Mutagenicity Assessment to Pesticide Adjuvants of Toluene, Chloroform, and Trichloroethylene by Ames Test
Abstract
:1. Introduction
2. Materials and Methods
2.1. Origin of Chemicals
2.2. Test Stains
2.3. Salmonella Typhimurium Reverse Mutation Test (Ames Test)
2.4. Statistical Analysis
3. Results
3.1. Negative Control Results
3.2. Ames Test for Toluene
3.3. Ames Test for Chloroform
3.4. Ames Test for Trichloroethylene
3.5. Ames Test for the Mixtures of Toluene, Chloroform, and Trichloroethylene
4. Discussion
4.1. Mutagenicity Analysis
4.2. Mutagenicity Change by S9 Liver Particles Metabolism
4.3. The Mixed Test Results
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- The State Bureau of Quality and Technical Supervision, Pesticide Emulsifiers-Terms. GB/T 17515-1998. Available online: https://std.samr.gov.cn/gb/search/gbDetailed?id=71F772D796E7D3A7E05397BE0A0AB82A (accessed on 26 July 2021).
- Pesticide Formulation and Adjuvant Technology. Available online: https://www.routledge.com/Pesticide-Formulation-and-Adjuvant-Technology/Foy-Pritchard/p/book/9780367448561 (accessed on 26 July 2021).
- Li, H.; Jiang, Z.; Cao, X.; Su, H.; Shao, H.; Jin, F.; Zheng, L.; El-Aty, A.M.A.; Wang, J. SPE/GC–MS Determination of 2-Pyrrolidone, N-Methyl-2-pyrrolidone, and N-Ethyl-2-pyrrolidone in Liquid Pesticide Formulations. Chromatographia 2018, 81, 359–364. [Google Scholar] [CrossRef]
- Kong, X.J.; Wei, X.; Wang, N.; Bu, Y.-Q.; Shan, Z.J. A Review on the Environmental Behavior of the Polyoxyethylene Type Nonionic Surfactants Adjuvants in Pesticides. J. Agric. Resour. Environ. 2017, 34, 197. [Google Scholar]
- Li, G.-R.; Yu, W.-Q.; Xiao, Z.J.; Gong, Y.K.; Lu, J.-L.; Wang, J.S. Research Status of Toxicity and Residual Detection of High-risk Pesticide Adjuvants. Agrochemicals 2019, 10, 26–30. [Google Scholar]
- Surgan, M.; Condon, M.; Cox, C. Pesticide Risk Indicators: Unidentified Inert Ingredients Compromise Their Integrity and Utility. Environ. Manag. 2010, 45, 834–841. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Sidhu, V.; Rong, Y.; Zheng, Y. Pesticide Pollution in Agricultural Soils and Sustainable Remediation Methods: A Review. Curr. Pollut. Rep. 2018, 4, 240–250. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency Office of Communications Education and Public Affairs. Terms of Environment: Glossary, Abbreviations, and Acronyms; United States Environmental Protection Agency: Washington, D.C. USA, 1993. [Google Scholar]
- List of Minimal Risk Inert Ingredients (List 4A) | Pesticides |; US EPA: Washington, DC, USA, 1993.
- Jiang, D.; Cheng, Z.; Chen, X.; Dong, F.; Xu, J.; Liu, X.; Wu, X.; Pan, X.; An, X.; Zheng, Y. Occurrences of eight common-used pesticide adjuvants in ten vegetable species and implications for dietary intake in North China. Food Chem. 2021, 347, 128984. [Google Scholar] [CrossRef]
- Li, G.; Yu, W.; Xiao, Z.; Long, M.; Tong, L.; Qiu, Y. A modified QuEChERS/GC–MS for simultaneous determination of 16 pesticide adjuvant residues in fruits and vegetables. SN Appl. Sci. 2020, 2, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.; Lin, F.; Zhang, R.; Yu, W.; Lu, N. Differential sensitivity of two green algae, Scenedesmus quadricauda and Chlorella vulgaris, to 14 pesticide adjuvants. Ecotoxicol. Environ. Saf. 2004, 58, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Mesnage, R.; Bernay, B.; Séralini, G. Ethoxylated adjuvants of glyphosate-based herbicides are active principles of human cell toxicity. Toxicology 2013, 313, 122–128. [Google Scholar] [CrossRef]
- Mesnage, R.; Defarge, N.; Spiroux, d.V.J.; Séralini, G. Major Pesticides Are More Toxic to Human Cells Than Their Declared Active Principles. BioMed Res. Int. 2014, 2014, 179691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mansano, A.S.; Moreira, R.A.; Pierozzi, M.; Oliveira, T.M.A.; Vieira, E.M.; Rocha, O.; Regali-Seleghim, M.H. Effects of diuron and carbofuran pesticides in their pure and commercial forms on Paramecium caudatum: The use of protozoan in ecotoxicology. Environ. Pollut. 2016, 213, 160–172. [Google Scholar] [CrossRef]
- Chen, L.; Yan, Q.; Zhang, J.; Yuan, S.; Liu, X. Joint Toxicity of Acetamiprid and Co-Applied Pesticide Adjuvants on Honeybees under Semifield and Laboratory Conditions. Environ. Toxicol. Chem. 2019, 38, 1940–1946. [Google Scholar] [CrossRef] [PubMed]
- Nobels, I.; Spanoghe, P.; Haesaert, G.; Robbens, J.; Blust, R.; Lin, B. Toxicity Ranking and Toxic Mode of Action Evaluation of Commonly Used Agricultural Adjuvants on the Basis of Bacterial Gene Expression Profiles. PLoS ONE 2011, 6, e24139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.X.; Zhang, Z.J.; Fei, Y.H.; Qian, Y.; Cui, X.X.; Guo, C.Y.; Ma, Z.-X. Preliminary Research of the Effect of Rainfall on Toluene Migration in Soil. J. Southwest Univ. Nat. Sci. Ed. 2015. [Google Scholar] [CrossRef]
- Spatial Distribution Characteristics and Vertical Migration Analysis of Trichloromethane in a Contaminated Site. Available online: https://en.cnki.com.cn/Article_en/CJFDTotal-KTAQ201802013.htm (accessed on 26 July 2021).
- Investigation of Volatile Organic Compounds Contamination in Soil of a Pesticide Production Site in Changzhou. Available online: https://en.cnki.com.cn/Article_en/CJFDTotal-IAOB201203016.htm (accessed on 26 July 2021).
- Fan, Y.; Zhou, Q.; Wang, Y.; Zhu, S. Toxic effects of BTEX in water on Daphnia magna and Limnodrilus hoffmeisteri and safety assessment of the aquatic environment. Acta Sci. Circumstantiae 2009, 29, 1485–1490. [Google Scholar]
- Bp, A.; Sr, A.; Yok, B.; Ag, C.; Mse, C.; Ad, C.; Aah, C.; Sa, D.; Hjk, E. Prophylactic efficacy of Boerhavia diffusa L. aqueous extract in toluene induced reproductive and developmental toxicity in Drosophila melanogaster. J. Infect. Public Health 2020, 13, 177–185. [Google Scholar]
- Ahmed, N.; Ok, Y.S.; Jeon, B.H.; Kim, J.R.; Chae, K.J.; Oh, S.E. Assessment of benzene, toluene, ethyl-benzene, and xylene (BTEX) toxicity in soil using sulfur-oxidizing bacterial (SOB) bioassay. Chemosphere 2019, 220, 651–657. [Google Scholar] [CrossRef]
- Bácsi, I.; Török, T.; B-Béres, V.; Török, P.; Vasas, G. Laboratory and microcosm experiments testing the toxicity of chlorinated hydrocarbons on a cyanobacterium strain (Synechococcus PCC 6301) and on natural phytoplankton assemblages. Hydrobiologia 2013, 710, 189–203. [Google Scholar] [CrossRef]
- Liang, J.C.; Hsu, T.C.; Henry, J.E. Cytogenetic assays for mitotic poisons. The grasshopper embryo system for volatile liquids. Mutat. Res. 1983, 113, 467–479. [Google Scholar] [CrossRef]
- Jin, H.M.; Ren, F.; Yue, C.; Cai, Y.X.; Chen, T.; Jiang, Y. Cardiac developmental toxicity of trichloroethylene. J. Environ. Occup. Med. 2018, 35, 14–18. [Google Scholar]
- China Food and Drug Administration. Safety and Technical Standards for Cosmetics; Military Medical Science Press: Beijing, China, 2015. [Google Scholar]
- Test Guideline for Testing of Chemicals: Bacterial Reverse Mutation Test 471. Available online: https://www.oecd-ilibrary.org/environment/test-no-471-bacterial-reverse-mutation-test_9789264071247-en (accessed on 26 July 2021).
- Wang, W.Q.; Duan, H.X.; Pei, Z.T.; Xu, R.R.; Qin, Z.T.; Zhu, G.C.; Sun, L.W. Evaluation by the Ames Assay of the Mutagenicity of UV Filters Using Benzophenone and Benzophenone-1. Int. J. Environ. Res. Public Health 2018, 15, 1907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ames, B.N.; Mccann, J.; Yamasaki, E. Methods for detecting carcinogens and mutagens with the Salmonella/mammalian-microsome mutagenicity test. Mutat. Res. /Environ. Mutagenesis Relat. Subj. 1975, 31, 347–363. [Google Scholar] [CrossRef]
- Maron, D.M. Revised methods for the Salmonella mutagenicity test. Mutat. Res. 1983, 113, 173–215. [Google Scholar] [CrossRef]
- Pelclová, D.; Černá, M.; Pastorková, A.; Vrbíková, V.; Procházka, B.; Hurychová, D.; Dlasková, Z.; Hornychová, M. Study of the Genotoxicity of Toluene. Arch. Environ. Health 2000, 55, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Khallef, M.; Cenkci, S.; Akyil, D.; Özkara, A.; Konuk, M.; Benouareth, D.E. Ames and random amplified polymorphic DNA tests for the validation of the mutagenic and/or genotoxic potential of the drinking water disinfection by-products chloroform and bromoform. J. Environ. Sci. Health A Tox. Hazard Subst. Environ. Eng. 2017, 53, 154–159. [Google Scholar] [CrossRef]
- Tabrez, S.; Ahmad, M. Genotoxicity of trichloroethylene in the natural milieu. Int. J. Hyg. Environ. Health 2012, 215, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Moore, M.M.; Harrington-Brock, K. Mutagenicity of trichloroethylene and its metabolites: Implications for the risk assessment of trichloroethylene. Environ. Health Perspect. Suppl. 2000, 108, 215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balasubramanyam, A.; Sailaja, N.; Mahboob, M.; Rahman, M.F.; Hussain, S.M.; Grover, P. In vitro mutagenicity assessment of aluminium oxide nanomaterials using the Salmonella/microsome assay. Toxicol. In Vitro 2010, 24, 1871–1876. [Google Scholar] [CrossRef]
- Xu, C.S.; Zhao, Q.Y.; Xu, T.; Li, M.H.; Li, J.; Ding, Y.; Wang, Y.W. Study on the Correlation between the Structure and Toxicity of Amines, Mercaptans and Halohydrocarbons. J. Life Sci. Res. 2015, 2, 68–71. [Google Scholar]
- da Silva Nunes-Halldorson, V.; Steiner, R.L.; Smith, G.B. Residual toxicity after biodegradation: Interactions among benzene, toluene, and chloroform—ScienceDirect. Ecotoxicol. Environ. Saf. 2004, 57, 162–167. [Google Scholar] [CrossRef]
- Prakash, J.; Nirmalakhandan, N.; Sun, B.; Peace, J. Toxicity of binary mixtures of organic chemicals to microorganisms. Water Res. 1996, 30, 1459–1463. [Google Scholar] [CrossRef]
- Eriksson, L.; Jonsson, J.; Berglind, R. External validation of a QSAR for the acute toxicity of halogenated aliphatic hydrocarbons. Environ. Toxicol. Chem. 1993, 12, 1185–1191. [Google Scholar] [CrossRef]
- Irving, R.M.; Elfarra, A.A. Mutagenicity of the cysteine S-conjugate sulfoxides of trichloroethylene and tetrachloroethylene in the Ames test. Toxicology 2013, 306, 157–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, H.Q.; Du, Y.; Zhang, Z.Y.; Jiang, W.J.; Guo, Y.M.; Lu, X.W.; Zhang, Y.M.; Sun, L.W. Acute Toxicity and Ecological Risk Assessment of Benzophenone and N,N-Diethyl-3 Methylbenzamide in Personal Care Products. Int. J. Environ. Res. Public Health 2016, 13, 925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, Y.; Wang, W.Q.; Pei, Z.T.; Ahmad, F.; Xu, R.R.; Zhang, Y.M.; Sun, L.W. Acute Toxicity and Ecological Risk Assessment of Benzophenone-3 (BP-3) and Benzophenone-4 (BP-4) in Ultraviolet (UV)-Filters. Int. J. Environ. Res. Public Health 2017, 14, 1414. [Google Scholar] [CrossRef] [Green Version]
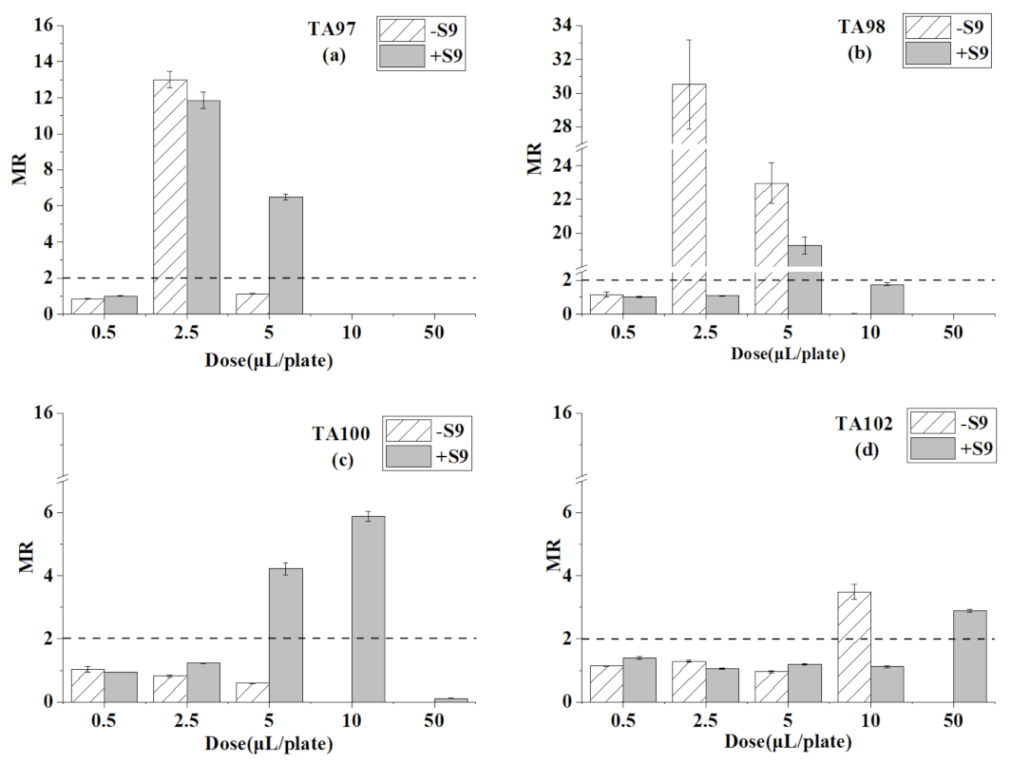
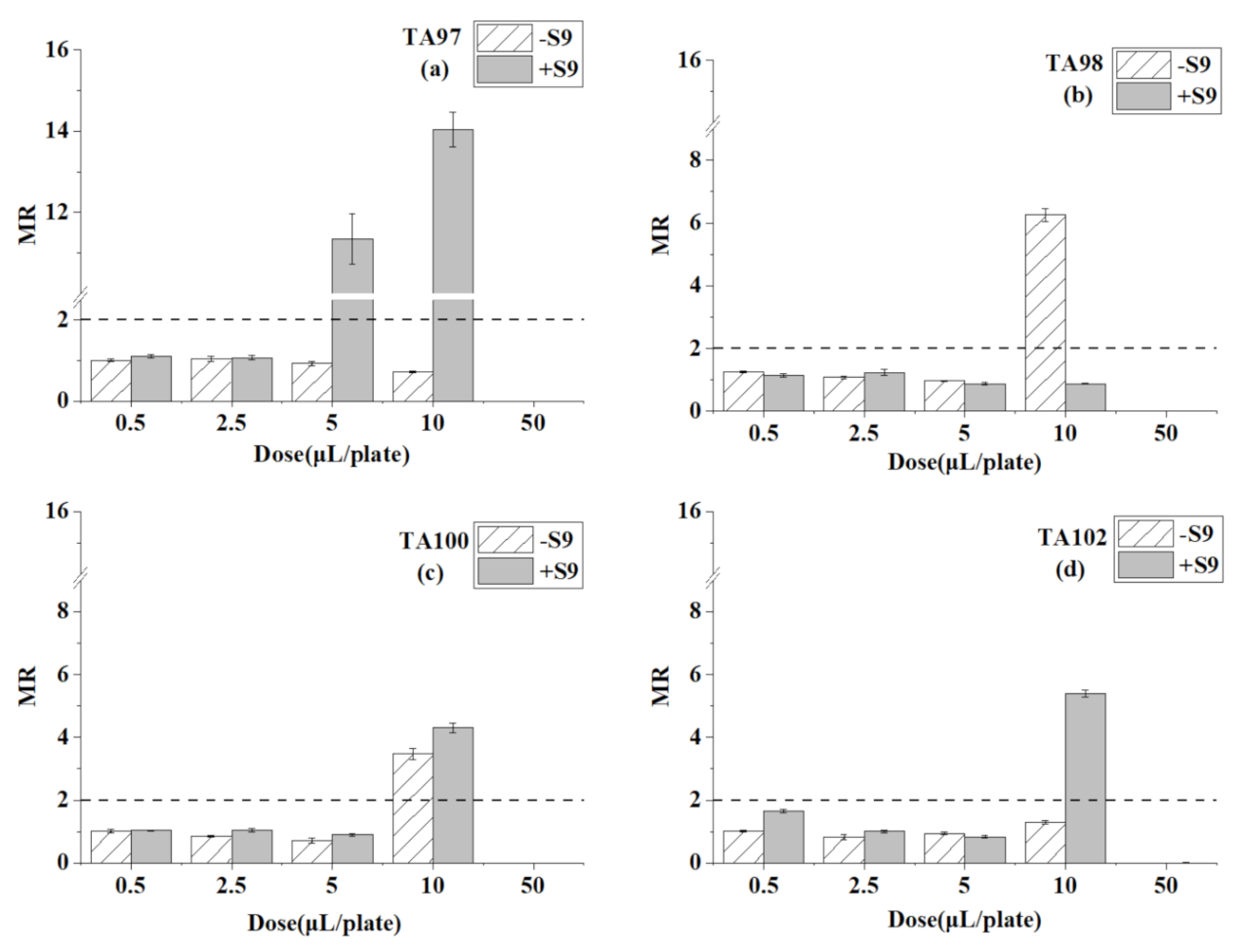
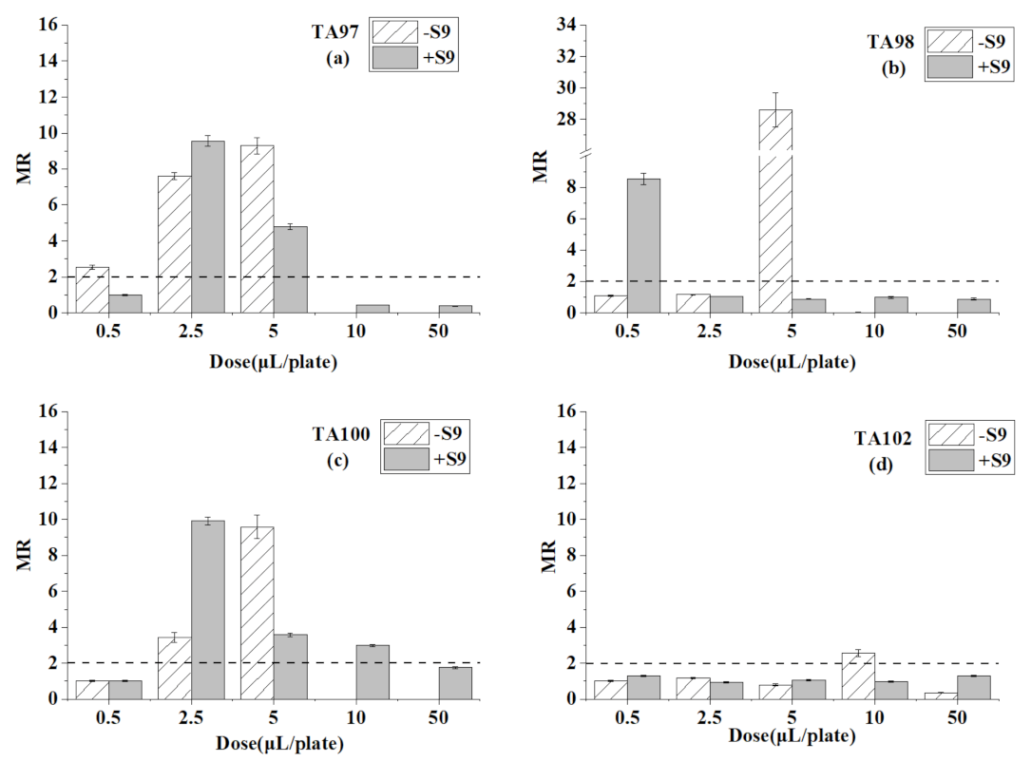
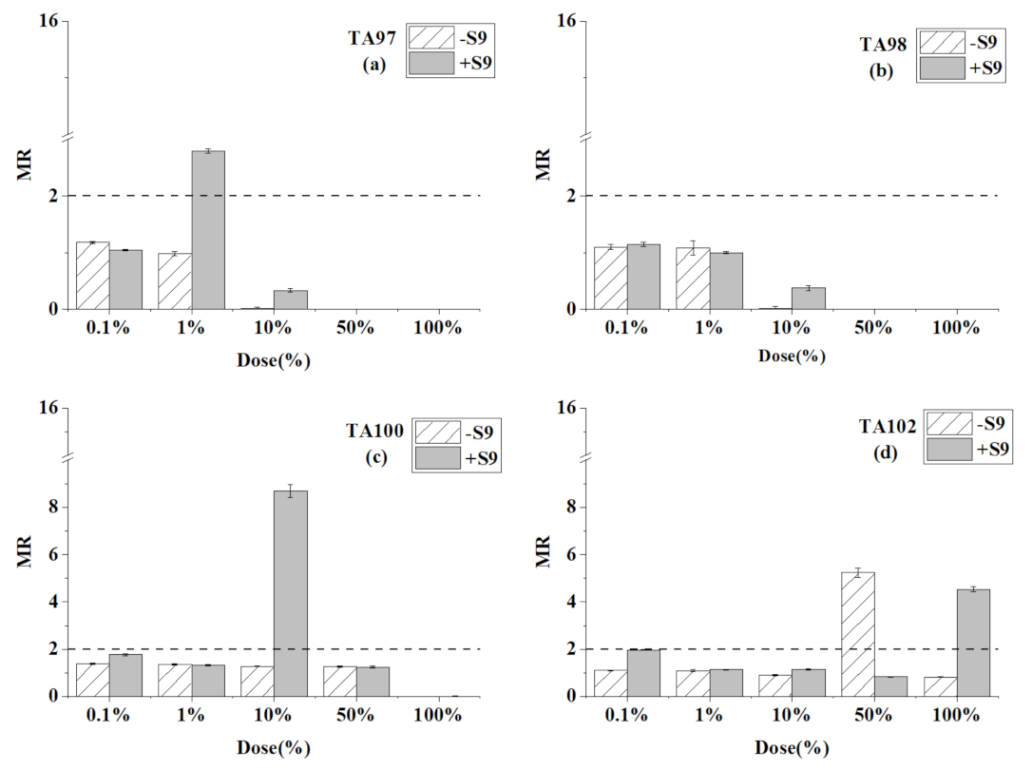
| Chemicals | Dose (μL/plate) | TA97 | TA98 | TA100 | TA102 | ||||
|---|---|---|---|---|---|---|---|---|---|
| -S9 | +S9 | -S9 | +S9 | -S9 | +S9 | -S9 | +S9 | ||
| DMSO | 100 | 109 ± 5 | 95 ± 3 | 31 ± 1 | 37 ± 1 | 185 ± 6 | 171 ± 6 | 256 ± 12 | 254 ± 5 |
| 0 | 107 ± 7 | 93 ± 3 | 33 ± 1 | 36 ± 2 | 191 ± 9 | 165 ± 10 | 221 ± 11 | 250 ± 5 | |
| toluene | 0.5 | 92 ± 4 | 94 ± 3 | 37 ± 4 | 37 ± 2 | 195 ± 17 | 159 ± 1 | 273 ± 2 | 355 ± 11 |
| 2.5 | 1400 ± 50 * | 1112 ± 43 * | 981 ± 84 * | 39 ± 1 | 155 ± 7 | 207 ± 2 | 308 ± 8 | 266 ± 7 | |
| 5 | 121 ± 2 | 609 ± 14* | 738 ± 39 * | 700 ± 18 * | 113 ± 3 | 708 ± 33 * | 229 ± 6 | 301 ± 8 | |
| 10 | 0 | 1 | 0 | 64 ± 3 | 0 | 987 ± 27 * | 834 ± 56 * | 282 ± 9 | |
| 50 | 0 | 0 | 0 | 0 | 0 | 19 ± 2 | 0 | 732 ± 11 * | |
| Chemicals | Dose (μL/plate) | TA97 | TA98 | TA100 | TA102 | ||||
|---|---|---|---|---|---|---|---|---|---|
| -S9 | +S9 | -S9 | +S9 | -S9 | +S9 | -S9 | +S9 | ||
| DMSO | 100 | 109 ± 5 | 95 ± 3 | 31 ± 1 | 37 ± 1 | 185 ± 6 | 171 ± 6 | 256 ± 12 | 254 ± 5 |
| 0 | 107 ± 7 | 93 ± 3 | 33 ± 1 | 36 ± 2 | 191 ± 9 | 165 ± 10 | 221 ± 11 | 250 ± 5 | |
| chloroform | 0.5 | 93 ± 6 | 104 ± 4 | 40 ± 1 | 42 ± 2 | 192 ± 9 | 175 ± 3 | 244 ± 4 | 420 ± 14 |
| 2.5 | 111 ± 6 | 100 ± 5 | 35 ± 1 | 45 ± 3 | 159 ± 5 | 175 ± 8 | 196 ± 16 | 257 ± 10 | |
| 5 | 100 ± 6 | 1065 ± 58 * | 31 ± 1 | 32 ± 2 | 132 ± 9 | 151 ± 8 | 224 ± 11 | 211 ± 8 | |
| 10 | 77 ± 3 | 1317 ± 39 * | 201 ± 6 * | 32 ± 1 | 653 ± 32 * | 723 ± 26 * | 310 ± 14 | 1360 ± 29 * | |
| 50 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 2 | |
| Chemicals | Dose (μL/plate) | TA97 | TA98 | TA100 | TA102 | ||||
|---|---|---|---|---|---|---|---|---|---|
| -S9 | +S9 | -S9 | +S9 | -S9 | +S9 | -S9 | +S9 | ||
| DMSO | 100 | 109 ± 5 | 95 ± 3 | 31 ± 1 | 37 ± 1 | 185 ± 6 | 171 ± 6 | 256 ± 12 | 254 ± 5 |
| 0 | 107 ± 7 | 93 ± 3 | 33 ± 1 | 36 ± 2 | 191 ± 9 | 165 ± 10 | 221 ± 11 | 250 ± 5 | |
| trichloro ethylene | 0.5 | 273 ± 14 * | 93 ± 4 | 35 ± 2 | 309 ± 13 * | 147 ± 8 | 172 ± 8 | 235 ± 7 | 329 ± 9 |
| 2.5 | 820 ± 22 * | 896 ± 28 * | 38 ± 1 | 38 ± 0 | 643 ± 53 * | 1664 ± 37 * | 281 ± 10 | 237 ± 8 | |
| 5 | 1000 ± 50 * | 449 ± 16 * | 920 ± 35 * | 32 ± 2 | 1803 ± 122 * | 601 ± 16 * | 191 ± 13 | 269 ± 8 | |
| 10 | 0 | 41 ± 1 | 1 | 36 ± 3 | 0 | 501 ± 12 * | 611 ± 47 * | 249 ± 10 | |
| 50 | 0 | 36 ± 1 | 0 | 32 ± 2 | 0 | 296 ± 9 | 87 ± 6 | 325 ± 10 | |
| Chemicals | Dose (μL/plate) | TA97 | TA98 | TA100 | TA102 | ||||
|---|---|---|---|---|---|---|---|---|---|
| -S9 | +S9 | -S9 | +S9 | -S9 | +S9 | -S9 | +S9 | ||
| DMSO | 100 | 109 ± 5 | 95 ± 3 | 31 ± 1 | 37 ± 1 | 185 ± 6 | 171 ± 6 | 256 ± 12 | 254 ± 5 |
| 0 | 107 ± 7 | 93 ± 3 | 33 ± 1 | 36 ± 2 | 191 ± 9 | 165 ± 10 | 221 ± 11 | 250 ± 5 | |
| The mixture | 0.1% | 111 ± 2 | 98 ± 1 | 40 ± 2 | 41 ± 2 | 232 ± 6 | 297 ± 8 | 277 ± 6 | 497 ± 4 |
| 1% | 92 ± 4 | 262 ± 3 * | 39 ± 5 | 36 ± 1 | 225 ± 5 | 221 ± 6 | 277 ± 10 | 287 ± 3 | |
| 10% | 1 | 31 ± 3 | 1 | 14 ± 2 | 213 ± 3 | 1459 ± 47 * | 229 ± 6 | 287 ± 4 | |
| 50% | 0 | 0 | 0 | 0 | 213 ± 3 | 208 ± 7 | 1320 ± 50 * | 210 ± 4 | |
| 100% | 0 | 0 | 0 | 0 | 0 | 1 | 208 ± 3 | 1144 ± 29 * | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Wang, W.; Pei, Z.; Wu, J.; Yu, R.; Zhang, Y.; Sun, L.; Gao, Y. Mutagenicity Assessment to Pesticide Adjuvants of Toluene, Chloroform, and Trichloroethylene by Ames Test. Int. J. Environ. Res. Public Health 2021, 18, 8095. https://doi.org/10.3390/ijerph18158095
Zhang J, Wang W, Pei Z, Wu J, Yu R, Zhang Y, Sun L, Gao Y. Mutagenicity Assessment to Pesticide Adjuvants of Toluene, Chloroform, and Trichloroethylene by Ames Test. International Journal of Environmental Research and Public Health. 2021; 18(15):8095. https://doi.org/10.3390/ijerph18158095
Chicago/Turabian StyleZhang, Jing, Wenqiang Wang, Zhoutao Pei, Jingya Wu, Ran Yu, Yimin Zhang, Liwei Sun, and Yuexiang Gao. 2021. "Mutagenicity Assessment to Pesticide Adjuvants of Toluene, Chloroform, and Trichloroethylene by Ames Test" International Journal of Environmental Research and Public Health 18, no. 15: 8095. https://doi.org/10.3390/ijerph18158095
APA StyleZhang, J., Wang, W., Pei, Z., Wu, J., Yu, R., Zhang, Y., Sun, L., & Gao, Y. (2021). Mutagenicity Assessment to Pesticide Adjuvants of Toluene, Chloroform, and Trichloroethylene by Ames Test. International Journal of Environmental Research and Public Health, 18(15), 8095. https://doi.org/10.3390/ijerph18158095







