Social Freezing: Pressing Pause on Fertility
Abstract
:1. Introduction
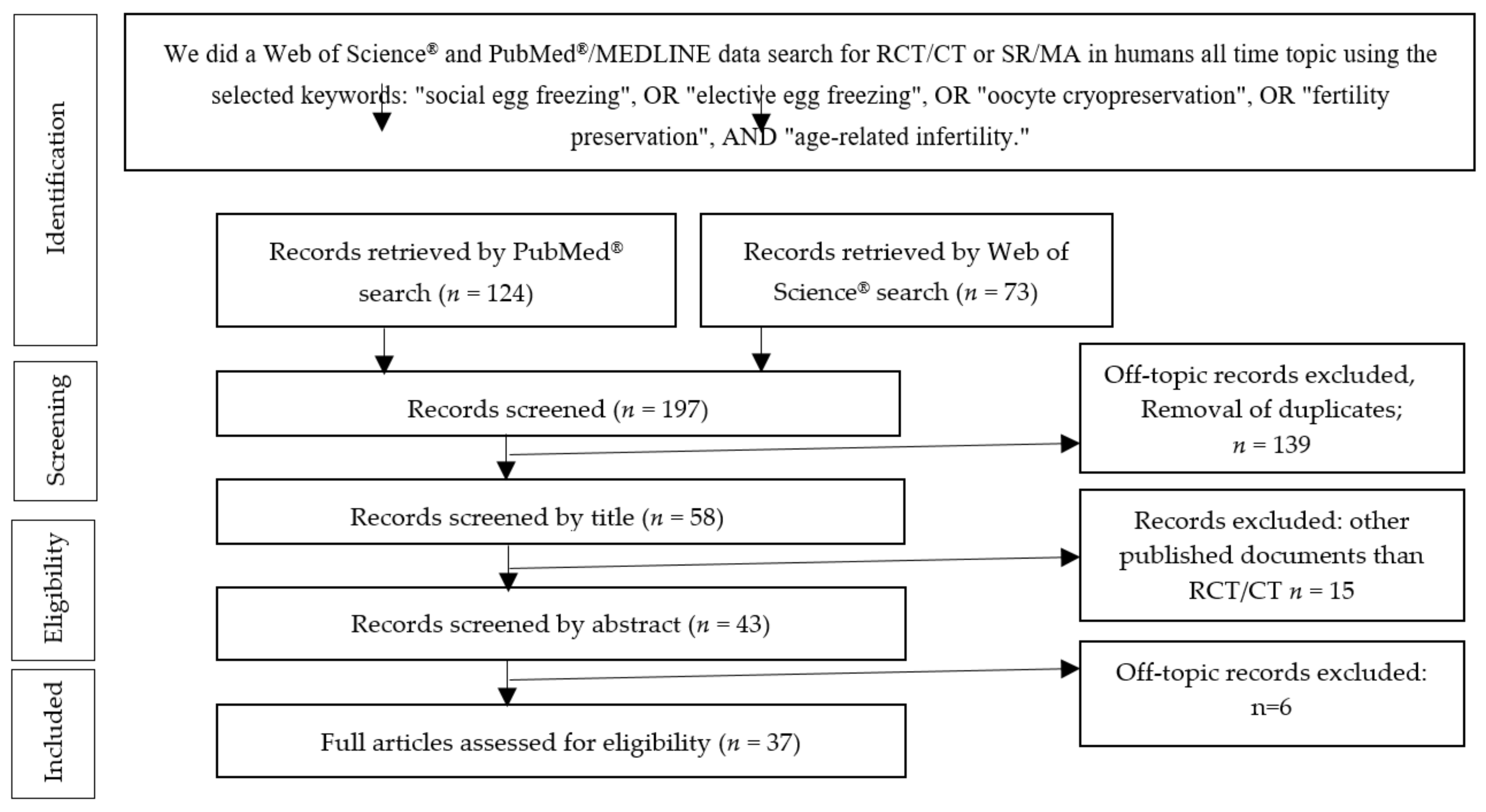
2. Materials and Methods
3. Results
3.1. Study Selection
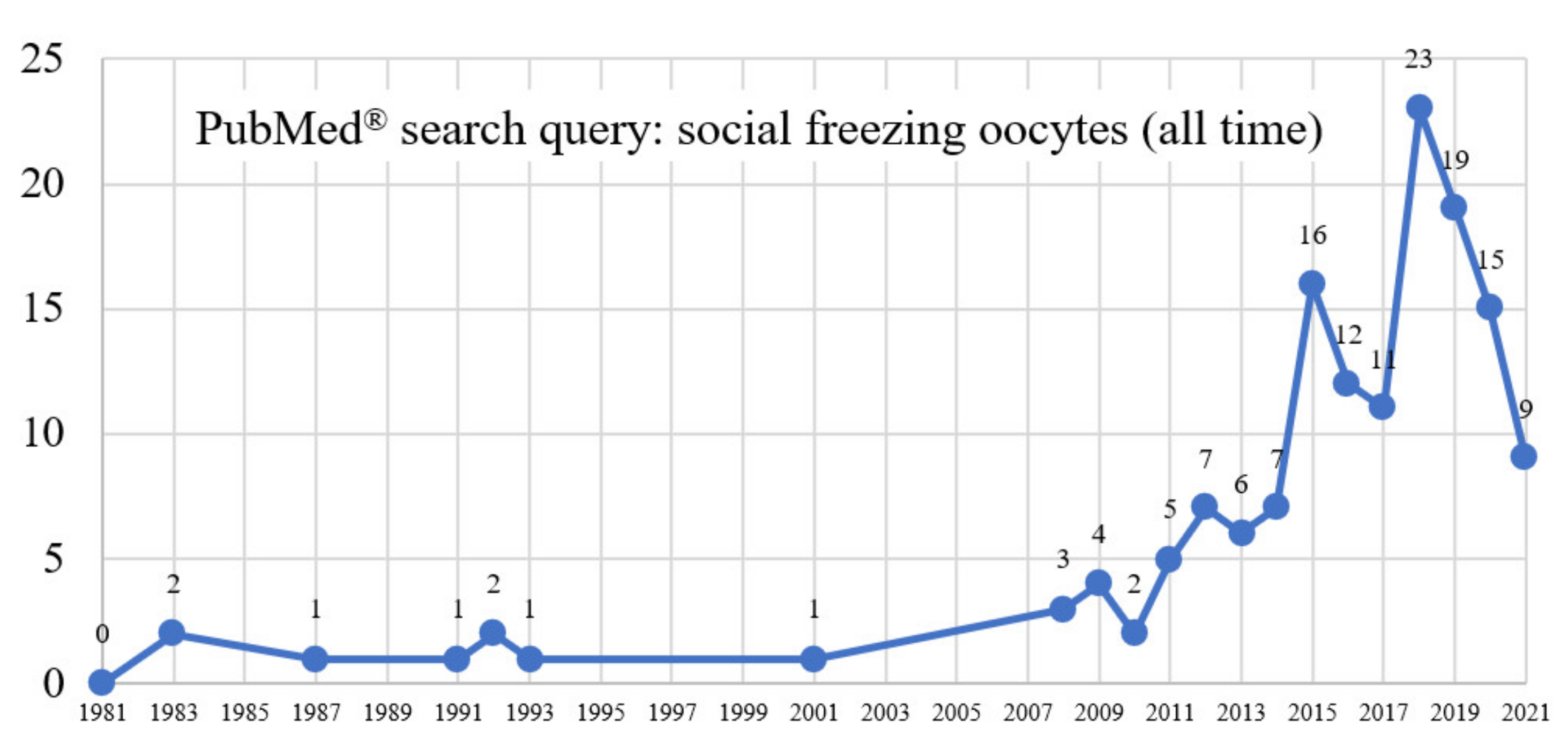
3.1.1. PubMed®/MEDLINE Search
3.1.2. Web of Science® Database Search
3.2. Fertility Preservation Techniques
3.2.1. Oocyte Cryopreservation
3.2.2. Embryo Cryopreservation
3.2.3. Ovarian Tissue Banking
3.2.4. Optimal Timing of Cryopreservation
3.2.5. The Optimal Number of Oocyte
3.2.6. The Quality of Oocytes
3.3. Safety of Freezing
3.4. The Usage Rate of Frozen Oocytes
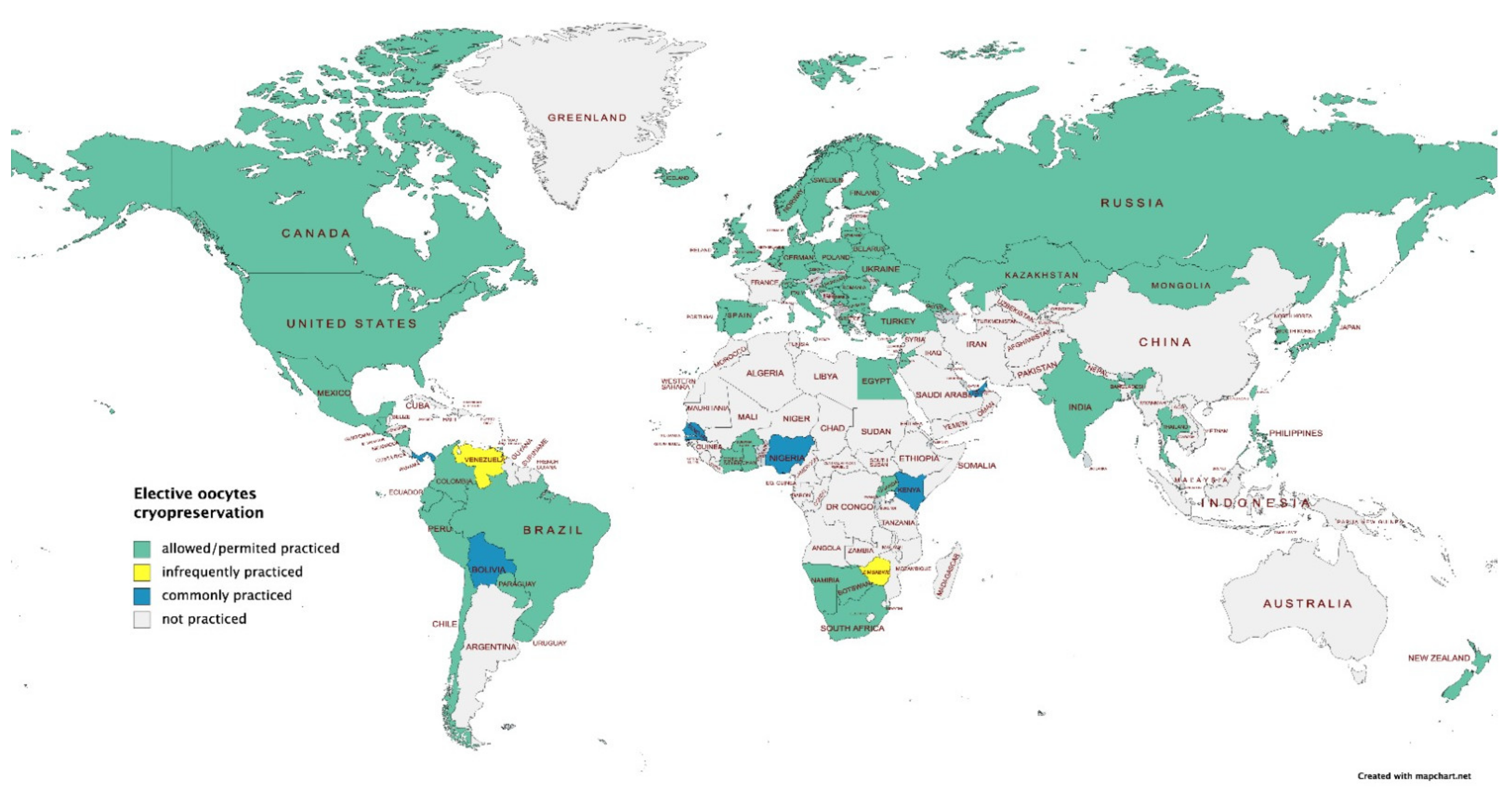
3.5. Ethical Considerations
3.6. Cost-Effectiveness
4. Discussion
5. Conclusions
Limitations
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ART | Assisted Reproduction Techniques |
| AMH | Anti-Müllerian hormone |
| ASRM | American Society for Reproductive Medicine |
| CCS | Clinical Case Series |
| CLBR | Cumulative Live Birth Rate |
| DRS | Decision Regret Scale |
| IVF | In Vitro Fertilization |
| MA | Meta-Analysis |
| OHSS | Ovarian Hyperstimulation Syndrome |
| PGT-A | Preimplantation Genetic Tests For Aneuploidy |
| PRISMA | Preferred Reporting Items for Systematic Review and Meta-Analysis |
| CT | Clinical Trial |
| RCT | Randomized Clinical Trial |
| SEF | Social Egg Freezing |
| SR | Systematic Review |
References
- Alteri, A.; Pisaturo, V.; Nogueira, D.; D’Angelo, A. Elective Egg Freezing without Medical Indications. Acta Obstet. Gynecol. Scand. 2019, 98, 647–652. [Google Scholar] [CrossRef] [Green Version]
- ESHRE. Female Fertility Preservation Guideline of the European Society of Human Reproduction and Embriology. 2020. Available online: https://www.eshre.eu/Guidelines-and-Legal/Guidelines/Female-fertility-preservation (accessed on 20 December 2020).
- Anderson, R.A.; Davies, M.C.; Lavery, S.A.; Royal College of Obstetricians and Gynaecologists. Elective Egg Freezing for Non-Medical Reasons: Scientific Impact Paper No. 63. BJOG Int. J. Obstet. Gynaecol. 2020, 127, e113–e121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nasab, S.; Ulin, L.; Nkele, C.; Shah, J.; Abdallah, M.E.; Sibai, B.M. Elective Egg Freezing: What Is the Vision of Women around the Globe? Future Sci. OA 2020, 6, FSO468. [Google Scholar] [CrossRef] [Green Version]
- Shkedi-Rafid, S.; Hashiloni-Dolev, Y. Egg Freezing for Age-Related Fertility Decline: Preventive Medicine or a Further Medicalization of Reproduction? Analyzing the New Israeli Policy. Fertil. Steril. 2011, 96, 291–294. [Google Scholar] [CrossRef] [PubMed]
- Lewis, E.I.; Missmer, S.A.; Farland, L.V.; Ginsburg, E.S. Public Support in the United States for Elective Oocyte Cryopreservation. Fertil. Steril. 2016, 106, 1183–1189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- American College of Obstetricians and Gynecologists Committee on Gynecologic Practice and Practice Committee. Female Age-Related Fertility Decline. Committee Opinion No. 589. Fertil. Steril. 2014, 101, 633–634. [Google Scholar] [CrossRef]
- Bachmann, G.; MacArthur, T.A.; Khanuja, K. Need for Comprehensive Counseling in Women Requesting Oocyte Cryopreservation. J. Womens Health 2002 2018, 27, 227–230. [Google Scholar] [CrossRef]
- Practice Committee of Society for Assisted Reproductive Technology; Practice Committee of American Society for Reproductive Medicine. Essential Elements of Informed Consent for Elective Oocyte Cryopreservation: A Practice Committee Opinion. Fertil. Steril. 2008, 90 (Suppl. S5), S134–S135. [Google Scholar] [CrossRef]
- Ben-Rafel, Z. The Dilemma of Social Oocyte Freezing: Usage Rate Is Too Low to Make It Cost-Effective. Reprod. Biomed. Online 2018, 37. [Google Scholar] [CrossRef] [Green Version]
- International Federation of Fertility Societies’ Surveillance (IFFS) 2019: Global Trends in Reproductive Policy and Practice, 8th Edition. Glob. Reprod. Health 2019, 4, e29. [CrossRef]
- Singer, P.; Wells, D. In Vitro Fertilisation: The Major Issues. J. Med. Ethics 1983, 9, 192–199. [Google Scholar] [CrossRef]
- Potdar, N.; Gelbaya, T.A.; Nardo, L.G. Oocyte Vitrification in the 21st Century and Post-Warming Fertility Outcomes: A Systematic Review and Meta-Analysis. Reprod. Biomed. Online 2014, 29, 159–176. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Velasco, J.A.; Domingo, J.; Cobo, A.; Martínez, M.; Carmona, L.; Pellicer, A. Five Years’ Experience Using Oocyte Vitrification to Preserve Fertility for Medical and Nonmedical Indications. Fertil. Steril. 2013, 99, 1994–1999. [Google Scholar] [CrossRef]
- Takahashi, N.; Harada, M.; Oi, N.; Izumi, G.; Momozawa, K.; Matsuzawa, A.; Osuga, Y. Preclinical validation of the new vitrification device possessing a feature of absorbing excess vitrification solution for the cryopreservation of human embryos. J. Obstet. Gynaecol. Res. 2020, 46, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Petropanagos, A.; Cattapan, A.; Baylis, F.; Leader, A. Social Egg Freezing: Risk, Benefits and Other Considerations. Can. Med. Assoc. J. 2015, 187, 666–669. [Google Scholar] [CrossRef] [Green Version]
- Argyle, C.E.; Harper, J.C.; Davies, M.C. Oocyte Cryopreservation: Where Are We Now? Hum. Reprod. Update 2016, 22, 440–449. [Google Scholar] [CrossRef] [Green Version]
- Lew, R.; Foo, J.; Kroon, B.; Boothroyd, C.; Chapman, M.; Australasian CREI Consensus Expert Panel on Trial evidence (ACCEPT) group. ANZSREI Consensus Statement on Elective Oocyte Cryopreservation. Aust. N. Z. J. Obstet. Gynaecol. 2019, 59, 616–626. [Google Scholar] [CrossRef] [PubMed]
- Mizrachi, Y.; Horowitz, E.; Farhi, J.; Raziel, A.; Weissman, A. Ovarian Stimulation for Freeze-All IVF Cycles: A Systematic Review. Hum. Reprod. Update 2020, 26, 118–135. [Google Scholar] [CrossRef]
- Milachich, T.; Shterev, A. Are There Optimal Numbers of Oocytes, Spermatozoa and Embryos in Assisted Reproduction? JBRA Assist. Reprod. 2016, 20, 142–149. [Google Scholar] [CrossRef]
- Barash, O.O.; Hinckley, M.D.; Rosenbluth, E.M.; Ivani, K.A.; Weckstein, L.N. High Gonadotropin Dosage Does Not Affect Euploidy and Pregnancy Rates in IVF PGS Cycles with Single Embryo Transfer. Hum. Reprod. Oxf. Engl. 2017, 32, 2209–2217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sekhon, L.; Shaia, K.; Santistevan, A.; Cohn, K.H.; Lee, J.A.; Beim, P.Y.; Copperman, A.B. The Cumulative Dose of Gonadotropins Used for Controlled Ovarian Stimulation Does Not Influence the Odds of Embryonic Aneuploidy in Patients with Normal Ovarian Response. J. Assist. Reprod. Genet. 2017, 34, 749–758. [Google Scholar] [CrossRef]
- Kuwayama, M.; Vajta, G.; Kato, O.; Leibo, S.P. Highly Efficient Vitrification Method for Cryopreservation of Human Oocytes. Reprod. Biomed. Online 2005, 11, 300–308. [Google Scholar] [CrossRef]
- Seyhan, A.; Akin, O.D.; Ertaş, S.; Ata, B.; Yakin, K.; Urman, B. A Survey of Women Who Cryopreserved Oocytes for Non-Medical Indications (Social Fertility Preservation). Reprod. Sci. 2021. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.Q.; Tan, A.W.K.; Lau, M.S.K.; Tan, H.H.; Nadarajah, S. Social Oocyte Freezing: A Survey among Singaporean Female Medical Students. J. Obstet. Gynaecol. Res. 2014, 40, 1345–1352. [Google Scholar] [CrossRef] [Green Version]
- Hammarberg, K.; Kirkman, M.; Pritchard, N.; Hickey, M.; Peate, M.; McBain, J.; Agresta, F.; Bayly, C.; Fisher, J. Reproductive Experiences of Women Who Cryopreserved Oocytes for Non-Medical Reasons. Hum. Reprod. 2017, 32, 575–581. [Google Scholar] [CrossRef]
- Wafi, A.; Nekkebroeck, J.; Blockeel, C.; De Munck, N.; Tournaye, H.; De Vos, M. A Follow-up Survey on the Reproductive Intentions and Experiences of Women Undergoing Planned Oocyte Cryopreservation. Reprod. Biomed. Online 2020, 40, 207–214. [Google Scholar] [CrossRef]
- Goswami, M.; Murdoch, A.P.; Haimes, E. To Freeze or Not to Freeze Embryos: Clarity, Confusion and Conflict. Hum. Fertil. 2015, 18, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Cattapan, A.; Baylis, F. Frozen in Perpetuity: “abandoned Embryos” in Canada. Reprod. Biomed. Soc. 2015, 1, 104–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adeleye, A.; Sauer, M. Managing the Unique Medical and Reproductive Needs of the Transgender Population. J. Reprod. Med. 2017, 62, 345–349. [Google Scholar]
- Montjean, D.; Pauly, V.; Gervoise-Boyer, M.; Amar-Hoffet, A.; Geoffroy-Siraudin, C.; Boyer, P. Is It Worth It to Cryopreserve Embryos with Blastulation Delay at Day 5? Zygote 2019, 27, 219–224. [Google Scholar] [CrossRef]
- Andersen, C.Y.; Kristensen, S.G. Novel Use of the Ovarian Follicular Pool to Postpone Menopause and Delay Osteoporosis. Reprod. Biomed. Online 2015, 31, 128–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balkenende, E.M.E.; van Rooij, F.B.; van der Veen, F.; Goddijn, M. Oocyte or Ovarian Tissue Banking: Decision-Making in Women Aged 35 Years or Older Facing Age-Related Fertility Decline. Reprod. Biomed. Online 2020, 41, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Hoekman, E.J.; Louwe, L.A.; Rooijers, M.; van der Westerlaken, L.A.J.; Klijn, N.F.; Pilgram, G.S.K.; de Kroon, C.D.; Hilders, C.G.J.M. Ovarian Tissue Cryopreservation: Low Usage Rates and High Live-Birth Rate after Transplantation. Acta Obstet. Gynecol. Scand. 2020, 99, 213–221. [Google Scholar] [CrossRef]
- Tsafrir, A.; Haimov-Kochman, R.; Margalioth, E.J.; Eldar-Geva, T.; Gal, M.; Bdolah, Y.; Imbar, T.; Hurwitz, A.; Ben-Chetrit, A.; Goldberg, D. Ovarian Stimulation for Oocyte Cryopreservation for Prevention of Age-Related Fertility Loss: One in Five Is a Low Responder. Gynecol. Endocrinol. Off. J. Int. Soc. Gynecol. Endocrinol. 2015, 31, 779–782. [Google Scholar] [CrossRef]
- Doyle, J.O.; Richter, K.S.; Lim, J.; Stillman, R.J.; Graham, J.R.; Tucker, M.J. Successful Elective and Medically Indicated Oocyte Vitrification and Warming for Autologous in Vitro Fertilization, with Predicted Birth Probabilities for Fertility Preservation According to Number of Cryopreserved Oocytes and Age at Retrieval. Fertil. Steril. 2016, 105, 459–466.e2. [Google Scholar] [CrossRef] [Green Version]
- Wennberg, A.-L. Social Freezing of Oocytes: A Means to Take Control of Your Fertility. Upsala J. Med. Sci. 2020, 125, 95–98. [Google Scholar] [CrossRef]
- Mesen, T.B.; Mersereau, J.E.; Kane, J.B.; Steiner, A.Z. Optimal Timing for Elective Egg Freezing. Fertil. Steril. 2015, 103, 1551–1556. [Google Scholar] [CrossRef] [Green Version]
- Cobo, A.; García-Velasco, J.; Domingo, J.; Pellicer, A.; Remohí, J. Elective and Onco-Fertility Preservation: Factors Related to IVF Outcomes. Hum. Reprod. 2018, 33, 2222–2231. [Google Scholar] [CrossRef]
- Cobo, A.; García-Velasco, J.A.; Coello, A.; Domingo, J.; Pellicer, A.; Remohí, J. Oocyte Vitrification as an Efficient Option for Elective Fertility Preservation. Fertil. Steril. 2016, 105, 755–764.e8. [Google Scholar] [CrossRef] [Green Version]
- Goldman, R.H.; Racowsky, C.; Farland, L.V.; Munné, S.; Ribustello, L.; Fox, J.H. Predicting the Likelihood of Live Birth for Elective Oocyte Cryopreservation: A Counseling Tool for Physicians and Patients. Hum. Reprod. 2017, 32, 853–859. [Google Scholar] [CrossRef]
- Wang, R.; Pan, W.; Jin, L.; Li, Y.; Geng, Y.; Gao, C.; Chen, G.; Wang, H.; Ma, D.; Liao, S. Artificial Intelligence in Reproductive Medicine. Reproduction 2019, 158, R139–R154. [Google Scholar] [CrossRef]
- Kaufmann, S.J.; Eastaugh, J.L.; Snowden, S.; Smye, S.W.; Sharma, V. The Application of Neural Networks in Predicting the Outcome of Fertilization. Hum. Reprod. 1997, 12, 1454–1457. [Google Scholar] [CrossRef] [Green Version]
- Meseguer, M.; Kruhne, U.; Laursen, S. Full in Vitro Fertilization Laboratory Mechanization: Toward Robotic Assisted Reproduction? Fertil. Steril. 2012, 97, 1277–1286. [Google Scholar] [CrossRef] [PubMed]
- Kort, J.; Behr, B. Biomechanics and Developmental Potential of Oocytes and Embryos. Fertil. Steril. 2017, 108, 738–741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faramarzi, A.; Khalili, M.A.; Omidi, M. Morphometric Analysis of Human Oocytes Using Time Lapse: Does It Predict Embryo Developmental Outcomes? Hum. Fertil. 2019, 22, 171–176. [Google Scholar] [CrossRef]
- Cavalera, F.; Zanoni, M.; Merico, V.; Bui, T.T.H.; Belli, M.; Fassina, L.; Garagna, S.; Zuccotti, M. A Neural Network-Based Identification of Developmentally Competent or Incompetent Mouse wn Oocytes. J. Vis. Exp. JoVE 2018. [Google Scholar] [CrossRef]
- Yanez, L.Z.; Han, J.; Behr, B.B.; Pera, R.A.R.; Camarillo, D.B. Human Oocyte Developmental Potential Is Predicted by Mechanical Properties within Hours after Fertilization. Nat. Commun. 2016, 7, 10809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dell’Aquila, M.E.; Cho, Y.S.; Martino, N.A.; Uranio, M.F.; Rutigliano, L.; Hinrichs, K. OMICS for the Identification of Biomarkers for Oocyte Competence, with Special Reference to the Mare as a Prospective Model for Human Reproductive Medicine; IntechOpen: London, UK, 2012. [Google Scholar] [CrossRef] [Green Version]
- Egea, R.R.; Puchalt, N.G.; Escrivá, M.M.; Varghese, A.C. OMICS: Current and Future Perspectives in Reproductive Medicine and Technology. J. Hum. Reprod. Sci. 2014, 7, 73–92. [Google Scholar] [CrossRef] [PubMed]
- Gunnala, V.; Schattman, G. Oocyte Vitrification for Elective Fertility Preservation: The Past, Present, and Future. Curr. Opin. Obstet. Gynecol. 2017, 29, 59–63. [Google Scholar] [CrossRef]
- Rybak, E.A.; Lieman, H.J. Egg Freezing, Procreative Liberty, and ICSI: The Double Standards Confronting Elective Self-Donation of Oocytes. Fertil. Steril. 2009, 92, 1509–1512. [Google Scholar] [CrossRef]
- Cobo, A.; Serra, V.; Garrido, N.; Olmo, I.; Pellicer, A.; Remohí, J. Obstetric and Perinatal Outcome of Babies Born from Vitrified Oocytes. Fertil. Steril. 2014, 102, 1006–1015.e4. [Google Scholar] [CrossRef] [PubMed]
- Goold, I.; Savulescu, J. In Favour of Freezing Eggs for Non-Medical Reasons. Bioethics 2009, 23, 47–58. [Google Scholar] [CrossRef]
- Chian, R.-C.; Huang, J.Y.J.; Tan, S.L.; Lucena, E.; Saa, A.; Rojas, A.; Ruvalcaba Castellón, L.A.; García Amador, M.I.; Montoya Sarmiento, J.E. Obstetric and Perinatal Outcome in 200 Infants Conceived from Vitrified Oocytes. Reprod. Biomed. Online 2008, 16, 608–610. [Google Scholar] [CrossRef]
- Hodes-Wertz, B.; Druckenmiller, S.; Smith, M.; Noyes, N. What Do Reproductive-Age Women Who Undergo Oocyte Cryopreservation Think about the Process as a Means to Preserve Fertility? Fertil. Steril. 2013, 100, 1343–1349. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, K.; Culley, L.; Hudson, N.; Mitchell, H.; Lavery, S. Oocyte Cryopreservation for Social Reasons: Demographic Profile and Disposal Intentions of UK Users. Reprod. Biomed. Online 2015, 31, 239–245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Platts, S.; Trigg, B.; Bracewell-Milnes, T.; Jones, B.P.; Saso, S.; Parikh, J.; Thum, M.Y. Exploring women’s attitudes, knowledge, and intentions to use oocyte freezing for non-medical reasons: A systematic review. Acta Obstet. Gynecol. Scand. 2021, 100, 383–393. [Google Scholar] [CrossRef]
- Greenwood, E.A.; Pasch, L.A.; Hastie, J.; Cedars, M.I.; Huddleston, H.G. To Freeze or Not to Freeze: Decision Regret and Satisfaction Following Elective Oocyte Cryopreservation. Fertil. Steril. 2018, 109, 1097–1104.e1. [Google Scholar] [CrossRef]
- Ethics Committee of the American Society for Reproductive Medicine. Planned Oocyte Cryopreservation for Women Seeking to Preserve Future Reproductive Potential: An Ethics Committee Opinion. Fertil. Steril. 2018, 110, 1022–1028. [Google Scholar] [CrossRef] [Green Version]
- Hickman, T.N.; McKenzie, L.J.; Schlenker, T.; Pinasco, M.A. First Successful Delivery in Texas Using Vitrified Human Oocytes: A Case Report. J. Reprod. Med. 2013, 58, 538–540. [Google Scholar]
- Belaisch-Allart, J.; Brzakowski, M.; Chouraqui, A.; Grefenstette, I.; Mayenga, J.-M.; Muller, E.; Belaid, Y.; Kulski, O. Social egg freezing: Which problems? Gynecol. Obstet. Fertil. 2013, 41, 518–520. [Google Scholar] [CrossRef]
- Frigout, L. Préservation Dite « Sociétale » de la Fertilité de la Femme. 2019. Available online: https://dumas.ccsd.cnrs.fr/dumas-03139017/document (accessed on 15 January 2021).
- Bénard, J.; Calvo, J.; Comtet, M.; Benoit, A.; Sifer, C.; Grynberg, M. Fertility preservation in women of the childbearing age: Indications and strategies. J. Gynecol. Obstet. Biol. Reprod. 2016, 45, 424–444. [Google Scholar] [CrossRef]
- Shkedi-Rafid, S.; Hashiloni-Dolev, Y. Egg Freezing for Non-Medical Uses: The Lack of a Relational Approach to Autonomy in the New Israeli Policy and in Academic Discussion. J. Med. Ethics 2012, 38, 154–157. [Google Scholar] [CrossRef]
- Kostenzer, J.; de Bont, A.; van Exel, J. Women’s Viewpoints on Egg Freezing in Austria: An Online Q-Methodology Study. BMC Med. Ethics 2021, 22, 4. [Google Scholar] [CrossRef]
- Harwood, K.A. On the Ethics of Social Egg Freezing and Fertility Preservation for Nonmedical Reasons. Medicolegal Bioeth. 2015, 5, 59–67. [Google Scholar] [CrossRef] [Green Version]
- Dondorp, W.J.; De Wert, G.M.W.R. Fertility Preservation for Healthy Women: Ethical Aspects. Hum. Reprod. 2009, 24, 1779–1785. [Google Scholar] [CrossRef] [Green Version]
- Mertes, H.; Pennings, G. Elective Oocyte Cryopreservation: Who Should Pay? Hum. Reprod. 2012, 27, 9–13. [Google Scholar] [CrossRef] [Green Version]
- Fritz, R.; Jindal, S. Reproductive Aging and Elective Fertility Preservation. J. Ovarian Res. 2018, 11, 66. [Google Scholar] [CrossRef]
- Van Loendersloot, L.L.; Moolenaar, L.M.; Mol, B.W.J.; Repping, S.; van der Veen, F.; Goddijn, M. Expanding Reproductive Lifespan: A Cost-Effectiveness Study on Oocyte Freezing. Hum. Reprod. 2011, 26, 3054–3060. [Google Scholar] [CrossRef] [Green Version]
- Hirshfeld-Cytron, J.; Grobman, W.A.; Milad, M.P. Fertility Preservation for Social Indications: A Cost-Based Decision Analysis. Fertil. Steril. 2012, 97, 665–670. [Google Scholar] [CrossRef]
- Daniluk, J.C.; Koert, E. Childless Women’s Beliefs and Knowledge about Oocyte Freezing for Social and Medical Reasons. Hum. Reprod. 2016, 31, 2313–2320. [Google Scholar] [CrossRef] [Green Version]
- Milman, L.W.; Senapati, S.; Sammel, M.D.; Cameron, K.D.; Gracia, C. Assessing Reproductive Choices of Women and the Likelihood of Oocyte Cryopreservation in the Era of Elective Oocyte Freezing. Fertil. Steril. 2017, 107, 1214–1222.e3. [Google Scholar] [CrossRef] [Green Version]
- Santo, E.V.E.; Dieamant, F.; Petersen, C.G.; Mauri, A.L.; Vagnini, L.D.; Renzi, A.; Zamara, C.; Oliveira, J.B.A.; Baruffi, R.L.R.; Franco, J.G. Social Oocyte Cryopreservation: A Portrayal of Brazilian Women. JBRA Assist. Reprod. 2017, 21, 101–104. [Google Scholar] [CrossRef]
- Inhorn, M.C.; Birenbaum-Carmeli, D.; Birger, J.; Westphal, L.M.; Doyle, J.; Gleicher, N.; Meirow, D.; Dirnfeld, M.; Seidman, D.; Kahane, A.; et al. Elective Egg Freezing and Its Underlying Socio-Demography: A Binational Analysis with Global Implications. Reprod. Biol. Endocrinol. RBE 2018, 16, 70. [Google Scholar] [CrossRef] [Green Version]
- Esfandiari, N.; Litzky, J.; Sayler, J.; Zagadailov, P.; George, K.; DeMars, L. Egg Freezing for Fertility Preservation and Family Planning: A Nationwide Survey of US Obstetrics and Gynecology Residents. Reprod. Biol. Endocrinol. RBE 2019, 17, 16. [Google Scholar] [CrossRef] [Green Version]
- Tozzo, P.; Fassina, A.; Nespeca, P.; Spigarolo, G.; Caenazzo, L. Understanding Social Oocyte Freezing in Italy: A Scoping Survey on University Female Students’ Awareness and Attitudes. Life Sci. Soc. Policy 2019, 15, 3. [Google Scholar] [CrossRef] [PubMed]
- Cardozo, E.R.; Turocy, J.M.; James, K.E.; Freeman, M.P.; Toth, T.L. Employee Benefit or Occupational Hazard? How Employer Coverage of Egg Freezing Impacts Reproductive Decisions of Graduate Students. FS Rep. 2020, 1, 186–192. [Google Scholar] [CrossRef]
- Johnston, M.; Fuscaldo, G.; Richings, N.M.; Gwini, S.; Catt, S. Cracked Open: Exploring Attitudes on Access to Egg Freezing. Sex. Reprod. Health Matters 2020, 28, 1758441. [Google Scholar] [CrossRef]
- Caughey, L.E.; White, K.M. Psychosocial Determinants of Women’s Intentions and Willingness to Freeze Their Eggs. Fertil. Steril. 2021, 115, 742–752. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.; Kim, Y.Y.; Noh, K.; Ku, S.-Y. A New Possibility in Fertility Preservation: The Artificial Ovary. J. Tissue Eng. Regen. Med. 2019, 13, 1294–1315. [Google Scholar] [CrossRef] [PubMed]
- Bioengineering and Evaluation of Artificial Ovaries for Fertility Preservation. Ph.D. Thesis. Available online: https://phd.leeds.ac.uk/project/226-bioengineering-and-evaluation-of-artificial-ovaries-for-fertility-preservation (accessed on 20 July 2021).
- Gallardo, M.; Saenz, J.; Risco, R. Human Oocytes and Zygotes Are Ready for Ultra-Fast Vitrification after 2 Minutes of Exposure to Standard CPA Solutions. Sci. Rep. 2019, 9, 15986. [Google Scholar] [CrossRef] [Green Version]
- Capodanno, F.; Daolio, J.; De Feo, G.; Falbo, A.; Morini, D.; Nicoli, A.; Braglia, L.; Villani, M.; La Sala, G.B.; Parmegiani, L.; et al. A Monocentric Analysis of the Efficacy of Extracellular Cryoprotectants in Unfrozen Solutions for Cleavage Stage Embryos. Reprod. Biol. Endocrinol. RBE 2019, 17, 84. [Google Scholar] [CrossRef]
- Practice Committee of the American Society for Reproductive Medicine. Fertility Preservation in Patients Undergoing Gonadotoxic Therapy or Gonadectomy: A Committee Opinion. Fertil. Steril. 2019, 112, 1022–1033. [Google Scholar] [CrossRef] [PubMed]
- Pennings, G.; Couture, V.; Ombelet, W. Social Sperm Freezing. Hum. Reprod. 2021, 36, 833–839. [Google Scholar] [CrossRef] [PubMed]
| Author | Perspectives/Counselling | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Year | Cases | Study Design | Age (Years) | Positive Attitude | Single | Higher Education | Costs Concerns | Number of OC | Birth | Usage Rate | Willing to Donate Eggs | |
| Hodes-Wertz [56] | 2013 | 183 | Survey | 23–46 38 ± 2.7 | 100% | 84% | - | - | 6–10 | 3 | 6% | 63% |
| Daniluk [73] | 2015 | 500 | Self-report questionnaire | 18–38 | 66% | 58.2% | 73% | 85.6% | - | - | - | 57.2–67% |
| Lewis [6] | 2016 | 1064 | Online questionnaire | 18–65 | 17.8% | 63.2% | - | - | - | - | - | - |
| Hammarberg [26] | 2016 | 95 | Survey | 37.1 * | - | 86% | 89% | - | 14.2 | 3 | 6% | - |
| Milman [74] | 2017 | 1000 | Cross-sectional | 34.5 * (28–38) | - | 23% | 13.6% | - | - | - | - | - |
| Santo [75] | 2017 | 444 | Survey | 33.2 ± 6.6 | 85.4% | 13.1% | 28.6% | 49.3% | - | - | - | - |
| Inhorn [76] | 2018 | 150 | Audio-recorded interview | 36.2–36.6 | - | 85% | 100% | - | - | 1 | 6% | - |
| Esfandiari [77] | 2019 | 103 | Survey | 26–30 | 44.7% | 23.3% | 100% | - | - | - | - | 57.2% |
| Tozzo [78] | 2019 | 930 | Survey | 18–35 | 19.5% | 48.7% | 100% | 48.4% | - | - | - | 42.5% |
| Cardozo [79] | 2020 | 171 | Survey | 21–45 | - | 50% | 100% | 59% | - | - | - | - |
| Johnston [80] | 2020 | 656 | Survey | 18–60 | >65% | 27.6% | 65.1% | - | - | - | - | - |
| Wafi [27] | 2020 | 138 | Online questionnaire | 35.7 ± 0.9 | - | 83% | 15% | - | 17.6 | 13 | 20.3% | 76.5% |
| Caughey [81] | 2021 | 234 | Online questionnaire | 25–43 | - | 22.2% | 36.8% | - | - | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varlas, V.N.; Bors, R.G.; Albu, D.; Penes, O.N.; Nasui, B.A.; Mehedintu, C.; Pop, A.L. Social Freezing: Pressing Pause on Fertility. Int. J. Environ. Res. Public Health 2021, 18, 8088. https://doi.org/10.3390/ijerph18158088
Varlas VN, Bors RG, Albu D, Penes ON, Nasui BA, Mehedintu C, Pop AL. Social Freezing: Pressing Pause on Fertility. International Journal of Environmental Research and Public Health. 2021; 18(15):8088. https://doi.org/10.3390/ijerph18158088
Chicago/Turabian StyleVarlas, Valentin Nicolae, Roxana Georgiana Bors, Dragos Albu, Ovidiu Nicolae Penes, Bogdana Adriana Nasui, Claudia Mehedintu, and Anca Lucia Pop. 2021. "Social Freezing: Pressing Pause on Fertility" International Journal of Environmental Research and Public Health 18, no. 15: 8088. https://doi.org/10.3390/ijerph18158088










