Early Detection Methods for Silicosis in Australia and Internationally: A Review of the Literature
Abstract
1. Introduction
- What methods are currently used in respiratory surveillance for occupational lung disease? Have they been validated?
- What alternative methods exist or are under investigation, and what evidence is there for the effectiveness of these methods?
- Is there evidence to support conducting a prospective cohort study to test the validity of alternative methods of early detection of silicosis?
2. Materials and Methods
2.1. Scoping Review
2.2. Grey Literature
2.3. Websites, Industry, Government, and Regulators
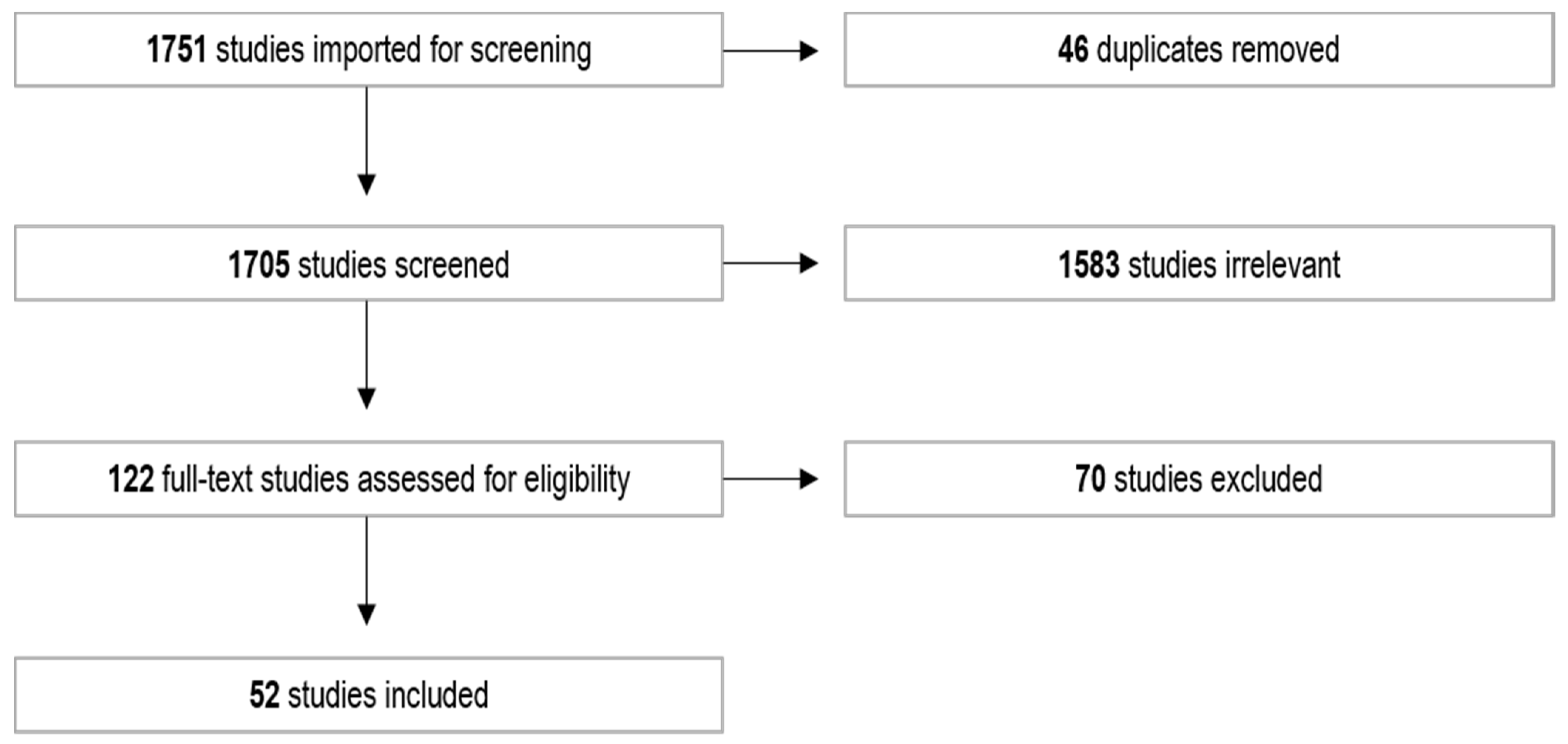
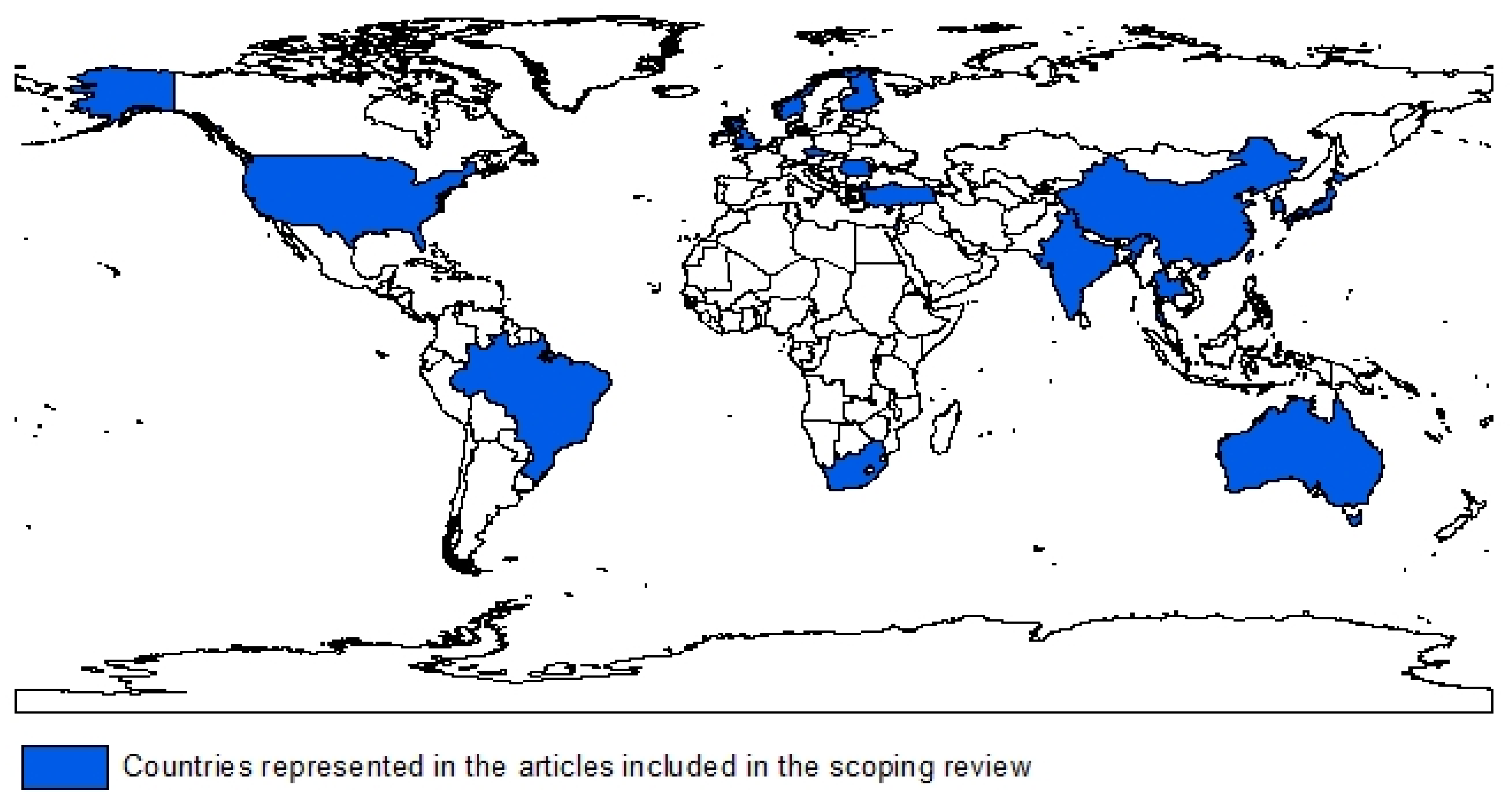
3. Results
Clinical Trials
4. Discussion
4.1. Spirometry
4.2. Imaging
4.3. Biomarkers
4.4. Exhaled Biomarkers
4.5. Summary of Methods
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Alif, S.M.; Glass, D.C.; Abramson, M.; Hoy, R.; Sim, M.R. Occupational Lung Diseases in Australia 2006–2019. Safe Work Australia 2020. Available online: https://www.safeworkaustralia.gov.au/doc/occupational-lung-diseases-australia-2006-2019 (accessed on 7 August 2020).
- De Vuyst, P.; Camus, P. The past and present of pneumoconioses. Curr. Opin. Pulm. Med. 2000, 6, 151–156. [Google Scholar] [CrossRef]
- Doganay, S.; Gocmen, H.; Yikilmaz, A.; Coskun, A. Silicosis due to denim sandblasting in young people: MDCT findings. Eurasian J. Med. 2010, 41, 21–23. [Google Scholar] [CrossRef] [PubMed]
- Chu, M.; Ji, X.; Chen, W.; Zhang, R.; Sun, C.; Wang, T.; Luo, C.; Gong, J.; Zhu, M.; Fan, J.; et al. A genome-wide association study identifies susceptibility loci of silica-related pneumoconiosis in Han Chinese. Hum. Mol. Genet. 2014, 23, 6385–6394. [Google Scholar] [CrossRef]
- Cohen, R.A. Resurgent coal mine dust lung disease: Wave of the future or a relic of the past? Occup. Environ. Med. 2016, 73, 715–716. [Google Scholar] [CrossRef]
- McBean, R.; Tatkovic, A.; Edwards, R.; Newbigin, K. What does coal mine dust lung disease look like? A radiological review following re-identification in Queensland. J. Med. Imaging Radiat. Oncol. 2020, 64, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Leung, C.C.; Yu, I.T.S.; Chen, W. Silicosis. Lancet 2012, 379, 2008–2018. [Google Scholar] [CrossRef]
- Greenberg, M.I.; Waksman, J.; Curtis, J. Silicosis: A review. Dis. Mon. 2007, 53, 394–416. [Google Scholar] [CrossRef] [PubMed]
- Lavoipierre, A. Silicosis Cases in Australia Rising, with Thousands Exposed to Unsafe Quantities of Silica in Past Decade 2019. Available online: https://www.abc.net.au/news/2019-12-26/silicosis-cases-rapidly-climbing-more-toxic-asbestos-expert-says/11826332 (accessed on 10 June 2020).
- McMahon, A. Silicosis: Here’s What You Need to Know about The Dust Lung Disease Killing Stonemasons 2018. Available online: https://www.abc.net.au/news/2018-10-12/what-is-the-dust-lung-disease-silicosis/10365604 (accessed on 5 July 2020).
- DNRME. Review of Respiratory Component of the Coal Mine Workers’ Health Scheme for the Queensland Department of Natural Resources and Mines. Monash Centre for Occupational and Environmental Health, Monash University 2016. Available online: https://www.dnrme.qld.gov.au/__data/assets/pdf_file/0009/383940/monash-qcwp-final-report-2016.pdf (accessed on 4 June 2020).
- Palabiyik, S.S.; Girgin, G.; Tutkun, E.; Yilmaz, O.H.; Baydar, T. Immunomodulation and oxidative stress in denim sandblasting workers: Changes caused by silica exposure. Arh. Za Hig. Rada I Toksikologiju. 2013, 64, 431–437. [Google Scholar] [CrossRef]
- Norboo, T.; Angchuk, P.T.; Yahya, M.; Kamat, S.R.; Pooley, F.D.; Corrin, B.; Kerr, I.H.; Bruce, N.; Ball, K.P. Silicosis in a Himalayan village population: Role of environmental dust. Thorax 1991, 46, 341–343. [Google Scholar] [CrossRef]
- Gudmundsson, G. Respiratory health effects of volcanic ash with special reference to Iceland. A review. Clin. Respir. J. 2011, 5, 2–9. [Google Scholar] [CrossRef]
- NIOSH. Health Effects of Occupational Exposure to Respirable Crystalline Silica; National Institute for Occupational Safety and Health (NIOSH), Department of Health and Human Services: Cincinnati, OH, USA, 2002. [Google Scholar]
- Akgun, M.; Araz, O.; Akkurt, I.; Eroglu, A.; Alper, F.; Saglam, L.; Mirici, A.; Gorguner, M.; Nemery, B. An epidemic of silicosis among former denim sandblasters. Eur. Respir. J. 2008, 32, 1295–1303. [Google Scholar] [CrossRef] [PubMed]
- Safe Work Australia. Crystalline Silica and Silicosis 2020. Available online: https://www.safeworkaustralia.gov.au/silica (accessed on 3 August 2020).
- Raymond, L.W.; Wintermeyer, S. Medical Surveillance of Workers Exposed to Crystalline Silica. J. Occup. Environ. Med. 2006, 48, 95–101. [Google Scholar] [CrossRef] [PubMed]
- RANZCR. Imaging of Occupational Lung Disease, Clinical Radiology, Position Statement. The Royal Australian and New Zealand College of Radiologists (RANZCR) 2019. Available online: https://www.ranzcr.com/search/silicosis-position-statement (accessed on 3 August 2020).
- Brilland, B.; Beauvillain, C.; Mazurkiewicz, G.; Rucay, P.; Roquelaure, Y.; Tabiasco, J.; Vinatier, E.; Riou, J.; Jeannin, P.; Renier, G.; et al. T Cell Dysregulation in Non-silicotic Silica Exposed Workers: A Step toward Immune Tolerance Breakdown. Front. Immunol. 2019, 10, 2743. [Google Scholar] [CrossRef] [PubMed]
- Naha, N.; Muhamed, J.; Pagdhune, A.; Sarkar, B.; Sarkar, K. Club cell protein 16 as a biomarker for early detection of silicosis. Indian J. Med. Res. 2020, 151, 319–325. [Google Scholar] [PubMed]
- RACP. Accelerated Silicosis. Royal Australasian College of Physicians (RACP) 2020. Available online: https://www.racp.edu.au/advocacy/division-faculty-and-chapter-priorities/faculty-of-occupational-environmental-medicine/accelerated-silicosis/faqs (accessed on 1 August 2020).
- ILO. ILO International Classification of Radiographs of Pneumoconioses: International Labour Organization (ILO). 2011. Available online: https://www.ilo.org/global/topics/safety-and-health-at-work/areasofwork/occupational-health/WCMS_108548/lang--en/index.htm (accessed on 6 April 2020).
- NIOSH. Specific Medical Tests or Examinations Published in the Literature for OSHA-Regulated Substances 2014. Available online: https://www.cdc.gov/niosh/docs/2005-110/nmed0205.html (accessed on 24 August 2020).
- NIOSH. Occupational Respiratory Disease Surveillance 2012. Available online: https://www.cdc.gov/niosh/topics/surveillance/ords/workermedicalmonitoring.html (accessed on 24 August 2020).
- Bandyopadhyay, A.; Majumdar, K.; Chakraborty, A.; Mitra, P.; Nag, S. CT-guided aspiration cytology of advanced silicosis and confirmation of the deposited zeolite nano particles through X ray diffraction: A novel approach. Diagn. Cytopathol. 2016, 44, 246–249. [Google Scholar] [CrossRef] [PubMed]
- Lewis, L.; Fishwick, D. Health surveillance for occupational respiratory disease. Occup. Med. 2013, 63, 322–334. [Google Scholar] [CrossRef] [PubMed][Green Version]
- McGowan, J.; Straus, S.; Moher, D.; Langlois, E.V.; O’Brien, K.K.; Horsley, T.; Aldcroft, A.; Zarin, W.; Garitty, C.M.; Hempel, S.; et al. Reporting scoping reviews—PRISMA ScR extension. J. Clin. Epidemiol. 2020, 123, 177–179. [Google Scholar] [CrossRef] [PubMed]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Godin, K.; Stapleton, J.; Kirkpatrick, S.I.; Hanning, R.M.; Leatherdale, S.T. Applying systematic review search methods to the grey literature: A case study examining guidelines for school-based breakfast programs in Canada. Syst. Rev. 2015, 4, 138. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.D. Lactate dehydrogenase as a biomarker for silica exposure-induced toxicity in agate workers. Occup. Environ. Med. 2014, 71, 578–582. [Google Scholar] [CrossRef]
- Alexopoulos, E.C.; Bouros, D.; Dimadi, M.; Serbescu, A.; Bakoyannis, G.; Kokkinis, F.P. Comparative analysis of induced sputum and bronchoalveolar lavage fluid (BALF) profile in asbestos exposed workers. J. Occup. Med. Toxicol. 2011, 6, 23. [Google Scholar] [CrossRef] [PubMed]
- Aslam, T.; Miele, A.; Chankeshwara, S.V.; Megia-Fernandez, A.; Michels, C.; Akram, A.R.; McDonald, N.; Hirani, N.; Haslett, C.; Bradley, M.; et al. Optical molecular imaging of lysyl oxidase activity—Detection of active fibrogenesis in human lung tissue. Chem. Sci. 2015, 6, 4946–4953. [Google Scholar] [CrossRef] [PubMed]
- Chao, J.; Wang, X.; Zhang, Y.; Zhu, T.; Zhang, W.; Zhou, Z.; Yang, J.; Han, B.; Cheng, Y.; Tu, X.; et al. Role of MCPIP1 in the Endothelial-Mesenchymal Transition Induced by Silica. Cell. Physiol. Biochem. 2016, 40, 309–325. [Google Scholar] [CrossRef]
- Chao, T.P.; Sperandio, E.F.; Ostolin, T.L.V.P.; Almeida, V.R.D.; Romiti, M.; Gagliardi, A.R.D.T.; Arantes, R.L.; Dourado, V.Z. Use of cardiopulmonary exercise testing to assess early ventilatory changes related to occupational particulate matter. Braz. J. Med. Biol. Res. 2018, 51. [Google Scholar] [CrossRef]
- Chu, M.; Wu, S.; Wang, W.; Mao, L.; Yu, Y.; Jiang, L.; Yuan, W.; Zhang, M.; Sang, L.; Huang, Q.; et al. miRNA sequencing reveals miRNA-4508 from peripheral blood lymphocytes as potential diagnostic biomarker for silica-related pulmonary fibrosis: A multistage study. Respirology 2020, 25, 511–517. [Google Scholar] [CrossRef]
- Chu, M.; Wu, S.; Wang, W.; Yu, Y.; Zhang, M.; Sang, L.; Tian, T.; Lu, Y.; Yuan, W.; Huang, Q.; et al. Functional variant of the carboxypeptidase M (CPM) gene may affect silica-related pneumoconiosis susceptibility by its expression: A multistage case-control study. Occup. Environ. Med. 2019, 76, 169–174. [Google Scholar] [CrossRef]
- Codorean, E.; Raducanu, A.; Albulescu, L.; Tanase, C.; Meghea, A.; Albulescu, R. Multiplex cytokine profiling in whole blood from individuals occupationally exposed to particulate coal species. Rom. Biotechnol. Lett. 2011, 16, 6748–6759. [Google Scholar]
- Corradi, M.; Gergelova, P.; Mutti, A. Use of exhaled breath condensate to investigate occupational lung diseases. Curr. Opin. Allergy Clin. Immunol. 2010, 10, 93–98. [Google Scholar] [CrossRef]
- Cox, C.W.; Lynch, D.A. Medical imaging in occupational and environmental lung disease. Curr. Opin. Pulm. Med. 2015, 21, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Dinescu, V.C.; Puiu, I.; Dinescu, S.N.; Tudorascu, D.; Bica, E.C.; Vasile, R.C.; Bunescu, M.G.; Romanescu, F.M.; Cioatera, N.; Rotaru, L.T.; et al. Early predictive biochemical, electrocardiographic and echocardiographic markers for cardiac damage in patients with pulmonic silicosis. Rev. Chim. 2019, 70, 63–68. [Google Scholar] [CrossRef]
- Ehrlich, R.I.; Myers, J.E.; Naude, J.M.T.W.; Thompson, M.L.; Churchyard, G.J. Lung function loss in relation to silica dust exposure in South African gold miners. Occup. Environ. Med. 2011, 68, 96–101. [Google Scholar] [CrossRef]
- Greabu, M.; Didilescu, A.; Puiu, L.; Miricescu, D.; Totan, A. Salivary antioxidant biomarkers in non-ferrous metals mine workers—A pilot study. J. Oral Pathol. Med. 2012, 41, 490–493. [Google Scholar] [CrossRef]
- Guo, L.; Ji, X.; Yang, S.; Hou, Z.; Luo, C.; Fan, J.; Ni, C.; Chen, F. Genome-wide analysis of aberrantly expressed circulating miRNAs in patients with coal workers’ pneumoconiosis. Mol. Biol. Rep. 2013, 40, 3739–3747. [Google Scholar] [CrossRef]
- Johnsen, H.L.; Bugge, M.D.; Føreland, S.; Kjuus, H.; Kongerud, J.; Søyseth, V. Dust exposure is associated with increased lung function loss among workers in the Norwegian silicon carbide industry. Occup. Environ. Med. 2013, 70, 803–809. [Google Scholar] [CrossRef]
- Kahraman, H.; Koksal, N.; Cinkara, M.; Ozkan, F.; Sucakli, M.H.; Ekerbicer, H. Pneumoconiosis in dental technicians: HRCT and pulmonary function findings. Occup. Med. 2014, 64, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Kamaludin, N.H.; Jalaludin, J.; Tamrin, S.B.M.; Akim, A.M. Biomarker of occupational airways inflammation for exposure to inorganic dust. Future Food 2018, 6, 23–28. [Google Scholar]
- Larici, A.R.; Mereu, M.; Franchi, P. Imaging in occupational and environmental lung disease. Curr. Opin. Pulm. Med. 2014, 20, 205–211. [Google Scholar] [CrossRef]
- Lee, S.; Honda, M.; Yamamoto, S.; Kumagai-Takei, N.; Yoshitome, K.; Nishimura, Y.; Sada, N.; Kon, S.; Otsuki, T. Role of nephronectin in pathophysiology of silicosis. Int. J. Mol. Sci. 2019, 20, 2581. [Google Scholar] [CrossRef]
- Lee, W.J.; Choi, B.S. Reliability and Validity of Soft Copy Images Based on Flat-panel Detector in Pneumoconiosis Classification. Comparison with the Analog Radiographs. Acad. Radiol. 2013, 20, 746–751. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.J.; Choi, B.S. Utility of digital radiography for the screening of pneumoconiosis as compared to analog radiography: Radiation dose, image quality, and pneumoconiosis classification. Health Phys. 2012, 103, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.J.; Choi, B.S.; Kim, S.J.; Park, C.K.; Park, J.S.; Tae, S.; Hering, K.G. Development of standard digital images for pneumoconiosis. J. Korean Med. Sci. 2011, 26, 1403–1408. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.J.; Wang, P.; Jiao, J.; Han, L.; Lu, Y.M. Differential gene expression associated with inflammation in peripheral blood cells of patients with pneumoconiosis. J. Occup. Health. 2016, 58, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Laney, A.S.; Wang, M.K.; Sun, X.; Zhou, S.; Shi, J.; Shi, H. Comparison of digital direct readout radiography with conventional film-screen radiography for the recognition of pneumoconiosis in dust-exposed Chinese workers. J. Occup. Health 2011, 53, 320–326. [Google Scholar] [CrossRef]
- Miao, R.; Ding, B.; Zhang, Y.; Xia, Q.; Li, Y.; Zhu, B. Proteomic profiling change during the early development of silicosis disease. J. Thorac. Dis. 2016, 8, 329–341. [Google Scholar] [CrossRef]
- Nardi, J.; Nascimento, S.; Göethel, G.; Gauer, B.; Sauer, E.; Fao, N.; Cestonaro, L.; Peruzzi, C.; Souza, J.; Garcia, S.C. Inflammatory and oxidative stress parameters as potential early biomarkers for silicosis. Clin. Chim. Acta 2018, 484, 305–313. [Google Scholar] [CrossRef]
- Okumura, E.; Kawashita, I.; Ishida, T. Development of CAD based on ANN analysis of power spectra for pneumoconiosis in chest radiographs: Effect of three new enhancement methods. Radiol. Phys. Technol. 2014, 7, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Ophir, N.; Shai, A.B.; Alkalay, Y.; Israeli, S.; Korenstein, R.; Kramer, M.R.; Fireman, E. Artificial stone dust-induced functional and inflammatory abnormalities in exposed workers monitored quantitatively by biometrics. ERS Monogr. 2016, 2, 00086-2015. [Google Scholar] [CrossRef]
- Pelclová, D.; Fenclova, Z.; Vlckova, S.; Lebedova, J.; Syslová, K.; Pecha, O.; Belacek, J.; Navratil, T.; Kuzma, M.; Kacer, P. Leukotrienes B4, C4, D4 and E4 in the exhaled breath condensate (EBC), blood and urine in patients with pneumoconiosis. Ind. Health 2012, 50, 299–306. [Google Scholar]
- Pelclova, D.; Fenclova, Z.; Syslova, K.; Vlčková, Š.; Lebedová, J.; Pecha, O.; Běláček, J.; Navrátil, T.; Kuzma, M.; Kačer, P. Oxidative stress markers in exhaled breath condensate in lung fibroses are not significantly affected by systemic diseases. Ind. Health 2011, 49, 746–754. [Google Scholar] [CrossRef]
- Sato, T.; Saito, Y.; Inoue, S.; Shimosato, T.; Takagi, S.; Kaneko, T.; Ishigatsubo, Y. Serum heme oxygenase-1 as a marker of lung function decline in patients with chronic silicosis. J. Occup. Environ. Med. 2012, 54, 1461–1466. [Google Scholar] [CrossRef]
- Sauni, R.; Oksa, P.; Lehtimäki, L.; Toivio, P.; Palmroos, P.; Nieminen, R.; Moilanen, E.; Uitti, J. Increased alveolar nitric oxide and systemic inflammation markers in silica-exposed workers. Occup. Environ. Med. 2012, 69, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Şener, M.U.; Şimşek, C.; Özkara, Ş.; Evran, H.; Bursali, İ.; Gökçek, A. Comparison of the international classification of high-resolution computed tomography for occupational and environmental respiratory diseases with the international labor organization international classification of radiographs of pneumoconiosis. Ind. Health 2019, 57, 495–502. [Google Scholar] [CrossRef]
- Sundararajan, R.; Xu, H.; Annangi, P.; Tao, X.; Sun, X.; Mao, L. A multiresolution support vector machine based algorithm for pneumoconiosis detection from chest radiographs 2010. In Proceedings of the 2010 IEEE International Symposium on Biomedical Imaging: From Nano to Macro, Rotterdam, The Netherlands, 14–17 April 2010. [Google Scholar]
- Syslova, K.; Kačer, P.; Kuzma, M.; Pankracova, A.; Fenclova, Z.; Vlčková, Š.; Lebedova, J.; Pelclova, D. LC-ESI-MS/MS method for oxidative stress multimarker screening in the exhaled breath condensate of asbestosis/silicosis patients. J. Breath Res. 2010, 4, 017104. [Google Scholar] [CrossRef] [PubMed]
- Trakultaweesuk, P.; Chaiear, N.; Boonsawat, W.; Khiewyoo, J.; Krisorn, P.; Silanun, K. Spirometry changes in normal or early ILO pneumoconiosis radiographs of sandstone-dust exposed workers: A preliminary result. J. Med. Assoc. Thail. 2017, 100, 1035–1044. [Google Scholar]
- Uygur, F.; Ornek, T.; Tanriverdi, H.; Altuntas, M.; Altinsoy, B.; Erboy, F.; Tor, M.; Atalay, F. Platelet Indices in Patients with Coal Workers’ Pneumoconiosis. Lung 2016, 194, 675–679. [Google Scholar] [CrossRef] [PubMed]
- Weissman, D.N. Role of chest computed tomography in prevention of occupational respiratory disease: Review of recent literature. Semin. Respir. Crit. Care Med. 2015, 36, 433–448. [Google Scholar] [CrossRef]
- Xing, J.; Huang, X.; Yang, L.; Liu, Y.; Zhang, H.; Chen, W. Comparison of high-resolution computerized tomography with film-screen radiography for the evaluation of opacity and the recognition of coal workers’ pneumoconiosis. J. Occup. Health 2014, 56, 301–308. [Google Scholar] [CrossRef]
- Xue, C.; Wu, N.; Li, X.; Qiu, M.; Du, X.; Ye, Q. Serum concentrations of Krebs von den Lungen-6, surfactant protein, D.; and matrix metalloproteinase-2 as diagnostic biomarkers in patients with asbestosis and silicosis: A case-control study. BMC Pulm. Med. 2017, 17, 144. [Google Scholar] [CrossRef]
- Yang, H.-Y.; Shie, R.-H.; Chang, C.-J.; Chen, P.-C. Development of breath test for pneumoconiosis: A case-control study. Respir. Res. 2017, 18, 1–8. [Google Scholar] [CrossRef]
- Young, C.; Barker, S.; Ehrlich, R.; Kistnasamy, B.; Yassi, A. Computer-aided detection for tuberculosis and silicosis in chest radiographs of gold miners of South Africa. Int. J. Tuberc. Lung Dis. 2020, 24, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Xu, H.; Zhu, Y.; Yang, C.; Sun, X.; Zhao, J. An Automatic Computer-Aided Detection Scheme for Pneumoconiosis on Digital Chest Radiographs. J. Digit. Imaging 2011, 24, 382–393. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.H.; Li, L.; Hao, C.; Ren, J.C.; Zhang, H.; Jiao, J.; Gao, L.; Ding, S.; Yao, S.; Yao, W.; et al. Screening and Preliminary Verification of a Phage Display Single-Chain Antibody Library Against Coal Workers’ Pneumoconiosis. J. Occup. Environ. Med. 2016, 58, 1264–1269. [Google Scholar] [CrossRef]
- Zhao, W.; Xu, R.; Hirano, Y.; Tachibana, R.; Kido, S.; Suganuma, N. Classification of pneumoconiosis on HRCT images for computer-aided diagnosis. IEICE Trans. Inf. Syst. 2013, 96, 836–844. [Google Scholar] [CrossRef]
- Zhu, L.; Zheng, R.; Jin, H.; Zhang, Q.; Zhang, W. Automatic detection and recognition of silicosis in chest radiograph. Bio.-Med. Mater. Eng. 2014, 24, 3389–3395. [Google Scholar] [CrossRef]
- TSANZ. Standards for Spirometry Training Courses Companion Document to Standards for the Delivery of Spirometry for Coal Mine Workers. Thoracic Society of Australia and New Zealand (TSANZ) 2017. Available online: https://www.dnrme.qld.gov.au/__data/assets/pdf_file/0004/1274422/tsanz-spirometry-training-standards.pdf (accessed on 22 June 2020).
- Tamura, T.; Suganuma, N.; Hering, K.G.; Vehmas, T.; Itoh, H.; Akira, M.; Takashima, Y.; Hirano, H.; Kusaka, Y. Relationships (I) of International Classification of High-resolution Computed Tomography for Occupational and Environmental Respiratory Diseases with the ILO International Classification of Radiographs of Pneumoconioses for parenchymal abnormalities. Ind. Health 2015, 53, 260–270. [Google Scholar] [CrossRef]
- Newbigin, K.; Parsons, R.; Deller, D.; Edwards, R.; McBean, R. Stonemasons with silicosis: Preliminary findings and a warning message from Australia. Respirology 2019, 24, 1220–1221. [Google Scholar] [CrossRef] [PubMed]
- Cox, C.W.; Rose, C.S.; Lynch, D.A. State of the art: Imaging of occupational lung disease. Radiology 2014, 270, 681–696. [Google Scholar] [CrossRef] [PubMed]
- Bacchus, L.; Shah, R.D.; Chung, J.H.; Crabtree, T.P.; Heitkamp, D.E.; Iannettoni, M.D.; Johnson, G.B.; Jokerst, C.; McComb, B.L.; Saleh, A.G.; et al. ACR Appropriateness Criteria Review ACR Appropriateness Criteria® Occupational Lung Diseases. J. Thorac. Imaging 2016, 31, W1–W3. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: Oxidants and antioxidants. Exp. Physiol. 1997, 82, 291–295. [Google Scholar] [CrossRef]
- Schulte, P.A. The use of biomarkers in surveillance, medical screening, and intervention. Mutat. Res./Fundam. Mol. Mech. Mutagen. 2005, 592, 155–163. [Google Scholar] [CrossRef]
- Thakkar, L.R.; Pingle, S.K.; Tumane, R.G.; Soni, P.N. Expression of Low Molecular Mass Proteins in Stone Quarry Workers. Natl. J. Lab. Med. 2013, 2, 8–11. [Google Scholar]
- Pandey, J.K.; Agarwal, D. Biomarkers: A potential prognostic tool for silicosis. Indian J. Occup. Environ. Med. 2012, 16, 101–107. [Google Scholar] [CrossRef]
- Horváth, I.; Barnes, P.J.; Loukides, S.; Sterk, P.J.; Högman, M.; Olin, A.C.; Amann, A.; Antus, B.; Baraldi, E.; Bikov, A.; et al. A European Respiratory Society technical standard: Exhaled biomarkers in lung disease. Eur. Respir. J. 2017, 49, 1600965. [Google Scholar] [CrossRef] [PubMed]
- Leese, E.; Staff, J.F.; Carolan, V.A.; Morton, J. Exhaled Breath Condensate: A Novel Matrix for Biological Monitoring to Assess Occupational Exposure to Respirable Crystalline Silica. Ann. Work. Expo. Health 2017, 61, 902–906. [Google Scholar] [CrossRef] [PubMed]
| Activity | Industries or Occupational Activities |
|---|---|
| Drilling | Construction, quarrying and related milling, mining and related milling, tunneling |
| Breaking and crushing | Construction, quarrying and related milling, mining and related milling, tunneling |
| Cutting | Arts, crafts, and sculpture, jewelry, construction, quarrying and related milling, grindstone production |
| Abrasive blasting and sand blasting | Boiler scaling, production of dental material, metal products, automobile repair (removal of paint and rust), arts, crafts, and sculpture, shipbuilding and repair, foundries, construction, quarrying and related milling, production of denim jeans, tombstone production |
| Grinding | Arts, crafts, and sculpture, jewelry, construction, quarrying and related milling |
| Sanding | Automobile repair (removal of paint and rust), construction |
| Excavation and digging | Agriculture, construction, quarrying and related milling, mining and related milling, tunneling |
| Hammering | Boiler scaling, construction |
| Casting and moulding | Jewelry, foundries, ceramics |
| Furnace installation and repair (refractory materials) | Iron and steel mills, foundries, glass |
| Cleaning (dry sweeping and brushing, pressurised air blowing) | Construction, arts, crafts, and sculpture, jewelry |
| Polishing and buffing | Production of dental material, arts, crafts, and sculpture, jewelry |
| Author (Year) | Objective/s | Type of Study (Cross-Sectional, Clinical Trial, Longitudinal, Review) | Population/Exposure (Years) | Population Size | Gender | Age (Years) | Respiratory Surveillance Method/S Used | Outcomes | Location |
|---|---|---|---|---|---|---|---|---|---|
| Aggarwal [31] | Investigate total lactate dehydrogenase (LDH) activity in blood samples as a non-invasive method to measure silica-induced toxicity. First study to estimate LDH activity in blood cells of silica-exposed agate workers and controls. Proposes LDH activity as a diagnostic tool for early silica exposure-induced cytotoxicity. | Cross-sectional | Silica-exposed agate workers | Exposed workers: 35 Control subjects: 27 | Exposed workers: 21 male | Exposed workers: 42 ± 11 | Blood sample | Blood cells: LDH activity significantly higher (~10×) in control subjects, suggesting the blood cells of exposed workers may have been damaged directly or indirectly by silica exposure | India |
| Exposure: 16 ± 8 | Control subjects:27 male | Control subjects: 31 ± 9 | Blood plasma: LDH activity is higher (~25×) in exposed workers, suggesting that silica exposure may have induced cellular and tissue injuries, with more extracellular LDH enzyme released into blood plasma | ||||||
| Alexopoulos et al. [32] | Compare cellular profiles of asbestosis-exposed workers using induced sputum (IS) and bronchoalveolar lavage fluid (BALF) to test the usefulness of IS in monitoring workers over an extended period. Validate screening tool for biological | Cross-sectional | Workers at a car brakes and clutches factory that uses chrysotile asbestos | Workers: 39 | Workers: 24 male | 37–53 | Questionnaire, Bronchoscopy, Induced sputum | Findings detected significant correlations between IS and BALF cellular profiles. This indicates that IS sampling, a less invasive and expensive method, may provide useful insights both for inhalation of dusts and inflammatory processes in the lung. | Romania |
| Exposure: >15 | |||||||||
| Absence of diagnosis of pneumoconiosis | |||||||||
| Aslam et al. [33] | Develop fluorescence-based analysis tool to monitor in real-time the LOX enzyme activities in vitro and in vivo of patients with fibrogenesis. These powerful tools are a simple and effective method of monitoring. | Cross-sectional | Human and asinine ex vivo tissue models | Human lung samples: 17 | N/A | Human lung samples: 55–81 | Lung samples from carcinoma resections, Biopsy, ex vivo asinine lung samples | Successful design of an activity-based fluorescent probe that quantifies in real-time the LOXF activity in fibrogenic conditions. This probe has the potential to image real-time LOXF activity within the lungs of patients. | United Kingdom |
| Number not stated | Asinine lungs: aged | ||||||||
| Brilland et al. [20] | Analyse the impact of crystalline silica on T cell phenotype and regulatory T cells (Tregs) frequency. | Prospective cohort | Workers with moderate to high levels of exposure | Exposed workers: 55 | Males | Exposed workers: 41.30 ± 6.52 | Clinical examination, chest X-ray, pulmonary function test, blood sampling | Alterations of the T cell compartment can be detected early during the course of crystalline silica exposure, hence preceding the development of silicosis or autoimmune diseases. | France |
| Two cohorts of HC; group 1 = 42 and group 2 = 45 | Control group 1: 41.67 ± 12.59 Control group 2: 42.06 ± 8.89 | ||||||||
| Chao et al. [34] | Investigate the mechanisms underlying endothelial-mesenchymal transition (EndMT) | Lab based | N/A | N/A | N/A | N/A | N/A—lab based | Findings suggest MCPIP1-induced EndMT in endothelial cells plays an important role in the development of silicosis. | China |
| Chao et al. [35] | Investigate if cardiopulmonary exercise testing may be better than spirometry when used to detect early signs of damage caused by occupational exposure to particulate matter. First study to focus on early detection in asymptomatic participants. | Cross-sectional | Male workers from the Epidemiology and Human Movement Study (EPIMOV). Completed a validated occupational respiratory questionnaire to determine occupational exposure to particulate matter. | Exposed = 52 | Male only | >18 | Male workers exhibited ventilatory alterations during exercise, even with normal pulmonary function at rest. Findings suggest ∆VT/∆InVE may be the most appropriate variable from the CPET to differentiate workers with incipient ventilatory changes. CPET may be useful in the prevention of occupational respiratory diseases. | Brazil | |
| Control = 83 | |||||||||
| Chu et al. [4] | To systematically evaluate genetic variants that were associated with pneumoconiosis susceptibility. | Three-stage case-control | Exposed coal and metalliferous underground miners | Cases: 202 | N/A | N/A | Physical examination, radiograph, genome samples | Identified a genome-wide significant association and two additional replicated associations for pneumoconiosis susceptibility in Han Chinese. | China |
| Control: 198 | |||||||||
| Chu et al. [36] | Identify miRNA as potential diagnostic biomarkers for silica-related pulmonary fibrosis. | Three-stage case-control | See Table 1 in article | Cases: 67 | See Table 1 in article | See Table 1 in article | Blood samples | miRNA-4508 may be a potential diagnostic marker for silica-related pulmonary fibrosis, and a functional variant of miRNA-4508 may affect susceptibility. | China |
| Controls: 67 | |||||||||
| Chu et al. [37] | Investigate the causal variants of chromosome 12q15 in silicosis susceptibility. | Case-control | Case: 24.58 ± 7.00 | Cases:177 | Case: Male 89.27% | Case: 67.70 ± 8.49 | Blood samples | A variant of the carboxypeptidase M (CPM) gene may increase silicosis susceptibility. Provide insight into the aetiology and biological mechanisms of silicosis. Assist in identification of high-risk individuals with occupational silica-exposure. | China |
| Control: 24.05 ± 5.69 | Healthy controls: 204 | Control: 84.80% | Control: 60.26 ± 6.35 | ||||||
| Codorean et al. [38] | Perform exploratory study on peripheral whole-blood to analyse early effects of exposure in coal fired power plants. | Cross-sectional | Three groups: 10 years; 20 years; control | N/A | N/A | N/A | Blood samples | This method is non-invasive and rapid and could be a useful tool in identifying early hazard before it is diagnosed clinically. | Romania |
| Corradi et al. [39] | Review EBC studies that investigate exposure and effect biomarkers in lung disease, particularly toxic metals | Review | N/A | N/A | N/A | N/A | Exhaled breath condensate (EBC) | Exhaled breath biomarkers have been shown to be capable of detecting and monitoring diseases of the respiratory system. | N/A |
| Cox and Lynch [40] | Provide review of recent developments in medical imaging of environmental lunch disease. | Review | N/A | N/A | N/A | N/A | Medical imaging | Medical imaging is useful in the diagnosis, epidemiological study and management of occupational lung disease. Studies that compare HRCT with film-screen radiography found CT was more sensitive. | N/A |
| Dinescu et al. [41] | Identify correlations between electrocardiographic and echocardiographic changes in patients with silicosis prior to chronic pulmonary heart disease occurring. | Prospective, descriptive, analytical | N/A | Cases: 67 | N/A | Cases: 477–8 years | Electrocardiograph, echocardiograph | Values of the right heart echocardiographic parameters at the upper limit of normality are early markers for cardiovascular damage in patients with silicosis. | Romania |
| Control: 25 | |||||||||
| Doganay et al. [3] | Assess MDCT findings of silicosis in denim sandblasters and define the role of MDCT in the early detection of silicosis. | Cross-sectional | Denim sandblasters | 12 male patients admitted to a pulmonary outpatient clinic between April-December 2009. | Male only | 19–25 years | CT | Silicosis may cause immediate mortality, especially in young people. MDCT can play an important role in the early detection of silicosis. | Turkey |
| 1.0–5.2 years | 21.2 ± 1.2 | ||||||||
| 3.7 ± 1.4 | |||||||||
| Ehrlich et al. [42] | Estimate the effect of respirable dust and quartz exposure on spirometric lung function. | Cross-sectional | Black South African gold miners | 520 mine workers | Not reported | 37–60 years | Questionnaires, X-ray, spirometry | Study demonstrated significant lung function loss attributable to dust exposure, mediated by silicosis, pulmonary TB and/or an independent dust effect. | South Africa |
| 6.3–34.5 years | Mean = 46.7 years | ||||||||
| Mean = 21.8 years | |||||||||
| Greabu et al. [43] | Evaluate the relationships between occupational exposure to mine dust and salivary antioxidants, blood uric acid and the possible implications for the causes of diseases caused by exposure. | Cross-sectional | Long-term occupational exposure in non-ferrous metal mines | Exposed workers: 30 | Not reported | Exposed: 44.3 (SD) 4.5 | Saliva samples | First study to describe saliva and serum parameters involved in antioxidant protection and metabolic regulations in non-ferrous metal miners. | Romania |
| Control: 30 | Control: 51.3 (SD) 5.6 | ||||||||
| Guo et al. [44] | Survey and identify differentially expressed circulating miRNAs by miRNA deep sequencing blood samples from patients at varying stages of CWP. | Case-control | N/A | Cases: 30 | N/A | N/A | Blood samples | Demonstrated that expressed circulating miRNAs showed dynamic expression patterns across diseased samples. This suggests these miRNAs may have critical roles in the occurrence and development of CWP. | China |
| Controls:10 n = 456 | |||||||||
| Johnsen et al. [45] | Investigate the relationship between dust exposure and annual change in lung function among Norwegian silicon carbide exposed workers using a quantitative job matrix (JEM) regarding total dust | Longitudinal cohort | Workers in Norwegian silicon carbide plants | See Table S2a,b. Examinations = 1499 | N/A | N/A | Questionnaires, spirometry, JEM (dust exposure matrix) | Dust exposure, expressed by quantitative JEM, was found to be associated with an increased yearly decline in FEV1. A dose-response relationship was found. | Norway |
| Kahraman et al. [46] | Document pulmonary function and prevalence of pneumoconiosis in dental prosthetic technicians | Cross-sectional | Dental prosthetic technicians 16.7 ± 8.4 (4–43) | n = 76 | Male | 32 ± 8, (18–55) | Physical examination, Pulmonary function test, HRCT | First prevalence study in dental prosthetic technicians using HRCT. Pneumoconiosis was detected in 46%, possible because HRCT is able to detect very early changes. | Turkey |
| Kamaludin et al. [47] | Determine biomarker to be used in diagnosis of occupational airways inflammation from occupational inorganic dust exposure. | Review | N/A | N/A | N/A | N/A | Biomarkers | Three biomarkers were identified. | Malaysia |
| Larici et al. [48] | Highlight the current role of imaging, describe classic as well as uncommon HRCT patterns helpful in guiding diagnosis. | Review | N/A | N/A | N/A | N/A | HRCT | HRCT is the best imaging modality. Imaging plays a role in diagnosis, surveillance, and prediction. | N/A |
| Lee et al. [49] | Review the roles of previously identified molecules in silicosis-related lung fibrosis from the literature. | Review | N/A | N/A | N/A | N/A | Biomarkers | Serum Npnt was higher in silicosis patients compared to healthy controls. Serum Npnt seems to play a role in progression of fibrosis with other cytokines, and may therefore be a suitable biomarker. | N/A |
| Lee and Choi [50] | Evaluate the reliability and validity of soft copy images based on flat-panel detector of digital radiography compared to analog radiographs in pneumoconiosis classification and diagnosis. | Cross-sectional | Retired workers exposed to inorganic dusts | n = 349 | N/a | 62.4 ± 7.8 | Digital and analog radiography | Flat-panel detector of digital radiography soft copy images showed more accurate and reliable results in pneumoconiosis classification and diagnosis than analog radiographs. | Korea |
| 19.8 ± 8.1 | |||||||||
| Lee and Choi [51] | Compare digital and analog radiography for screening of pneumoconiosis with respect to radiation dose, image quality, and classification. | Cross-sectional | Exposed to inorganic dust | n = 531 | Male | 61.1 ± 8.3 (43–79) | Digital and analog radiography | Compared to analog radiography, digital radiography provides improved image quality with a significant reduction of up to 23.6% in radiation dose and more accurate pneumoconiosis classification. | Korea |
| 19.5 ± 8.2 (3–45) | |||||||||
| Lee et al. [52] | Develop an improved set of standard digital images to be used in the recognition and classification of pneumoconiosis. | Cross-sectional | Exposed to inorganic dust | n = 531 | Male | 63.1 ± 7.9 (42–84) | Digital and analog radiography | A set of 120 standard digital images was developed with more various pneumoconiosis findings than the ILO SARS. They can be used for the digital reference images for recognition and classification of pneumoconiosis. | Korea |
| 19.5 ± 8.2 (3–45) | |||||||||
| Lewis and Fishwick [27] | Identify areas of good practice within respiratory health surveillance and to formulate recommendations for practice | Review | N/A | N/A | N/A | N/A | N/A | Respiratory health surveillance remains relatively disparate and would benefit from standardisation. | N/A |
| Liu et al. [53] | Study expression changes in inflammation-related genes in peripheral blood of patients with pneumoconiosis and explore the possibility of these genes as biomarkers. | Cross-sectional | N/A | Various populations | Male | Various populations | Blood samples | IL6 was identified as being possibly involved in the development of pneumoconiosis. | China |
| Mao et al. [54] | Evaluate the applicability of digital radiography. | Cross-sectional | Dust exposed workers | 192 | Male 95.3% | Mean = 55.7 | Film screen and digital radiographs | Findings demonstrate that digital systems are equivalent to traditional film-screen radiography in the recognition and classification of small opacities. | China |
| McBean et al. [6] | Understand the radiological presentation of individuals diagnosed with coal mine dust lung disease since 2015 in Queensland. | Case series | Individuals identified as having coal mine dust lung disease (CMDLD) since 2015 | 79 | Male | Mean = 58.9 years (range: 35–90) | Questionnaires, X-ray and/or CT, spirometry | First study in over 30 years to investigate the radiological presentation of CMDLD in QLD, and the first ever to incorporate HRCT. Approximately 30% of subjects had advanced disease. Findings of interest included the high burden of opacities observed and the presence of RCS-related features in the majority of subjects. | Australia |
| Mean: 26.2 years (range: 6–45) | |||||||||
| Miao et al. [55] | Conduct proteomic profiling for the early stages of silicosis to investigate the pathophysiology and to identify potential candidate proteins for early diagnosis. | Case-control | Dust-exposed workers without silicosis; silicosis patients; Healthy controls | 45 | N/A | 55–64 | X-ray, blood sample | A number of proteins involved in silicosis development were identified, with a large number of proteins and peptides being dramatically altered during early development. This may contribute to future work to identify potential biomarkers. | China |
| Nardi et al. [56] | Evaluate inflammatory and oxidative stress parameters as potential early biomarkers for RCS exposure. | Case-control | CS exposed miners | Workers exposed to CS = 38 | Male | Various, see Table 1 in article | Blood sample, anthropometric measurements, | For the first time, this study suggested L-selectin surface protein expression in lymphocytes might be a potential biomarker for monitoring CS toxicity in workers with at least 16 years exposure. | Brazil |
| With silicosis = 24 | |||||||||
| Unexposed workers = 30 | |||||||||
| Okumura et al. [57] | Investigate the effects of parameters on overall classification performance. Develop enhancement methods to reduce false-positive and false-negative values in a CAD scheme for pneumoconiosis. | Retrospective, cross-sectional | N/A | N/A | N/A | N/A | Chest radiographs | Successfully developed a CAD system using three new enhancement methods for classification of pneumoconiosis chest radiographs. | Japan |
| Ophir et al. [58] | Screen exposed workers using quantitative biometric monitoring of functional and inflammatory parameters. | Case-control | Artificial stone workers | Exposed workers: 68 | Male | Exposed workers: 48.6 ± 11.4 | Questionnaires, PFT, induced sputum | Reports first application of XRF technology for quantifying elements in biological samples. PFT were significantly lower for exposed workers. Also IS in exposed workers showed significantly higher neutrophilic inflammation. Particle size in IS of exposed workers was similar to the artificial stone dust. | Israel |
| Up to 20 years | Controls: 48 | Controls: 38.0 ± 17.1 | |||||||
| Palabiyik et al. [12] | Investigate if occupational silica exposure results in alterations in neopterin levels, tryptophan degradation, and activities of superoxide dismutase and catalase, agents in the antioxidant defense system. | Case-control | Denim sandblasting workers | Silicosis patients: 55 | Male | Silicosis patients: 30 ± 1 (21–48) | Questionnaires, PFT, blood samples | Denim sandblasters exposed to silica had increased neopterin levels and tryptophan degradation confirming the possibility of their use as indicators of cellular immune response. | Turkey |
| 33.6 ± 23.8 (2 to 120) months | Controls: 22 | Controls: 36 ± 10 (18–52) | |||||||
| Pelclová et al. [59] | Evaluate the potential impact of lung fibrosis on the levels of oxidative stress markers in blood and urine of workers exposed to silica. | Case-control | Various occupations with exposure | Asbestos exposed workers: 45 | Asbestos exposed workers: 24 male | Asbestos exposed workers: 69.6 ± 2.0 | Questionnaires, physical examination, X-ray, CT, blood sample, urine sample, lung function, EBC | 8-isoprostane appears to be the optimal oxidative stress marker for respiratory disorders. HNE can be used as a marker for pneumoconiosis. Findings support the suggestion that EBC can contribute to a better understanding of the pathogenesis of silicosis. | Czech Republic |
| Silica exposed workers: 37 | Silica exposed workers: 36 male | Silica exposed workers: 69.1 ± 2.9 | |||||||
| Controls: 29 | Controls: 20 male | Controls: 67.0 ± 4.6 | |||||||
| Pelclová et al. [60] | Measure multiple markers in the EBC, plasma and urine of exposed workers to determine the possible impact of systemic disease, pharmaceuticals and diet on EBC levels. | Case-control | Various occupations with exposure | Asbestos exposed workers: 45 | Asbestos exposed workers: 24 male | Asbestos exposed workers: 69.6 ± 2.0 | Questionnaires, physical examination, X-ray, CT, blood sample, urine sample, EBC | Findings suggest that for the detection of pneumoconiosis EBC is the most useful compared to plasma and urine. | Czech Republic |
| Silica exposed workers: 37 | Silica exposed workers: 36 male | Silica exposed workers: 69.1 ± 2.9 | |||||||
| Controls: 27 | Controls: 18 male | Controls: 66.0 ± 6.9 | |||||||
| Sato et al. [61] | Identify predictive factors of excess decline in FEV1 in patients with chronic silicosis. | Cross-sectional | Exposed workers | n = 33 | Male | 73.5 ± 5.7 | Questionnaires, X-ray, spirometry, blood samples | Serum H01 may be a useful marker of lung function decline and disease progression in silicosis patients. | Japan |
| 21.9 ± 12.3 | |||||||||
| Sauni et al. [62] | Investigate responses to silica exposure, by testing the effects of silica dust on exhaled nitric oxide. | Case-control | Exposed workers in prefabrication factories, quarries and stone-cutting industry | Exposed workers: 94 | Male | Exposed workers: 60.4 (40–78) | Exhaled NO, blood samples, spirometry | Measurement of nitric oxide concentration, plasma cytokine and adipokine levels appears to offer a novel method of demonstrating the inflammatory effects of silica exposure. Measurement of exhaled NO is safe, easy to perform and inexpensive. | Finland |
| 31.0 (SD8.1) | Controls: 35 | Controls: 62.1 (49–72) | |||||||
| Şener et al. [63] | Compare the ability of chest X-ray (ILO classification) and HRCT (ICOERD) to make an early diagnosis of pneumoconiosis. | Retrospective, cross-sectional | Various exposed workers diagnosed with pneumoconiosis | 83 | Male | 44.46 ± 11.45 | Chest X-rays, CT, PFT | ILO categories and ICOERD grades were significantly correlated. HRCT performed better when detecting pneumoconiosis in an early stage, however not in evaluating pulmonary functions. | Turkey |
| Sundararajan et al. [64] | Investigate a method to automatically detect pneumoconiosis on the basis of digital chest X-rays. | Cross-sectional | N/A | N/A | N/A | N/A | Chest X-rays | The method successfully allows practitioners to classify normal versus pneumoconiosis patients. | N/A |
| Syslová et al. [65] | Determine concentration levels of oxidative stress biomarkers in the EBC of patients with pneumoconiosis. | Clinical study | Pneumoconiosis patients with exposure to silica or asbestos for 22 ± 6 years | n = 10 | Male | Patients: 69 ± 8 | EBC, | There was a statistically significant difference in biomarkers’ concentration levels between the pneumoconiosis patients and the control subjects. | Czech Republic |
| Control: 67 ± 4 | |||||||||
| Trakultaweesuk et al. [66] | Estimate FEV1 decline at one year follow-up among workers with normal or early abnormal ILO classified chest X-rays. | Descriptive, longitudinal | Exposed sandstone workers (median exposure 6.5 years) | n = 52 | Female (65.4%) | 48 ± 8.9 (27–65) | Questionnaire, spirometry, chest X-ray | A significant loss of lung function was found, despite being only a one-year follow-up. Spirometry was found to be effective in monitoring the effect of exposure on sandstone workers. | Thailand |
| Uygur et al. [67] | Investigate the relationship between platelet indices and CWP. | Case-control | Retired coal miners | Retired workers with CWP: 97 | Male | Retired workers with CWP: 61.9 ± 4.8 | Questionnaire, blood sample, chest X-ray | Platelet indices may be considered as biomarkers for the progression of pneumoconiosis. | Turkey |
| 20.5 ± 3.6 | Controls: 50 | Controls: 62.3 ± 1.9 | |||||||
| Weissman [68] | Provide update on literature relevant to using CT as a tool for preventing occupational respiratory disease. | Review | N/A | N/A | N/A | N/A | N/A | Although HRCT is more sensitive than X-ray there are insufficient data to determine the effectiveness of HRCT in improving individual outcomes. However, if HRCT is used to screen populations, the ICOERD classification has been shown to be an important tool. | N/A |
| Xing et al. [69] | Compare film-screen radiography and HRCT for the recognition of the profusion of small opacities and to evaluate the role of HRCT in CWP diagnosis. | Cross-sectional | Coal miners | Coal miners with CWP: 96 | Male | Coal miners with CWP: 49.01 ± 6.16 | Film-screen radiography, HRCT | HRCT was more sensitive than film-screen radiography in recognising the profusion of small opacities. Findings provide evidence of the advantages of HRCT in diagnosis of pneumoconiosis. | China |
| Healthy coal miners: 67 | Healthy coal miners: 47.12 ± 7.35 | ||||||||
| Controls:37 | Controls: 46.67 ± 6.76 | ||||||||
| Xue et al. [70] | Investigate and evaluate the diagnostic values of pneumocyte-derived biomarkers in various pneumoconioses. | Case-control | Patients with asbestosis/silicosis and dust exposed workers | Patients with asbestosis: 43 | Patients with asbestosis: 19 male | Patients with asbestosis: 68.2 ± 8.6 | HRCT, X-ray, blood samples, pulmonary function test | The combination of KL-6, SP-D and MMP-2 may improve the diagnostic sensitivity for asbestosis and silicosis. | China |
| Patients with silicosis: 45 | Patients with silicosis: 23 male | Patients with silicosis: 65.1 ± 11.3 | |||||||
| Dust exposed workers: 40 | Dust exposed workers: 21 male | Dust exposed workers: 63.1 ± 8.7 | |||||||
| Controls; 45 | Controls; 22 male | Controls; 65.6 ± 11.4 | |||||||
| Yang et al. [71] | Develop a breath test to detect pneumoconiosis using volatile organic compounds generated from lipid peroxidation. | Case-control | Exposed stone workers | Cases:25 | Cases: 68.0% | Cases: 60.0 (9.2) | Questionnaires, physical examination, X-ray, pulmonary function test, fractional exhaled nitric oxide test, blood and urine samples. | Analysis of VOCs in breath is a novel respiratory screening method. Three VOCs were identified as constituting a distinct fingerprint in the breath of pneumoconiosis patients, demonstrating exhaled breath could be used in screening. | Taiwan |
| Cases: 19.8 (14.5) | Controls:154 | Control: 46.1% | Controls: 50.3 (11.8) | ||||||
| Controls: 17.6 (13.8) | |||||||||
| Young et al. [72] | Evaluate the use of CAD to diagnose both TB and silicosis in a population with a high burden of both diseases. | Quantitative | N/A | N/A | N/A | N/A | X-ray | Using CAD as a mass screening tool for TB and silicosis shows promise, however current ability to differentiate between the two is limited. The successful use of CAD to streamline the process of detection requires knowledge of the local context. | South Africa |
| Yu et al. [73] | Establish an automated scheme for CAD of pneumoconiosis in X-rays | Quantitative | N/A | Normal: 300 | N/A | N/A | X-ray | Findings show high classification performances. The fully automated scheme developed in this study has a higher accuracy and a more convenient interaction compared to previous methods. Scheme may be helpful to clinicians using CAD for mass chest screening and interpreting and differentiating between normal and pneumoconiosis cases. | China |
| Pneumoconiosis: 125 | |||||||||
| Zhang et al. [74] | Construct a phage display human antibody library (PDHAL) against pneumoconiosis for the diagnosis and treatment of CWP. | Case-control | N/A | Patients with pneumoconiosis: 25 | Male | Coal workers: 37.51 ± 6.75 | Blood samples, BALF | A PDHAL against CWP was established and six strong positive clones were selected, sequenced and identified. Protective factors were identified. Serum and antibodies that could be used as potential biomarkers for the diagnosis and treatment of CWP were identified. | China |
| Coal workers with CWP: 558 | Controls: 36.88 ± 9.39 | ||||||||
| Coal workers without CWP: 309 | |||||||||
| Control: 393 | |||||||||
| Zhao et al. [75] | Describe a CAD method to classify pneumoconiosis on HRCT images. | Quantitative | N/A | Subjects:112 | N/A | N/A | HRCT | Findings indicate that the method developed could be helpful in classifying pneumoconiosis on HRCT. | Japan |
| HRCT scans: 175 | |||||||||
| Zhu et al. [76] | Propose a multi-scale opacity detection approach to detect suspected opacities from X-ray | Quantitative | N/A | N/A | N/A | N/A | X-ray | Findings demonstrate the approach to be effective in detecting and recognising silicosis opacity. The approach successfully revealed changes in silicosis pathology and may be adopted as an appropriate tool for automatic silicosis diagnosis. | China |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Austin, E.K.; James, C.; Tessier, J. Early Detection Methods for Silicosis in Australia and Internationally: A Review of the Literature. Int. J. Environ. Res. Public Health 2021, 18, 8123. https://doi.org/10.3390/ijerph18158123
Austin EK, James C, Tessier J. Early Detection Methods for Silicosis in Australia and Internationally: A Review of the Literature. International Journal of Environmental Research and Public Health. 2021; 18(15):8123. https://doi.org/10.3390/ijerph18158123
Chicago/Turabian StyleAustin, Emma K., Carole James, and John Tessier. 2021. "Early Detection Methods for Silicosis in Australia and Internationally: A Review of the Literature" International Journal of Environmental Research and Public Health 18, no. 15: 8123. https://doi.org/10.3390/ijerph18158123
APA StyleAustin, E. K., James, C., & Tessier, J. (2021). Early Detection Methods for Silicosis in Australia and Internationally: A Review of the Literature. International Journal of Environmental Research and Public Health, 18(15), 8123. https://doi.org/10.3390/ijerph18158123






