sebajang, H. YouTube videos as a source of information on colorectal cancer: What do our patients learn? J. Cancer Educ. 2019, 34, 1160–1166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brar, J.; Ferdous, M.; Abedin, T.; Turin, T.C. Online Information for Colorectal Cancer Screening: A Content Analysis of YouTube Videos. J. Cancer Educ. 2020, 35, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Zapka, J.G.; Lemon, S.C.; Puleo, E.; Estabrook, B.; Luckmann, R.; Erban, S. Patient education for colon cancer screening: A randomized trial of a video mailed before a physical examination. Ann. Intern. Med. 2004, 141, 683–692. [Google Scholar] [CrossRef] [PubMed]
- Gabel, P.; Edwards, A.; Kirkegaard, P.; Larsen, M.B.; Andersen, B. The LEAD trial—The effectiveness of a decision aid on decision making among citizens with lower educational attainment who have not participated in FIT-based colorectal cancer screening in Denmark: A randomised controlled trial. Patient Educ. Couns. 2020, 103, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Farooqui, M.; Hassali, M.A.; Knight, A.; Shafie, A.A.; Farooqui, M.A.; Saleem, F.; Haq, N.-u.; Aljadhey, H. A qualitative exploration of Malaysian cancer patients’ perceptions of cancer screening. BMC Public Health 2013, 13, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Outcome Measures | Operational Definition | |
|---|---|---|
| Primary | CRC-screening completion rate | Completion rate or participation rate or adherence rate to either FOBT or colonoscopy or sigmoidoscopy uptake. |
| Secondary | Spoiled kit return rate | Spoiled FOBT kits are samples which cannot be analyzed for one reason or another (e.g., no date on the specimen). |
| CRC-screening awareness and belief | Awareness score was calculated by summing the points earned for all awareness items. A scale assessing influence of religious beliefs on medical-decision-making, such as CRC screening, was used. | |
| Ability to state CRC-screening test preference | Assessment on the post-program survey by asking patients which CRC-screening test they would want if all tests were free. | |
| Readiness to receive CRC screening | Determined by comparing patients’ readiness stage after the intervention program to their baseline stage. | |
| Ordered CRC-screening tests | Determined by patients’ chart review. | |
| No. | Author (Year) | Country | Study Design (Follow-Up) | Participants (Sample Size) | Type of e-Media Decision-Aid Used | E-media Decision-Aid Content (Duration) | Comparison | Main Outcome Measures | Results |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Walsh et al. (2020) | San Francisco, United States | RCT (14 months) | Patients with upcoming follow-up or preventive appointments (508, 6 clinics) | Interactive multimedia program (“Video Doctor” and “Provider Alert” paper, named “PreView” (Preventive Video Education in Waiting Areas)) |
| Video about healthy lifestyle |
|
|
| 2 | Bartholomew et al. (2019) | Auckland, New Zealand | RCT (3 months) | Māori and Pacific residents, non-adherent to initial screening invitation (5271, clinic numbers not mentioned) | Video (DVD and a reminder letter) |
| d Usual reminder letter |
|
|
| 3 | Gwede et al. (2019) | Florida, United States | RCT (3 months) | Latinos population (76, 2 clinics) | Video (DVD with fotonovela booklet and FIT kit, called “LCARES” (Latinos CRC Awareness, Research, Education, and Screening)) |
| Booklet about CRC-screening promotion in Spanish and a FIT kit |
|
|
| 4 | Besharati et al. (2017) | Hamadan City, Iran | Cluster RCT (4 months) | Iranian adults (248, 8 clinics) | Video ((educational package consists of video, discussion, role play, a reminder pack of postcards, and pamphlet). Group 1: Educational package and free FOBT; Group 2: only Educational package; Group 3: free FOBT) |
| Usual care given with survey about determinants of CRC-screening behavior |
|
|
| 5 | Reuland et al. (2017) | North Carolina and New Mexico, United States | RCT (6 months) | Vulnerable low income population (265, 2 clinics) | Video (decision aid and patient navigation (employees of the clinic or its affiliated health system)) |
| Video on food safety and d usual care |
|
|
| 6 | Larkey et al. (2015) | Arizona, United States | RCT (3 months) | Low-income patients (545, 15 clinics) | Video |
| Usual care given with instrument estimating level of personal cancer risk based on the Harvard Cancer Risk Index |
|
|
| 7 | Tu et al. (2014) | Washington, United States | Quasi experiment (24 months) | Vietnamese immigrants (1260, 2 clinics) | Video ((DVD) and a pamphlet) |
| Usual care (FOBT ordered by primary care providers) |
|
|
| 8 | Davis et al. (2013) | Louisiana, United States | Quasi experiment (12 months) | Low-income, uninsured patients in predominantly rural areas (961, 8 clinics) | Video (Educational strategy (enhanced usual care, pamphlet, video, and FOBT instructions). Group 1: Educational strategy; Group 2: Educational strategy and nurse support to encourage CRC-screening completion) |
| Enhanced usual care (recommendation for CRC screening and FOBT kit) |
|
|
| 9 | Miller et al. (2011) | North Carolina, United States | RCT (6 months) | Patients scheduled for routine visits (264, 1 clinic) | Interactive multimedia web-based program (named “CHOICE” (Communicating Health Options through Interactive Computer Education, version 6.0 W)) |
| Interactive web-based program about prescription drug refılls and safety |
|
|
| 10 | Aragones et al. (2010) | New York, United States | RCT (3 months) | Latino immigrants (65, 1 clinic) | Video ((and a brochure for patient) with a paper-based reminder for their physicians) |
| d Usual care |
|
|
| Study (Year) | Outcome (Intervention vs. Control) | Outcome Summary | |||
|---|---|---|---|---|---|
| Higher CRC-Screening Completion Rate with Statistical Significance | Higher CRC-Screening Completion Rate without Statistical Significance | Lower CRC-Screening Completion rate with Statistical Significance | Lower CRC-Screening Completion Rate without Statistical Significance | ||
| Walsh et al. (2020) | √ | Null | |||
| Bartholomew et al. (2019) | √ | Not effective | |||
| Gwede et al. (2019) | √ | Null | |||
| Besharati et al. (2017) | √ | Effective | |||
| Reuland et al. (2017) | √ | Effective | |||
| Larkey et al. (2015) | √ | Null | |||
| Tu et al. (2014) | √ | Null | |||
| Davis et al. (2013) | √ | Effective | |||
| Miller et al. (2011) | √ | Null | |||
| Aragones et al. (2010) | √ | Effective | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramli, N.S.; Manaf, M.R.A.; Hassan, M.R.; Ismail, M.I.; Nawi, A.M. Effectiveness of Colorectal Cancer Screening Promotion Using E-Media Decision Aids: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2021, 18, 8190. https://doi.org/10.3390/ijerph18158190
Ramli NS, Manaf MRA, Hassan MR, Ismail MI, Nawi AM. Effectiveness of Colorectal Cancer Screening Promotion Using E-Media Decision Aids: A Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health. 2021; 18(15):8190. https://doi.org/10.3390/ijerph18158190
Chicago/Turabian StyleRamli, Nur Suhada, Mohd Rizal Abdul Manaf, Mohd Rohaizat Hassan, Muhamad Izwan Ismail, and Azmawati Mohammed Nawi. 2021. "Effectiveness of Colorectal Cancer Screening Promotion Using E-Media Decision Aids: A Systematic Review and Meta-Analysis" International Journal of Environmental Research and Public Health 18, no. 15: 8190. https://doi.org/10.3390/ijerph18158190
APA StyleRamli, N. S., Manaf, M. R. A., Hassan, M. R., Ismail, M. I., & Nawi, A. M. (2021). Effectiveness of Colorectal Cancer Screening Promotion Using E-Media Decision Aids: A Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health, 18(15), 8190. https://doi.org/10.3390/ijerph18158190






