Environmental Safety Analysis of Red Mud-Based Cemented Backfill on Groundwater
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Bleeding Experiments and Results
- (1)
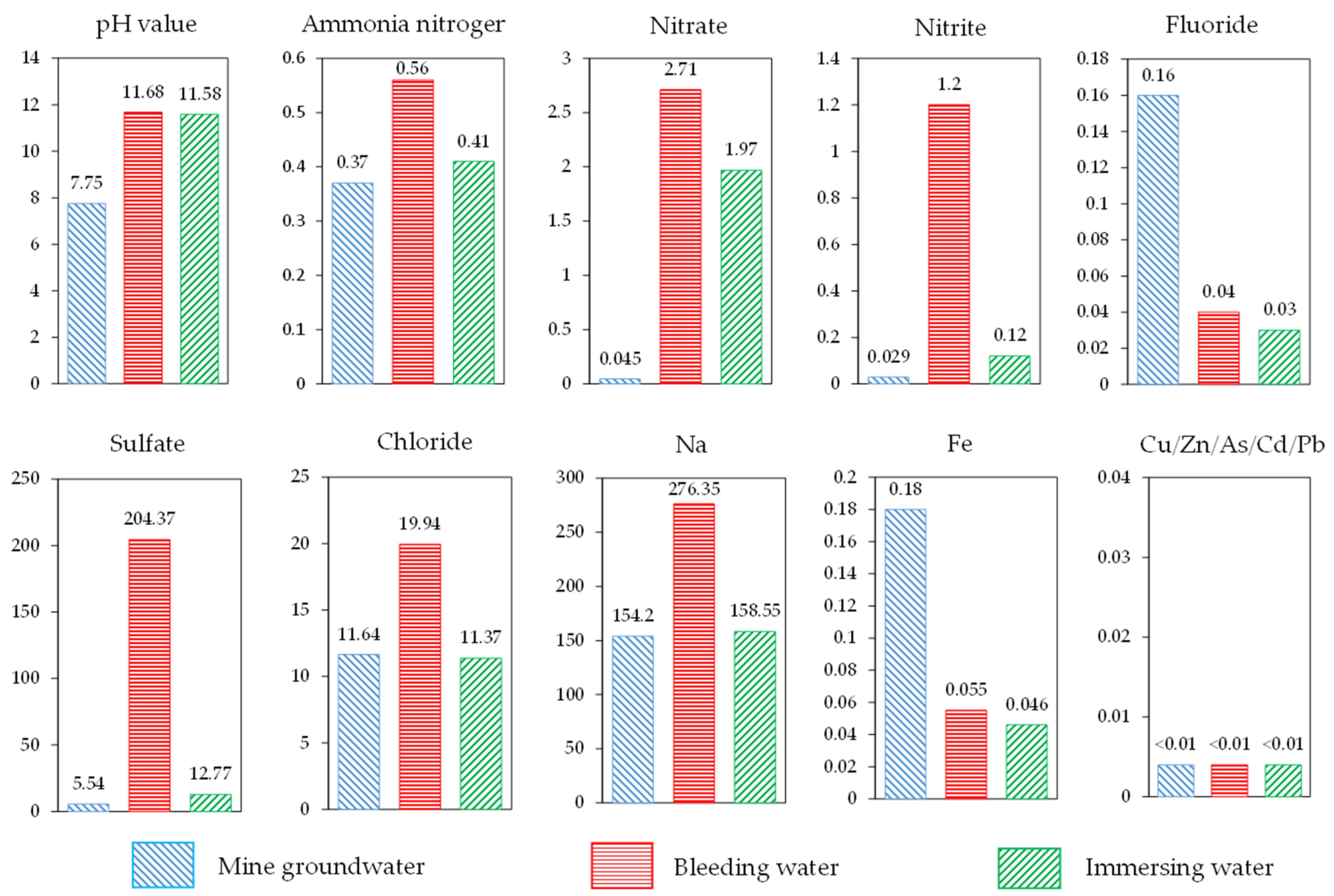
- Because the RMCB was prepared with alkaline BRM, slag powder, and lime, the pH of the RMCB was about 11.68, which was higher than the value of 7.75 measured in the mine groundwater and slightly lower than the value of 12.1 for fresh BRM.
- (2)
- The concentrations of ammonia nitrogen, nitrate, nitrite, sulfate, chloride, and sodium ions detected in the bleeding water of RMCB were all higher than those in mine groundwater, which is consistent with the phenomenon in which BRM forms white efflorescence on the surface and leads to soil salinization. The primary reason is that a large number of salt agents are added in the Bayer process [36].
- (3)
- The concentrations of fluoride and iron detected in the bleeding water of RMCB were lower than those in the mine groundwater. This is because the AFT and C-S-H gels produced by slag powder and lime generated the effects of inclusion and solidification, thus inhibiting the precipitation of such contaminants [37].
- (4)
- No heavy metal ions, such as copper, zinc, arsenic, cadmium, or lead, were detected in the bleeding water of the RMCB or the mine groundwater. The concentrations of ammonia nitrogen, nitrite, and sodium detected in the bleeding water of RMCB belonged to grade IV of the Groundwater Quality Standard [38], the sulfate concentration belonged to grade III, and the other contaminants all belonged to grade I or grade II.
3.2. Immersion Experiments and Results
- (1)
- Under the long-term effects of groundwater immersion, the pH of the soaking water was about 11.58, which was slightly lower than the values of 11.68 for the bleeding water and 12.1 for the fresh BRM.
- (2)
- The concentrations of ammonia nitrogen, nitrate, nitrite, sulfate, and sodium ions detected in the soaking water of the RMCB were still higher than those in the mine groundwater, which was consistent with the results of the bleeding experiments. The reason was that a large number of salt agents are added in the Bayer process.
- (3)
- The concentrations of fluoride, chloride, and iron detected in the soaking water of the RMCB were lower than those in the mine groundwater, which was also caused by the encapsulation and solidification of the AFT and C-S-H gels. Copper, zinc, arsenic, cadmium, lead, and other heavy metal ions were also not detected in the soaking water of the RMCB and the mine groundwater.
- (4)
- Compared with the results of the bleeding experiments, the concentrations of ammonia nitrogen, nitrate, nitrite, sulfate, chloride, sodium, and iron ions detected in the soaking water decreased by 26.8%, 27.3%, 90.0%, 93.8%, 43.0%, 42.6%, and 16.4%, respectively. Except for the concentrations of ammonia nitrogen, nitrite, and sodium ions, which belonged to grade III of the Groundwater Quality Standard, the other contaminants all belonged to grade I.
3.3. Toxic Leaching Experiments and Results
- (1)
- In both the fresh BRM or RMCB, the contents of heavy metal ions in the leaching solution were all lower than the standard toxicity limit specified in the Chinese National Standard for the identification of leaching of hazardous solid waste (GB5085.3-2007) [42], indicating that neither the BRM nor the RMCB was a hazardous solid waste.
- (2)
- Small amounts of heavy metal ions, such as Cr6+, As, Cu, Zn, Pb, and Ni, were detected in the leaching solution of the BRM, which was consistent with the fact that the content of rare earth elements in BRM in Shanxi province is relatively high.
- (3)
- After adding PO 42.5 cement, only Cr6+ and As were detected in the leaching solution of the RMCB, and their detected concentrations were 73.3% and 57.1% lower than those in the BRM, respectively. The SEM images of the RMCB after adding PO 42.5 cement show that the AFT and C-S-H gels generated the effects of inclusion and solidification, thus inhibiting the precipitation of heavy metal ions (see Figure 3c).
- (4)
- After adding slag powder and lime, there were also small amounts of Cr6+ and As that were detected in the leaching solution of the RMCB, and their detected concentrations were 20.0% and 33.3% lower, respectively, than those in the RMCB to which PO 42.5 cement was added. This indicated that the hydration products produced by the slag powder and lime were more developed and compact than the PO 42.5 cement, thus showing the more obvious effects of solidification, encapsulation, and inhibition of the precipitation of heavy metal ions.
3.4. Environmental Safety Analysis of RMCB on Groundwater
3.4.1. Establishment of the Fuzzy Factor Set and Evaluation Set
3.4.2. Calculation of the Weight Matrix of the Evaluation Factor
3.4.3. Fuzzy Comprehensive Evaluation Results for Water Quality
4. Discussion
- (1)
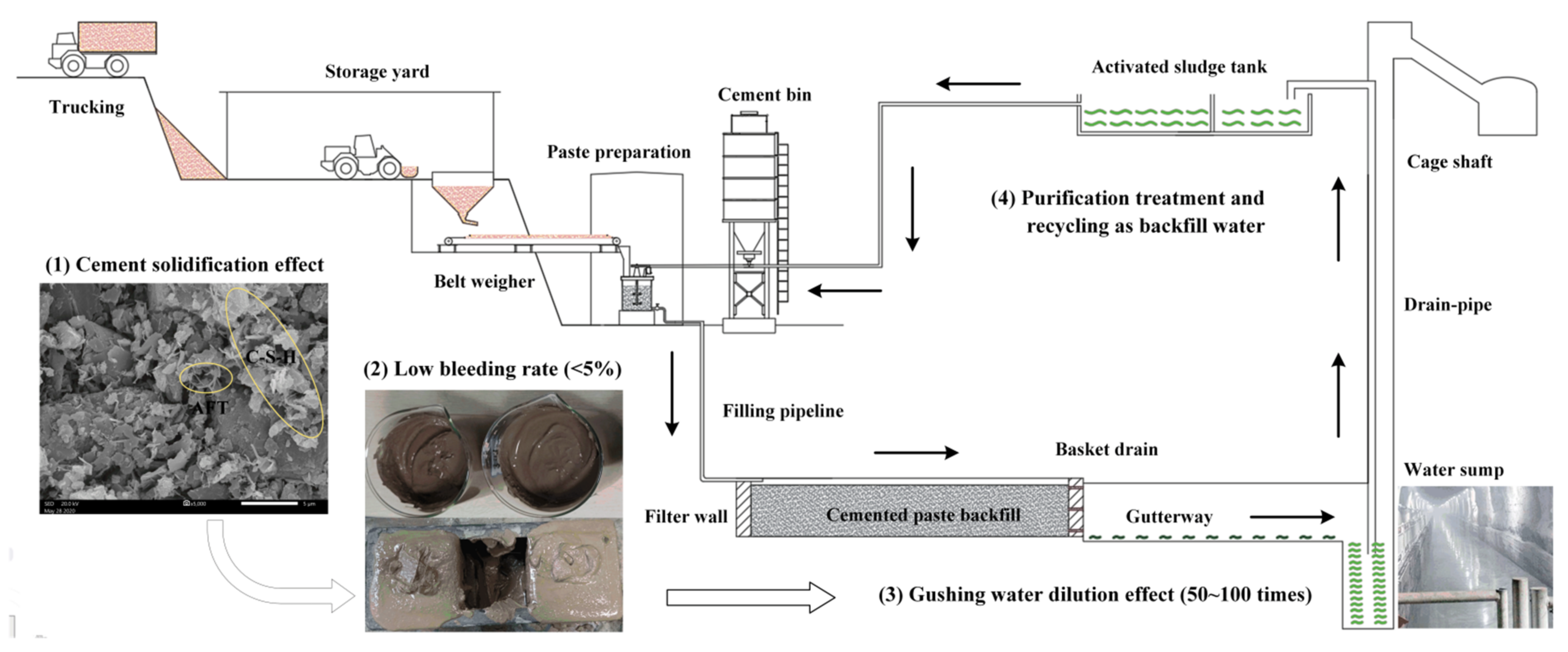
- A large amount of cementitious material is added to BRM, which will produce abundant hydration products such as AFT and C-S-H gel during the process of the hydration reaction [54]. These hydration products can significantly encapsulate and solidify harmful substances in the BRM and inhibit their precipitation [55]. The toxic leaching results show that the leaching concentrations of chromium, selenium, fluorine, arsenic, lead, and vanadium in RMCB could be reduced by more than 70% compared with those in fresh BRM.
- (2)
- To improve the backfilling effect, RMCB is prepared as a paste with a solid mass concentration of 60% and a bleeding rate of 4%. Less than 0.1 t/h of drainage water, which is only 1% of the normal water inflow in a mine, will be secreted through the pre-buried drainage hole in the filter wall.
- (3)
- After entering the water sump through the gutterway, the bleeding water of the RMCB will be mixed with gushing water caused by underground mining in order to engender a dilution effect. Due to the wave of the inflow of underground water, the dilution ratio can reach at least 50–100 times. The water quality after dilution can reach grade I, and the pH and concentrations of ammonia nitrogen, nitrite, and sodium can be reduced by more than 99%.
- (4)
- The water gushing into the water sump will be discharged into a surface sewage tank for centralized purification, where it will be reused as backfilling water or underground production water to achieve the goal of zero emissions [56].
5. Conclusions
- (1)
- As one of the main industrial solid wastes, there are large amounts of NaOH and chemically bound alkaloids that remain in BRM and are difficult to remove. These components react with Ca2+ in PO 42.5 cement and form hydrated garnet, resulting in slow solidification speed and low early strength of RMCB. After using a lower-cost industrial solid waste, S95 slag powder, as a cementing material and lime as an activator, RMCB with a cement–sand ratio of 1:6 and a solid mass concentration of 60% initially solidified within 12 h, and the 7 d compressive strength could reach 1.1 MPa.
- (2)
- The concentrations of ammonia nitrogen, nitrite, and sodium detected in the bleeding water of the RMCB slurry belonged to grade IV of the Groundwater Quality Standard, and the sulfate concentration belonged to grade III. Because only a few Cr6+ and As were detected, the RMCB was obviously not hazardous solid waste, and the concentrations of Cr6+ and As were lower than those in fresh BRM by 73.3% and 57.1%, respectively.
- (3)
- The fuzzy comprehensive evaluation method was used to carry out the coupling calculation, fuzzy analysis, and comprehensive evaluation of the water quality with respect to RMCB. The results show that the membership degree of the bleeding water of the RMCB reached the grade III standard, that is, the discharged contaminants had a medium influence on the groundwater quality, and the water could be used as industrial and agricultural water. Similarly, the membership degree of the soaking water of the RMCB reached the grade II standard, that is, the pollutants in the soaking water had a low degree of impact on the groundwater quality, and the water could be used as centralized drinking water.
- (4)
- The SEM images of the RMCB showed that a large number of hydration products, such as AFT and C-S-H gel, were generated during the hydration reaction. Under the actions of encapsulation, solidification, and inhibition of precipitation resulting from these hydration products, the bleeding rate of the RMCB paste was only 4%, and the leaching concentrations of harmful substances could be reduced by more than 70%. After flowing into the water sump through the gutterway, the bleeding water can be further diluted by at least 50–100 times to reach the grade I standard, and can finally be discharged into a surface sewage tank for centralized purification and recycling.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chi, Y.; Gu, G.; Yu, H.; Chen, C. Laser surface alloying on aluminum and its alloys: A review. Opt. Lasers Eng. 2018, 100, 23–37. [Google Scholar] [CrossRef]
- Paramguru, R.K.; Rath, P.C.; Misra, V.N. Trends in red mud utilization—A review. Miner. Process. Extr. Metall. Rev. 2004, 26, 1–29. [Google Scholar] [CrossRef]
- Patel, S.; Pal, B.K. Current status of an industrial waste: Red mud an overview. Ijltemas 2015, 4, 1–16. [Google Scholar]
- Samal, S.; Ray, A.K.; Bandopadhyay, A. Proposal for resources, utilization and processes of red mud in India—A review. Int. J. Miner. Process. 2013, 118, 43–55. [Google Scholar] [CrossRef]
- Khairul, M.A.; Zanganeh, J.; Moghtaderi, B. The composition, recycling and utilisation of Bayer red mud. Resour. Conserv. Recycl. 2019, 141, 483–498. [Google Scholar] [CrossRef]
- Zhu, X.; Li, W.; Guan, X. An active dealkalization of red mud with roasting and water leaching. J. Hazard. Mater. 2015, 286, 85–91. [Google Scholar] [CrossRef]
- Gelencsér, A.; Kováts, N.; Turóczi, B.; Rostási, Á.; Hoffer, A.; Imre, K.; Nyirő-Kósa, I.; Csákberényi-Malasics, D.; Tóth, Á.; Czitrovszky, A.; et al. The red mud accident in Ajka (Hungary): Characterization and potential health effects of fugitive dust. Environ. Sci. Technol. 2011, 45, 1608–1615. [Google Scholar] [CrossRef]
- Uzinger, N.; Anton, Á.D.; Ötvös, K.; Tamás, P.; Anton, A. Results of the clean-up operation to reduce pollution on flooded agricultural fields after the red mud spill in Hungary. Environ. Sci. Pollut. Res. 2015, 22, 9849–9857. [Google Scholar] [CrossRef]
- Taneez, M.; Hurel, C. A review on the potential uses of red mud as amendment for pollution control in environmental media. Environ. Sci. Pollut. Res. Int. 2019, 22, 22106–22125. [Google Scholar] [CrossRef]
- Mayes, W.M.; Burke, I.T.; Gomes, H.I.; Anton, Á.D.; Molnár, M.; Feigl, V.; Ujaczki, É. Advances in understanding environmental risks of red mud after the Ajka spill, Hungary. J. Sustain. Metall. 2016, 2, 332–343. [Google Scholar] [CrossRef] [Green Version]
- De Resende, E.C.; Carvalho, I.D.R.G.; Schlaf, M.; Guerreiro, M.C. Red Mud waste from the Bayer process as a catalyst for the desulfurization of hydrocarbon fuels. RSC Adv. 2014, 4, 47287–47296. [Google Scholar] [CrossRef]
- Wang, K.; Liu, Y.; Lyu, G.; Li, X.; Chen, X. A review of comprehensive utilization of high-iron red mud of China. Light Met. 2020, 2020, 65–71. [Google Scholar]
- Zhang, T.A.; Wang, Y.; Lu, G.; Liu, Y.; Zhang, W.; Zhao, Q. Comprehensive utilization of red mud: Current research status and a possible way forward for non-hazardous treatment. In Proceedings of the TMS Annual Meeting & Exhibition, Phoenix, AZ, USA, 11–15 March 2018; Springer: Cham, Switzerland, 2018; pp. 135–141. [Google Scholar]
- Liu, Y.; Zuo, K.; Yang, G.; Shang, Z.; Zhang, J. Recovery of ferric oxide from Bayer red mud by reduction roasting-magnetic separation process. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2016, 31, 404–407. [Google Scholar] [CrossRef]
- Liu, X.; Han, Y.; He, F.; Li, Y.; Gao, P.; Li, W. Research Status on Hazards and Comprehensive Utilization of Red Mud. Met. Mine 2018, 47, 7. [Google Scholar]
- Gray, C.W.; Dunham, S.J.; Dennis, P.G.; Zhao, F.J.; McGrath, S.P. Field evaluation of in situ remediation of a heavy metal contaminated soil using lime and red-mud. Environ. Pollut. 2006, 1423, 530–539. [Google Scholar] [CrossRef]
- Gupta, V.K.; Gupta, M.; Sharma, S. Process development for the removal of lead and chromium from aqueous solutions using red mud—An aluminum industry waste. Water Res. 2001, 355, 1125–1134. [Google Scholar] [CrossRef]
- Lin, J.; Zhu, X.; Yang, K.; Peng, J. Optimization of Microwave-Assisted Oxidation Roasting of Oxide–Sulphide Zinc Ore with Addition of Manganese Dioxide Using Response Surface Methodology. High Temp. Mater. Process. 2019, 38, 301–308. [Google Scholar] [CrossRef]
- Nadaroglu, H.; Kalkan, E.; Demir, N. Removal of copper from aqueous solution using red mud. Desalination 2010, 251, 90–95. [Google Scholar] [CrossRef]
- Liu, W.; Yang, J.; Xiao, B. Application of Bayer red mud for iron recovery and building material production from alumosilicate residues. J. Hazard. Mater. 2009, 161, 474–478. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Lyu, F.; Sun, W.; Zhang, H.; Wang, L.; Wang, Y. Progress on the industrial applications of red mud with a focus on China. Minerals 2020, 10, 773. [Google Scholar] [CrossRef]
- Cheng, Y.; Xu, L.; Jiang, Z.; Liu, C.; Zhang, Q.; Zou, Y.; Chen, Y.; Li, J.; Liu, X. Feasible low-cost conversion of red mud into magnetically separated and recycled hybrid SrFe12O19@ NaP1 zeolite as a novel wastewater adsorbent. Chem. Eng. J. 2020, 417, 128090. [Google Scholar] [CrossRef]
- Sun, W.; Wang, H.; Hou, K. Control of waste rock-tailings paste backfill for active mining subsidence areas. J. Clean. Prod. 2018, 171, 567–579. [Google Scholar] [CrossRef]
- Li, X.; Zhou, Y.; Zhu, Q.; Zhou, S.; Min, C.; Shi, Y. Slurry preparation effects on the cemented phosphogypsum backfill through an orthogonal experiment. Minerals 2019, 9, 31. [Google Scholar] [CrossRef] [Green Version]
- Yang, B.; Jin, J.; Yin, X.; Wang, X.; Yang, H. Effect of Concentration and Suspension Agent (HPMC) on Properties of Coal Gangue and Fly Ash Cemented Filling Material. Shock. Vib. 2021, 2021, 6643773. [Google Scholar]
- Zhu, L.; Ni, W.; Zhang, X.; Huang, X. Performance and microstructure of cemented whole-tailings backfilling materials based on red mud, slag and cement. J. Univ. Sci. Technol. Beijing 2010, 32, 838–842. (In Chinese) [Google Scholar]
- Chen, S.; Du, Z.; Zhang, Z.; Yin, D.; Feng, F.; Ma, J. Effects of red mud additions on gangue-cemented paste backfill properties. Powder Technol. 2020, 367, 833–840. [Google Scholar] [CrossRef]
- Lan, G.A.O.; Jihong, L.I.; Denghong, W.; Xiaoyun, X.; Chengwei, Y.; Meizhi, H.A.N. Outline of metallogenic regularity of bauxite deposits in China. Acta Geol. Sin. Engl. Ed. 2015, 89, 2072–2084. [Google Scholar] [CrossRef]
- Den Hond, R.; Hiralal, I.; Rijkeboer, A. Alumina yield in the Bayer process past, present and prospects. In Essential Readings in Light Metals; Springer: Cham, Switzerland, 2016; pp. 528–533. [Google Scholar]
- Wang, P.; Liu, D.Y. Physical and chemical properties of sintering red mud and bayer red mud and the implications for beneficial utilization. Materials 2012, 5, 1800–1810. [Google Scholar] [CrossRef] [Green Version]
- Hua, Y.; Heal, K.V.; Friesl-Hanl, W. The use of red mud as an immobiliser for metal/metalloid-contaminated soil: A review. J. Hazard. Mater. 2017, 325, 17–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, D.; Wu, C.; Yang, Q.; Zhang, W.; Lu, Z.; Wang, P.; Li, J.; Ding, Q. Insights on the molecular structure evolution for tricalcium silicate and slag composite: From 29Si and 27Al NMR to molecular dynamics. Compos. Part B Eng. 2020, 202, 108401. [Google Scholar] [CrossRef]
- Yang, C.; Dong, J.; Ren, L.; Fan, Y.; Li, B.; Hu, W. Influencing factors on the stabilization of colloid biliquid aphrons and its effectiveness used for density modification of DNAPLs in subsurface environment. Colloids Surf. A Physicochem. Eng. Asp. 2018, 553, 439–445. [Google Scholar] [CrossRef]
- Matalkah, F.; Xu, L.; Wu, W.; Soroushian, P. Mechanochemical synthesis of one-part alkali aluminosilicate hydraulic cement. Mater. Struct. 2017, 50, 1–12. [Google Scholar] [CrossRef]
- Li, S.; Zhang, R.; Feng, R.; Hu, B.; Wang, G.; Yu, H. Feasibility of recycling Bayer process red mud for the safety backfill mining of layered soft bauxite under coal seams. Minerals 2021, 11, 722. [Google Scholar] [CrossRef]
- Raiguel, S.; Dehaen, W.; Binnemans, K. Extraction of gallium from simulated Bayer process liquor by Kelex 100 dissolved in ionic liquids. Dalton Trans. 2020, 49, 3532–3544. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Li, H.; Yang, X. Effect and characterization of the nucleation CSH seed on the reactivity of granulated blast furnace slag powder. Constr. Build. Mater. 2020, 238, 117726. [Google Scholar] [CrossRef]
- Wen, X.; Lu, J.; Wu, J.; Lin, Y.; Luo, Y. Influence of coastal groundwater salinization on the distribution and risks of heavy metals. Sci. Total Environ. 2019, 652, 267–277. [Google Scholar] [CrossRef]
- Gu, C.; Zhang, Y.; Peng, Y.; Leng, P.; Zhu, N.; Qiao, Y.; Li, Z.; Li, F. Spatial Distribution and Health Risk Assessment of Dissolved trace elements in Groundwater in southern china. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Intrakamhaeng, V.; Clavier, K.A.; Roessler, J.G.; Townsend, T.G. Limitations of the toxicity characteristic leaching procedure for providing a conservative estimate of landfilled municipal solid waste incineration ash leaching. J. Air Waste Manag. Assoc. 2019, 69, 623–632. [Google Scholar] [CrossRef]
- Zhang, H.; He, P.-J.; Shao, L.-M.; Li, X.-J. Test method standard for leaching toxicity of solid wastes-roll over leaching procedure, 1997. J. Mater. Cycles Waste Manag. 2008, 10, 7–13. [Google Scholar] [CrossRef]
- China, E.P.A. Identification Standard for Hazardous Wastes-Identification for Extraction Procedure Toxicity (GB5085. 3-1996); China Environmental Science Press: Beijing, China, 1996. [Google Scholar]
- Wu, X.; Hu, F. Analysis of ecological carrying capacity using a fuzzy comprehensive evaluation method. Ecol. Indic. 2020, 113, 106243. [Google Scholar] [CrossRef]
- Carrasco, G.; Molina, J.L.; Patino-Alonso, M.C.; Castillo, M.D.C.; Vicente-Galindo, M.P.; Galindo-Villardón, M.P. Water quality evaluation through a multivariate statistical HJ-Biplot approach. J. Hydrol. 2019, 577, 123993. [Google Scholar] [CrossRef]
- Xu, S.; Cui, Y.; Yang, C.; Wei, S.; Dong, W.; Huang, L.; Liu, C.; Ren, Z.; Wang, W. The fuzzy comprehensive evaluation (FCE) and the principal component analysis (PCA) model simulation and its applications in water quality assessment of Nansi Lake Basin, China. Environ. Eng. Res. 2021, 26, 222–232. [Google Scholar] [CrossRef]
- Xie, Q.; Ni, J.Q.; Su, Z. Fuzzy comprehensive evaluation of multiple environmental factors for swine building assessment and control. J. Hazard. Mater. 2017, 340, 463–471. [Google Scholar] [CrossRef]
- Xu, X.; Xu, H.; Wen, C.; Li, J.; Hou, P.; Zhang, J. A belief rule-based evidence updating method for industrial alarm system design. Control Eng. Pract. 2018, 81, 73–84. [Google Scholar] [CrossRef]
- Feng, Y.; Hua, Z.; Liu, G. Partial reduction algorithms for fuzzy relation systems. Knowl. Based Syst. 2020, 188, 105047. [Google Scholar] [CrossRef]
- Mellander, P.E.; Jordan, P.; Bechmann, M.; Fovet, O.; Shore, M.M.; McDonald, N.T.; Gascuel-Odoux, C. Integrated climate-chemical indicators of diffuse pollution from land to water. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef]
- Zheng, G.; Li, K.; Bu, W.; Wang, Y. Fuzzy comprehensive evaluation of human physiological state in indoor high temperature environments. Build. Environ. 2019, 150, 108–118. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, B.; Teng, D.; Yang, W.; Qiu, D. Comprehensive evaluation method of groundwater environment in a mining area based on fuzzy set theory. Geosyst. Eng. 2018, 21, 103–112. [Google Scholar] [CrossRef]
- Deng, D.Q.; Liu, L.; Yao, Z.L.; Song, K.I.; Lao, D.Z. A practice of ultra-fine tailings disposal as filling material in a gold mine. J. Environ. Manag. 2017, 196, 100–109. [Google Scholar] [CrossRef]
- Wang, L.; Hu, G.; Lyu, F.; Yue, T.; Tang, H.; Han, H.; Yang, Y.; Liu, R.; Sun, W. Application of red mud in wastewater treatment. Minerals 2019, 9, 281. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Chen, L.; Guo, B.; Tsang, D.C.; Huang, L.; Ok, Y.S.; Mechtcherine, V. Red mud-enhanced magnesium phosphate cement for remediation of Pb and As contaminated soil. J. Hazard. Mater. 2020, 400, 123317. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.S.; Dai, J.G.; Wang, L.; Tsang, D.C.; Poon, C.S. Influence of lead on stabilization/solidification by ordinary Portland cement and magnesium phosphate cement. Chemosphere 2018, 190, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Li, Y.; Guo, D.; Yao, L.; Yang, T.; Li, T. Failure mechanism and control technology of water-immersed roadway in high-stress and soft rock in a deep mine. Int. J. Min. Sci. Technol. 2017, 27, 245–252. [Google Scholar] [CrossRef]


| Composition (%) | CaO | SiO2 | Fe2O3 | Al2O3 | MgO | SO3 | Loss on Ignition |
|---|---|---|---|---|---|---|---|
| BRM | 2.57 | 19 | 34.5 | 23 | 0.2 | - | - |
| PO 42.5 | 63.20 | 20.9 | 2.77 | 5.45 | 2.7 | 2.54 | - |
| Slag powder | 37.85 | 29.55 | 0.65 | 15.22 | 4.97 | 2.01 | 1.17 |
| Metal Element | Cr6+ | As | Cu | Zn | Pb | Ni | Ag | Ba | Se | Cd | Hg | Be |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GB5085.3-2007 | 5 | 5 | 100 | 5 | 100 | 5 | 5 | 100 | 1 | 1 | 0.1 | 0.02 |
| BRM | 0.131 | 0.007 | 0.03 | 0.02 | 0.01 | 0.01 | <0.01 | <0.01 | <0.01 | <0.001 | <0.0001 | <0.001 |
| RMCB (PO42.5) | 0.035 | 0.003 | <0.01 | <0.01 | <0.001 | <0.001 | <0.01 | <0.01 | <0.01 | <0.001 | <0.0001 | <0.001 |
| RMCB (Slag) | 0.028 | 0.002 | <0.01 | <0.01 | <0.001 | <0.001 | <0.01 | <0.01 | <0.01 | <0.001 | <0.0001 | <0.001 |
| Factor | Ci | Si | Ci/Si | αi | |
|---|---|---|---|---|---|
| pH value | 11.680 | 8.700 | 1.343 | 5.008 | 0.2681 |
| Ammonia nitrogen | 0.560 | 0.724 | 0.773 | 0.1545 | |
| Nitrate | 2.710 | 17.400 | 0.156 | 0.0311 | |
| Nitrite | 1.200 | 2.142 | 0.560 | 0.1119 | |
| Fluoride | <0.050 | 1.400 | 0.035 | 0.0071 | |
| Sulfate | 204.370 | 230.000 | 0.889 | 0.1774 | |
| Chloride | 19.940 | 230.000 | 0.087 | 0.0173 | |
| Na | 276.350 | 250.000 | 1.105 | 0.2207 | |
| Fe | 0.055 | 0.920 | 0.060 | 0.0119 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Zhang, Y.; Feng, R.; Yu, H.; Pan, J.; Bian, J. Environmental Safety Analysis of Red Mud-Based Cemented Backfill on Groundwater. Int. J. Environ. Res. Public Health 2021, 18, 8094. https://doi.org/10.3390/ijerph18158094
Li S, Zhang Y, Feng R, Yu H, Pan J, Bian J. Environmental Safety Analysis of Red Mud-Based Cemented Backfill on Groundwater. International Journal of Environmental Research and Public Health. 2021; 18(15):8094. https://doi.org/10.3390/ijerph18158094
Chicago/Turabian StyleLi, Shuai, Yulin Zhang, Ru Feng, Haoxuan Yu, Jilong Pan, and Jiwei Bian. 2021. "Environmental Safety Analysis of Red Mud-Based Cemented Backfill on Groundwater" International Journal of Environmental Research and Public Health 18, no. 15: 8094. https://doi.org/10.3390/ijerph18158094
APA StyleLi, S., Zhang, Y., Feng, R., Yu, H., Pan, J., & Bian, J. (2021). Environmental Safety Analysis of Red Mud-Based Cemented Backfill on Groundwater. International Journal of Environmental Research and Public Health, 18(15), 8094. https://doi.org/10.3390/ijerph18158094







