Affective and Enjoyment Responses to Sprint Interval Exercise at Different Hypoxia Levels
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experimental Design
2.3. Main Outcome Measures
2.4. Sprint Interval Exercise
2.5. Statistical Analysis
3. Results
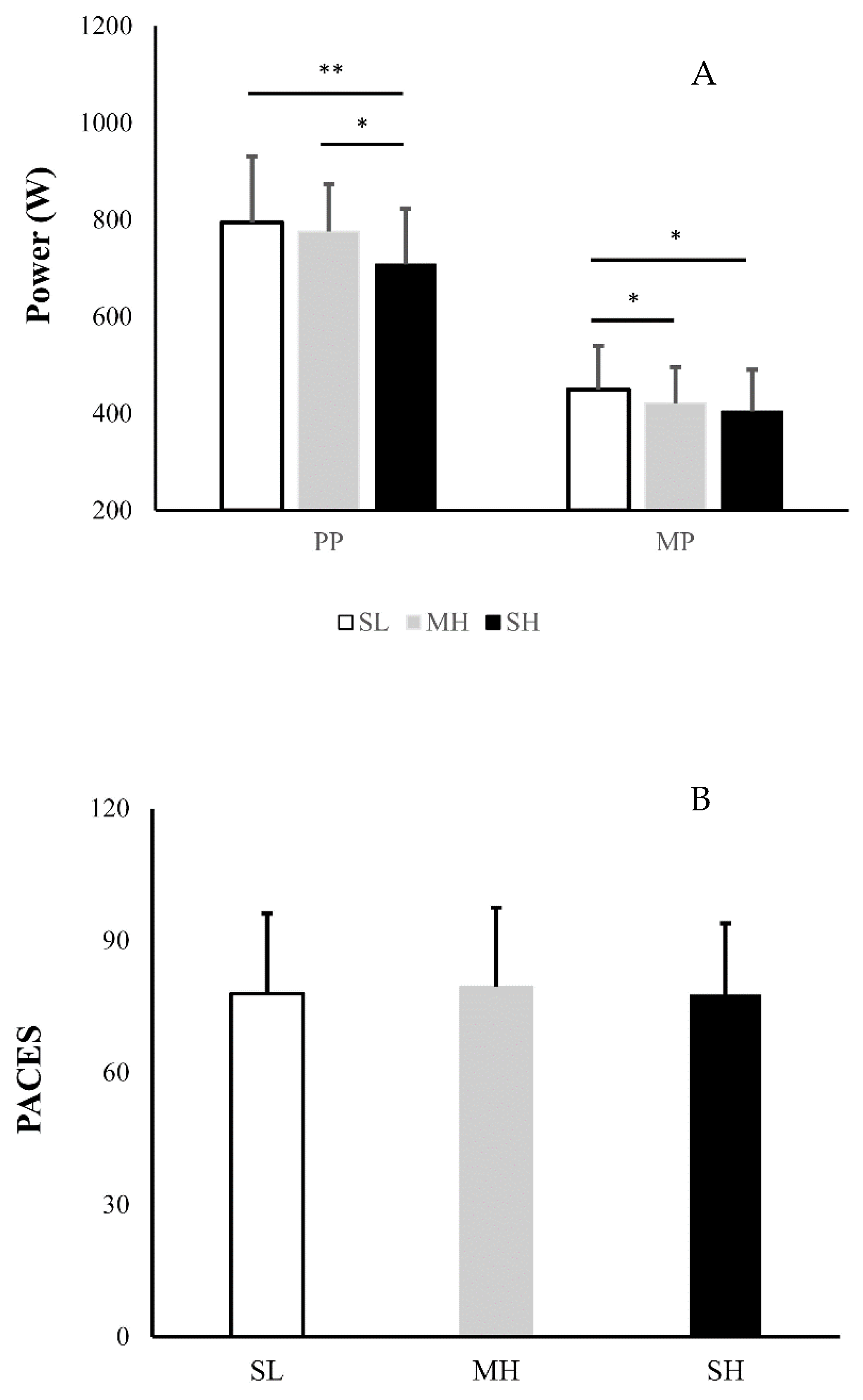
3.1. Daily Physical Activity and Physiological Responses
3.2. Affective Responses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Millet, G.P.; Roels, B.; Schmitt, L.; Woorons, X.; Richalet, J.P. Combining Hypoxic Methods for Peak Performance. Sports Med. 2010, 40, 1–25. [Google Scholar] [CrossRef]
- Hobbins, L.G.; Hunter, S.; Gaoua, N.; Girard, O. Normobaric hypoxic conditioning to maximize weight loss and ameliorate cardio-metabolic health in obese populations: A systematic review. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2017, 313, R251–R264. [Google Scholar] [CrossRef] [Green Version]
- Camacho-Cardenosa, A.; Camacho-Cardenosa, M.; Brazo-Sayavera, J.; Timón, R.; González-Custodio, A.; Olcina, G. Repeated sprint in hypoxia as a time-metabolic efficient strategy to improve physical fitness of obese women. Eur. J. Appl. Physiol. 2020, 120, 1051–1061. [Google Scholar] [CrossRef]
- Camacho-Cardenosa, A.; Camacho-Cardenosa, M.; Burtscher, M.; Martínez-Guardado, I.; Timon, R.; Brazo-Sayavera, J.; Olcina, G. High-Intensity Interval Training in Normobaric Hypoxia Leads to Greater Body Fat Loss in Overweight/Obese Women than High-Intensity Interval Training in Normoxia. Front. Physiol. 2018, 9, 60. [Google Scholar] [CrossRef]
- Kong, Z.; Shi, Q.; Nie, J.; Tong, T.K.; Song, L.; Yi, L.; Hu, Y. High-Intensity Interval Training in Normobaric Hypoxia Improves Cardiorespiratory Fitness in Overweight Chinese Young Women. Front. Physiol. 2017, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McLean, B.D.; Gore, C.J.; Kemp, J. Application of live low-train high’for enhancing normoxic exercise performance in team sport athletes. Sports Med. 2014, 44, 1275–1287. [Google Scholar] [CrossRef]
- Ekkekakis, P.; Zenko, Z.; Ladwig, M.; Hartman, M. Affective Determinants of Health Behavior. In Affect as a Potential Determinant of Physical Activity and Exercise: Critical Appraisal of an Emerging Research Field; Oxford University Press: New York, NY, USA, 2018. [Google Scholar]
- Rhodes, R.E.; Fiala, B.; Conner, M. A Review and Meta-Analysis of Affective Judgments and Physical Activity in Adult Populations. Ann. Behav. Med. 2009, 38, 180–204. [Google Scholar] [CrossRef] [PubMed]
- Weyland, S.; Finne, E.; Krell-Roesch, J.; Jekauc, D. (How) Does Affect Influence the Formation of Habits in Exercise? Front. Psychol. 2020, 11, 578108. [Google Scholar] [CrossRef]
- Ekkekakis, P.; Parfitt, G.; Petruzzello, S. The Pleasure and Displeasure People Feel When they Exercise at Different Intensities. Sports Med. 2011, 41, 641–671. [Google Scholar] [CrossRef] [PubMed]
- Niven, A.; Laird, Y.; Saunders, D.H.; Phillips, S.M. A systematic review and meta-analysis of affective responses to acute high intensity interval exercise compared with continuous moderate- and high-Intensity exercise. Health Psychol. Rev. 2020, 2020, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, B.R.R.; Santos, T.; Kilpatrick, M.; Pires, F.O.; Deslandes, A. Affective and enjoyment responses in high intensity interval training and continuous training: A systematic review and meta-analysis. PLoS ONE 2018, 13, e0197124. [Google Scholar] [CrossRef] [Green Version]
- Stork, M.J.; Banfield, L.E.; Gibala, M.J.; Ginis, K.A.M. A scoping review of the psychological responses to interval exercise: Is interval exercise a viable alternative to traditional exercise? Health Psychol. Rev. 2017, 11, 324–344. [Google Scholar] [CrossRef] [PubMed]
- Townsend, L.K.; Islam, H.; Dunn, E.; Eys, M.; Robertson-Wilson, J.; Hazell, T.J. Modified sprint interval training protocols. Part II. Psychological responses. Appl. Physiol. Nutr. Metab. 2017, 42, 347–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brocherie, F.; Millet, G.P.; Girard, O. Psychophysiological Responses to Repeated-Sprint Training in Normobaric Hypoxia and Normoxia. Int. J. Sports Physiol. Perform. 2017, 12, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Warnier, G.; Benoit, N.; Naslain, D.; Lambrecht, S.; Francaux, M.; Deldicque, L. Effects of Sprint Interval Training at Different Altitudes on Cycling Performance at Sea-Level. Sports 2020, 8, 148. [Google Scholar] [CrossRef] [PubMed]
- Balsom, P.D.; Gaitanos, G.C.; Ekblom, B.; Sjödin, B. Reduced oxygen availability during high intensity intermittent exercise impairs performance. Acta Physiol. Scand. 1994, 152, 279–285. [Google Scholar] [CrossRef]
- Girard, O.; Brocherie, F.; Morin, J.-B.; Millet, G.P. Neuro-mechanical determinants of repeated treadmill sprints - Usefulness of an “hypoxic to normoxic recovery” approach. Front. Physiol. 2015, 6, 260. [Google Scholar] [CrossRef] [Green Version]
- Pollak, K.A.; Swenson, J.D.; VanHaitsma, T.A.; Hughen, R.W.; Jo, D.; Light, K.C.; Schweinhardt, P.; Amann, M.; Light, A.R. Exogenously applied muscle metabolites synergistically evoke sensations of muscle fatigue and pain in human subjects. Exp. Physiol. 2014, 99, 368–380. [Google Scholar] [CrossRef] [PubMed]
- Billaut, F.; Kerris, J.P.; Rodriguez, R.F.; Martin, D.T.; Gore, C.J.; Bishop, D.J. Interaction of Central and Peripheral Factors during Repeated Sprints at Different Levels of Arterial O2 Saturation. PLoS ONE 2013, 8, e77297. [Google Scholar] [CrossRef] [Green Version]
- Kong, Z.; Fan, X.; Sun, S.; Song, L.; Shi, Q.; Nie, J. Comparison of High-Intensity Interval Training and Moderate-to-Vigorous Continuous Training for Cardiometabolic Health and Exercise Enjoyment in Obese Young Women: A Randomized Controlled Trial. PLoS ONE 2016, 11, e0158589. [Google Scholar] [CrossRef]
- Ekkekakis, P. Pleasure and displeasure from the body: Perspectives from exercise. Cogn. Emot. 2003, 17, 213–239. [Google Scholar] [CrossRef] [PubMed]
- Haile, L.; Gallagher, M.; Robertson, R.J. Perceived Exertion Laboratory Manual; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Stanley, D.M.; Cumming, J. Are we having fun yet? Testing the effects of imagery use on the affective and enjoyment responses to acute moderate exercise. Psychol. Sport Exerc. 2010, 11, 582–590. [Google Scholar] [CrossRef]
- Kendzierski, D.; DeCarlo, K.J. Physical activity enjoyment scale: Two validation studies. J. Sport Exerc. Psychol. 1991, 13, 50–64. [Google Scholar] [CrossRef]
- Shi, Q.; Tong, T.K.; Sun, S.; Kong, Z.; Chan, C.K.; Liu, W.; Nie, J. Influence of recovery duration during 6-s sprint interval exercise on time spent at high rates of oxygen uptake. J. Exerc. Sci. Fit. 2018, 16, 16–20. [Google Scholar] [CrossRef]
- Brooks GAS. Nutrition and Metabolism; Springer: New York, NY, USA, 2014. [Google Scholar]
- Cohen, J. Statistical Power Analysis for the Behavioural Sciences; Academic Press: New York, NY, USA, 1969. [Google Scholar]
- Cumming, G. Understanding the New Statistics: Effect Sizes, Confidence Intervals, and Meta-Analysis; Routledge: New York, NY, USA, 2012. [Google Scholar]
- Cohen, J. A power primer. Psychol. Bull. 1992, 112, 155. [Google Scholar] [CrossRef]
- Hobbins, L.; Gaoua, N.; Hunter, S.; Girard, O. Psycho-physiological responses to perceptually-regulated interval runs in hypoxia and normoxia. Physiol. Behav. 2019, 209, 112611. [Google Scholar] [CrossRef]
- Ekkekakis, P.; Petruzzello, S.J. Analysis of the affect measurement conundrum in exercise psychology: I. Fundamental issues. Psychol. Sport Exerc. 2000, 1, 71–88. [Google Scholar] [CrossRef]
- Marin, D.P.; Astorino, T.A.; Martinatto, F.; Ragazzini, F.T.; Bispo, R.E.; Foschini, D.; Otton, R. Comparison of perceptual responses between different upper-body sprint interval exercise protocols. Physiol. Behav. 2019, 210. [Google Scholar] [CrossRef] [PubMed]
- Marques, M.; Alves, E.; Henrique, N.; Franchini, E. Positive Affective and Enjoyment Responses to Four High-Intensity Interval Exercise Protocols. Percept. Mot. Ski. 2020, 127, 742–765. [Google Scholar] [CrossRef] [PubMed]
- Niven, A.; Thow, J.; Holroyd, J.; Turner, A.P.; Phillips, S.M. Comparison of affective responses during and after low volume high-intensity interval exercise, continuous moderate- and continuous high-intensity exercise in active, untrained, healthy males. J. Sports Sci. 2018, 36, 1993–2001. [Google Scholar] [CrossRef] [Green Version]
- Decker, E.S.; Ekkekakis, P. More efficient, perhaps, but at what price? Pleasure and enjoyment responses to high-intensity interval exercise in low-active women with obesity. Psychol. Sport Exerc. 2017, 28, 1–10. [Google Scholar] [CrossRef]
- Foster, C.; Farland, C.V.; Guidotti, F.; Harbin, M.; Roberts, B.; Schuette, J.; Tuuri, A.; Doberstein, S.T.; Porcari, J.P. The Effects of High Intensity Interval Training vs Steady State Training on Aerobic and Anaerobic Capacity. J. Sports Sci. Med. 2015, 14, 747–755. [Google Scholar] [PubMed]
- McKie, G.L.; Islam, H.; Townsend, L.K.; Robertson-Wilson, J.; Eys, M.; Hazell, T.J. Modified sprint interval training protocols: Physiological and psychological responses to 4 weeks of training. Appl. Physiol. Nutr. Metab. 2018, 43, 595–601. [Google Scholar] [CrossRef]
- Astorino, T.A.; Clausen, R.; Marroquin, J.; Arthur, B.; Stiles, K. Similar perceptual responses to reduced exertion high intensity interval training (REHIT) in adults differing in cardiorespiratory fitness. Physiol. Behav. 2020, 213, 112687. [Google Scholar] [CrossRef] [PubMed]
- Bradley, C.; Niven, A.; Phillips, S.M. Self-reported tolerance of the intensity of exercise influences affective responses to and intentions to engage with high-intensity interval exercise. J. Sports Sci. 2019, 37, 1472–1480. [Google Scholar] [CrossRef] [Green Version]
- Ekkekakis, P.; Hall, E.E.; Petruzzello, S.J. Some like It Vigorous: Measuring Individual Differences in the Preference for and Tolerance of Exercise Intensity. J. Sport Exerc. Psychol. 2005, 27, 350–374. [Google Scholar] [CrossRef]
- Kong, Z.; Hu, M.; Liu, Y.; Shi, Q.; Zou, L.; Sun, S.; Zhang, H.; Nie, J. Affective and Enjoyment Responses to Short-Term High-Intensity Interval Training with Low-Carbohydrate Diet in Overweight Young Women. Nutrients 2020, 12, 442. [Google Scholar] [CrossRef] [Green Version]
- Tempest, G.D.; Parfitt, G. Prefrontal oxygenation and the acoustic startle eyeblink response during exercise: A test of the dual-mode model. Psychophysiology 2017, 54, 1070–1080. [Google Scholar] [CrossRef]
- Davidson, R. Anxiety and affective style: Role of prefrontal cortex and amygdala. Biol. Psychiatry 2002, 51, 68–80. [Google Scholar] [CrossRef]
- Tempest, G.; Parfitt, G. Self-reported tolerance influences prefrontal cortex hemodynamics and affective responses. Cogn. Affect. Behav. Neurosci. 2016, 16, 63–71. [Google Scholar] [CrossRef]
- Hargreaves, E.; Stych, K. Exploring the peak and end rule of past affective episodes within the exercise context. Psychol. Sport Exerc. 2013, 14, 169–178. [Google Scholar] [CrossRef]
- Oussaidene, K.; Prieur, F.; Bougault, V.; Borel, B.; Matran, R.; Mucci, P. Cerebral oxygenation during hyperoxia-induced increase in exercise tolerance for untrained men. Eur. J. Appl. Physiol. 2013, 113, 2047–2056. [Google Scholar] [CrossRef] [PubMed]
- Faiss, R.; Girard, O.; Millet, G.P. Advancing hypoxic training in team sports: From intermittent hypoxic training to repeated sprint training in hypoxia: Table 1. Br. J. Sports Med. 2013, 47, i45–i50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buchheit, M.; Laursen, P.B. High-intensity interval training, solutions to the programming puzzle. Sports Med. 2013, 43, 313–338. [Google Scholar] [CrossRef]
| SL | MH | SH | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | |||||||
| HR (bpm) | 68 | ±8 | 150 | ±12 * | 72 | ±8 † | 155 | ±12 * | 79 | ±11 †‡ | 152 | ±13 * |
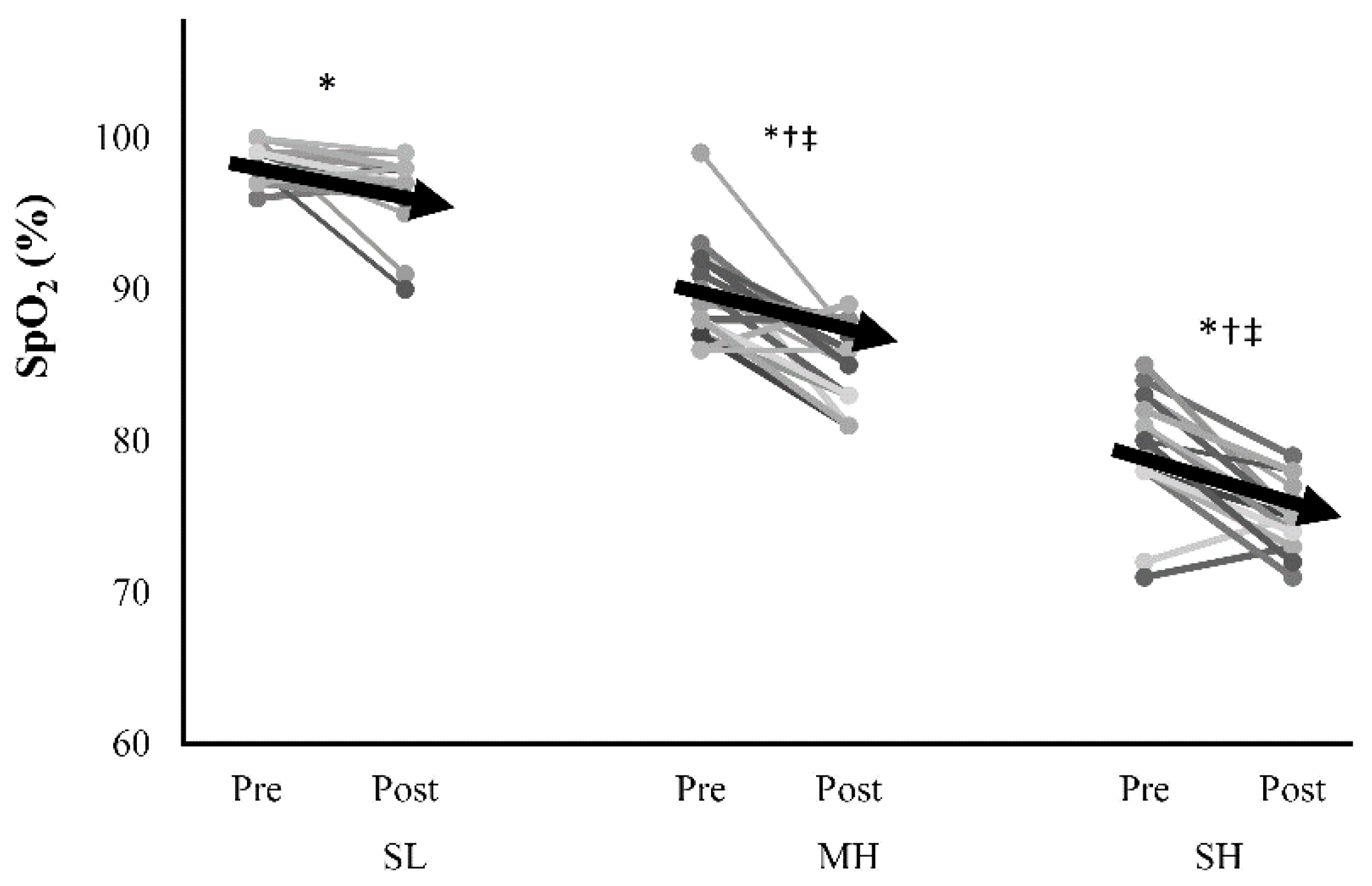
| SpO2 (%) | 98 | ±1 | 96 | ±1 * | 90 | ±3 †,‡ | 86 | ±3 * | 79 | ±3 †‡ | 75 | ±3 * |
| RPE | 0.4 | ±0.8 | 7.2 | ±2.9 * | 0.3 | ±0.4 | 6.8 | ±2.2 * | 0.6 | ±1.0 | 7.4 | ±2.4 * |
| FS | 1.7 | ±1.3 | 1.7 | ±1.5 | 1.0 | ±1.7 | 1.1 | ±1.5 | 1.2 | ±1.3 | 1.4 | ±1.3 |
| FAS | 2.3 | ±1.2 | 4.0 | ±1.2 * | 2.6 | ±1.5 | 3.9 | ±1.4 * | 2.6 | ±1.4 | 3.6 | ±1.4 * |
| EES | 4.0 | ±2.0 | 3.9 | ±1.5 | 3.7 | ±1.9 | 3.8 | ±1.3 | 4.2 | ±0.7 | 3.8 | ±1.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, Z.; Hu, M.; Sun, S.; Zou, L.; Shi, Q.; Jiao, Y.; Nie, J. Affective and Enjoyment Responses to Sprint Interval Exercise at Different Hypoxia Levels. Int. J. Environ. Res. Public Health 2021, 18, 8171. https://doi.org/10.3390/ijerph18158171
Kong Z, Hu M, Sun S, Zou L, Shi Q, Jiao Y, Nie J. Affective and Enjoyment Responses to Sprint Interval Exercise at Different Hypoxia Levels. International Journal of Environmental Research and Public Health. 2021; 18(15):8171. https://doi.org/10.3390/ijerph18158171
Chicago/Turabian StyleKong, Zhaowei, Mingzhu Hu, Shengyan Sun, Liye Zou, Qingde Shi, Yubo Jiao, and Jinlei Nie. 2021. "Affective and Enjoyment Responses to Sprint Interval Exercise at Different Hypoxia Levels" International Journal of Environmental Research and Public Health 18, no. 15: 8171. https://doi.org/10.3390/ijerph18158171
APA StyleKong, Z., Hu, M., Sun, S., Zou, L., Shi, Q., Jiao, Y., & Nie, J. (2021). Affective and Enjoyment Responses to Sprint Interval Exercise at Different Hypoxia Levels. International Journal of Environmental Research and Public Health, 18(15), 8171. https://doi.org/10.3390/ijerph18158171






