Evaluation of Groundwater Quality for Human Consumption and Irrigation in Relation to Arsenic Concentration in Flow Systems in a Semi-Arid Mexican Region
Abstract
:1. Introduction
2. Materials and Methods
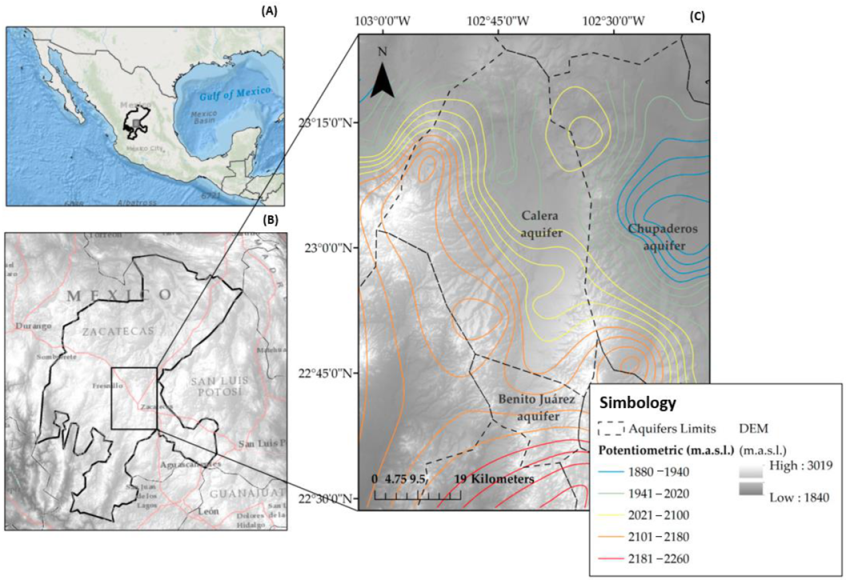
2.1. Study Area
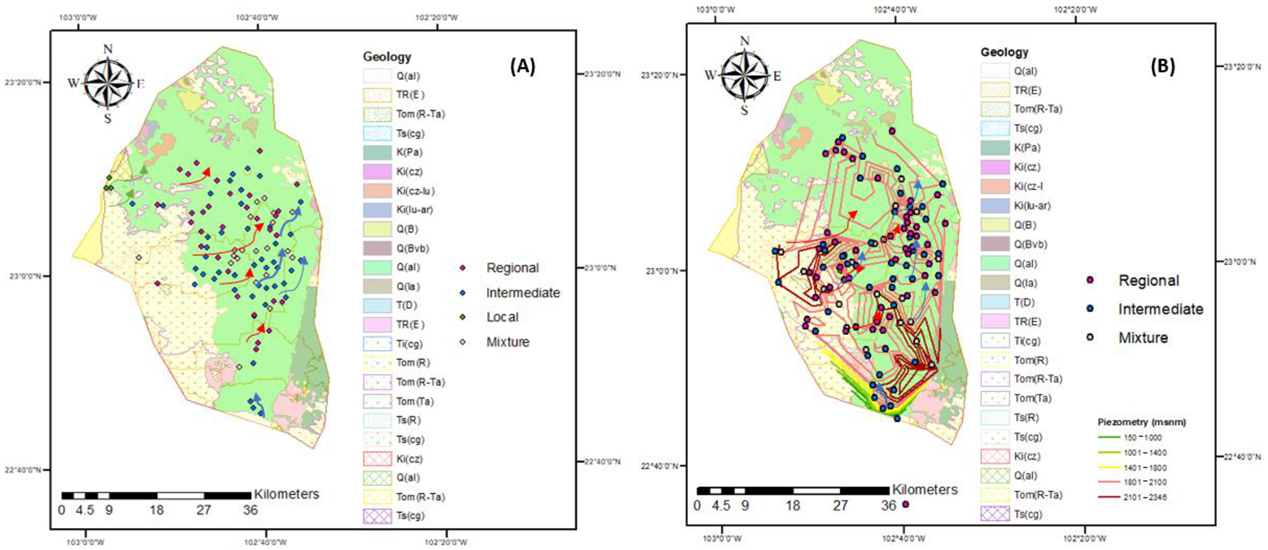
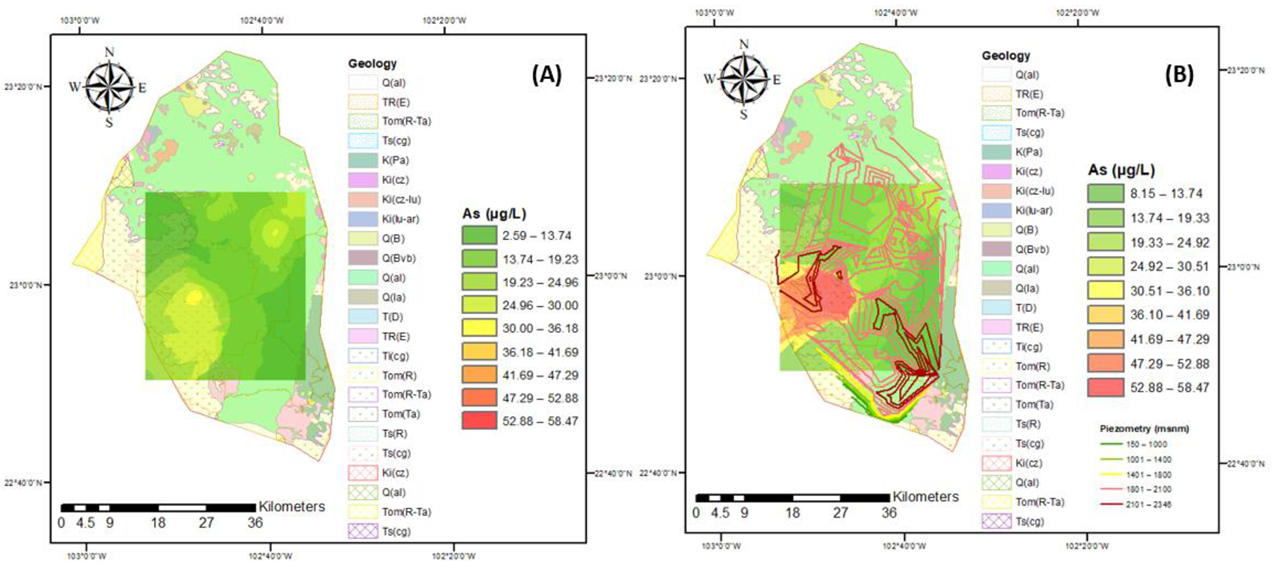
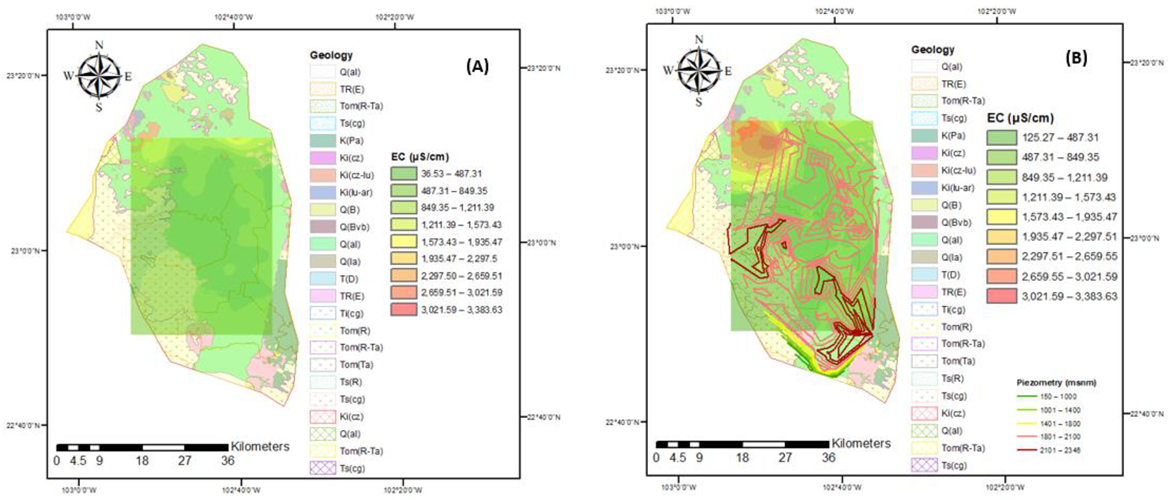
2.2. Geological Features
2.3. Sample Collection and Concentration Determination
2.4. Data Analysis
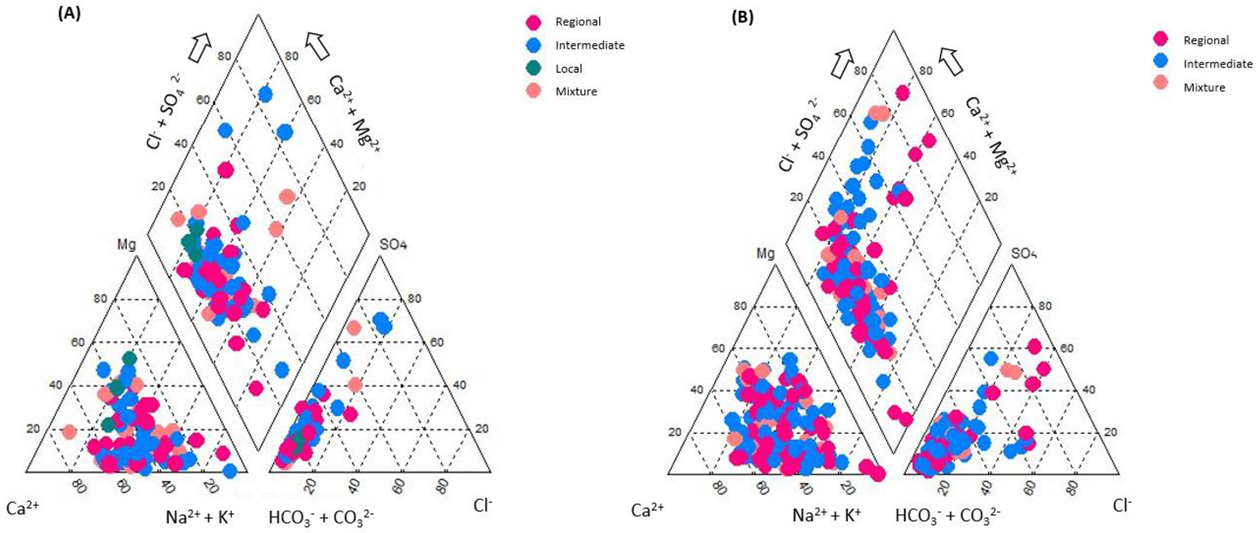
2.4.1. Flow Systems Identification
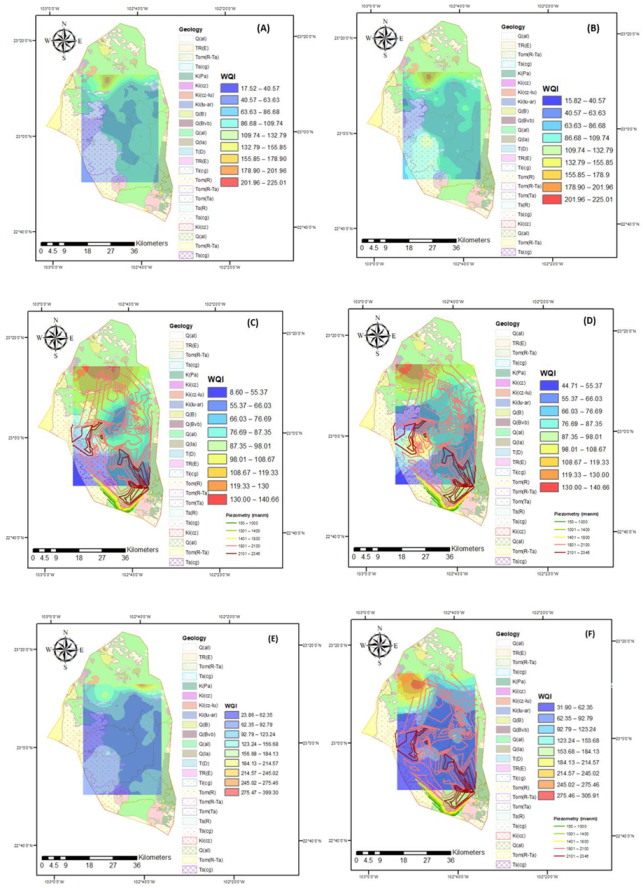
2.4.2. Drinking Water Quality
2.4.3. Irrigation Water Quality
2.4.4. Statistical Analysis
3. Results and Discussion
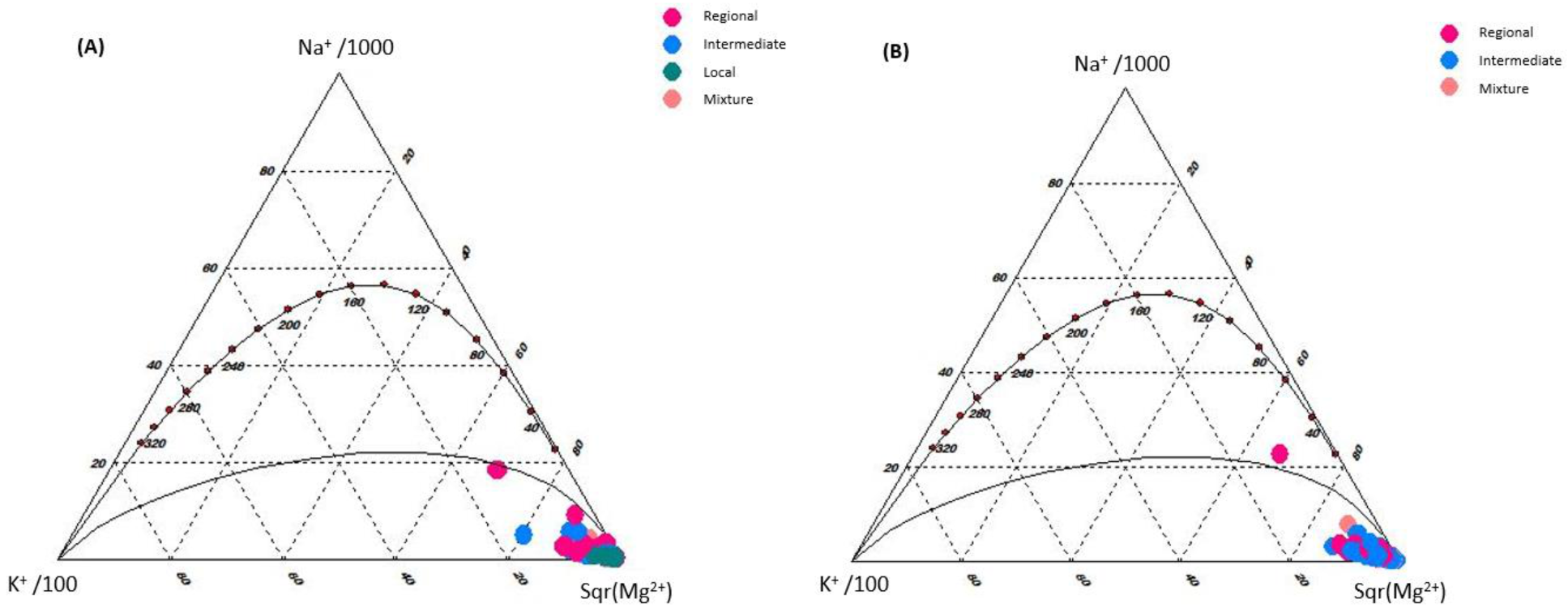
3.1. Identification of Flow Systems
3.2. Water Quality for Human Use
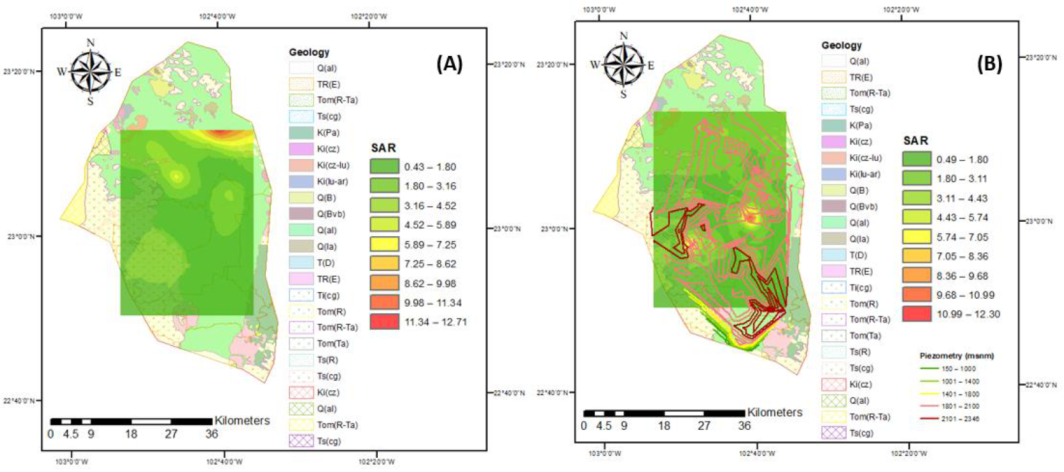
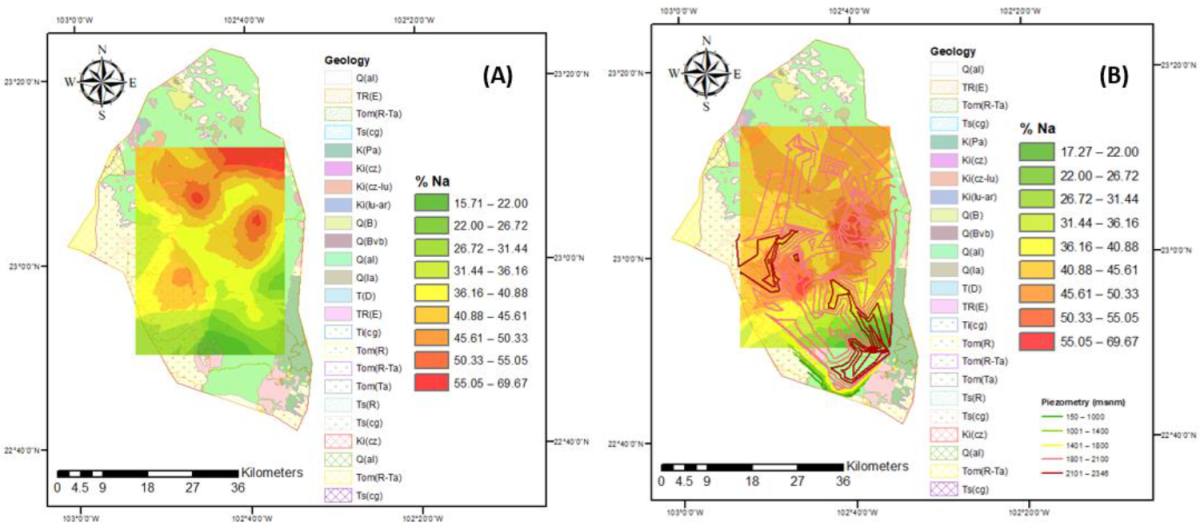
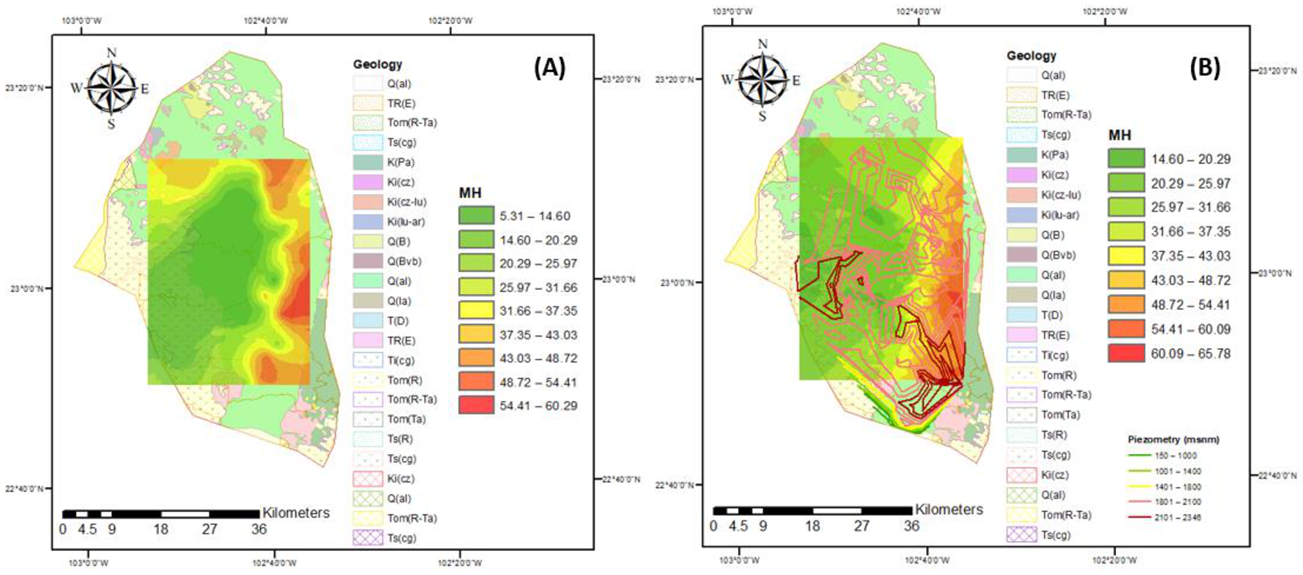
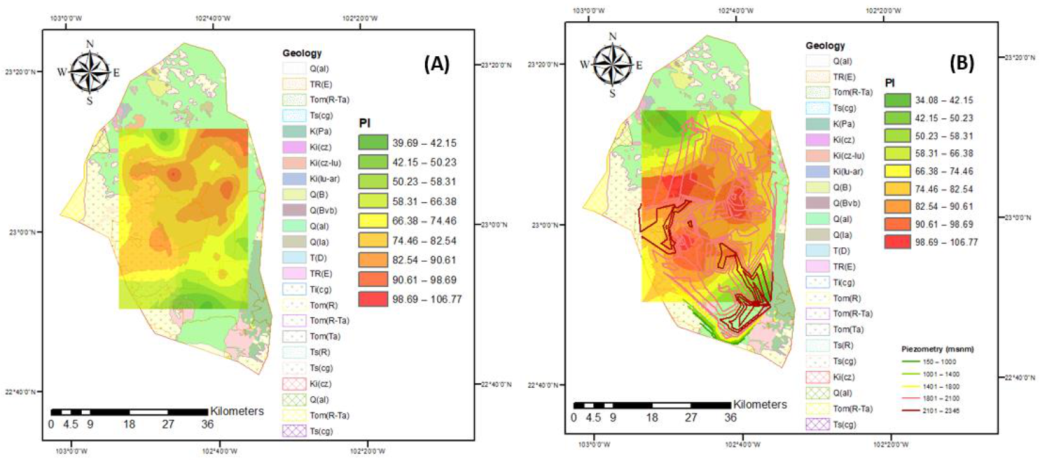
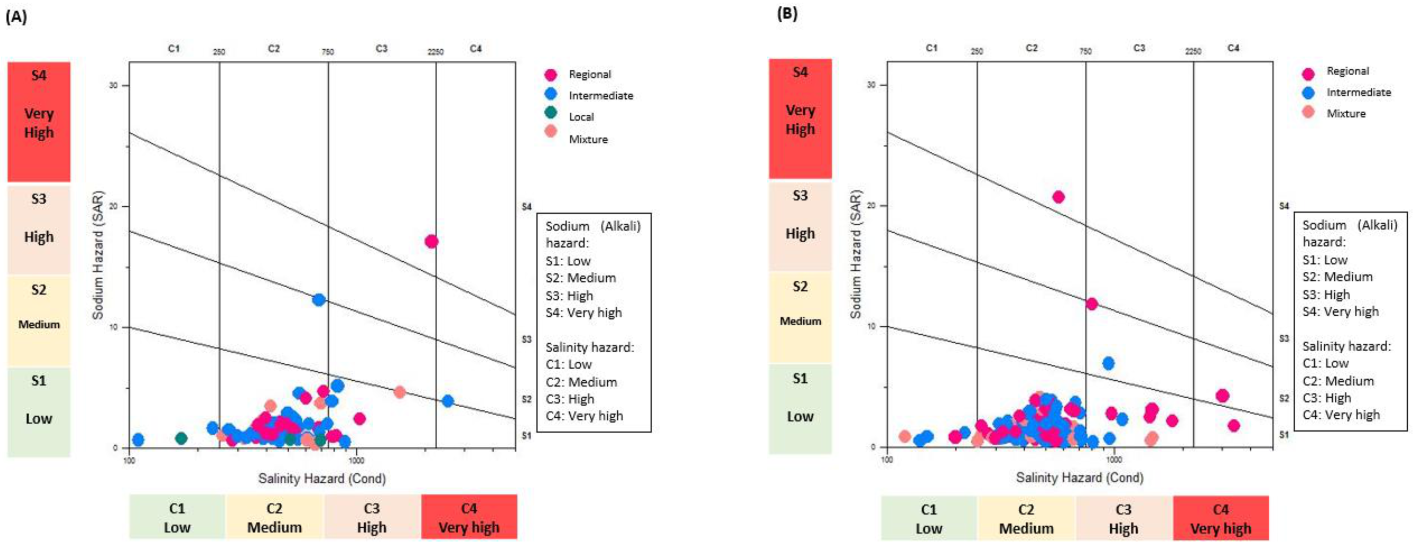
3.3. Irrigation Water Quality
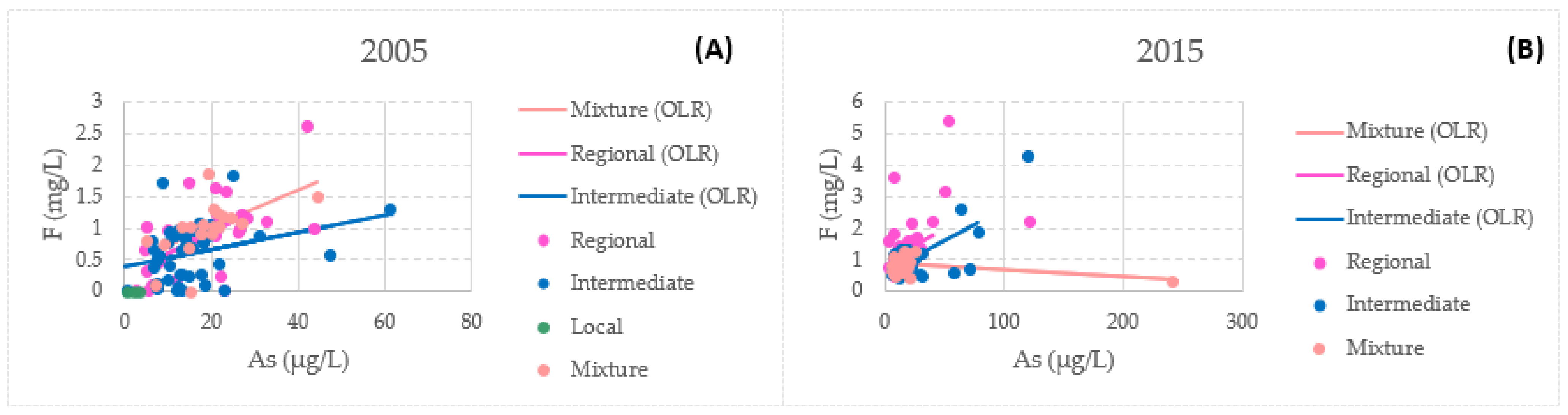
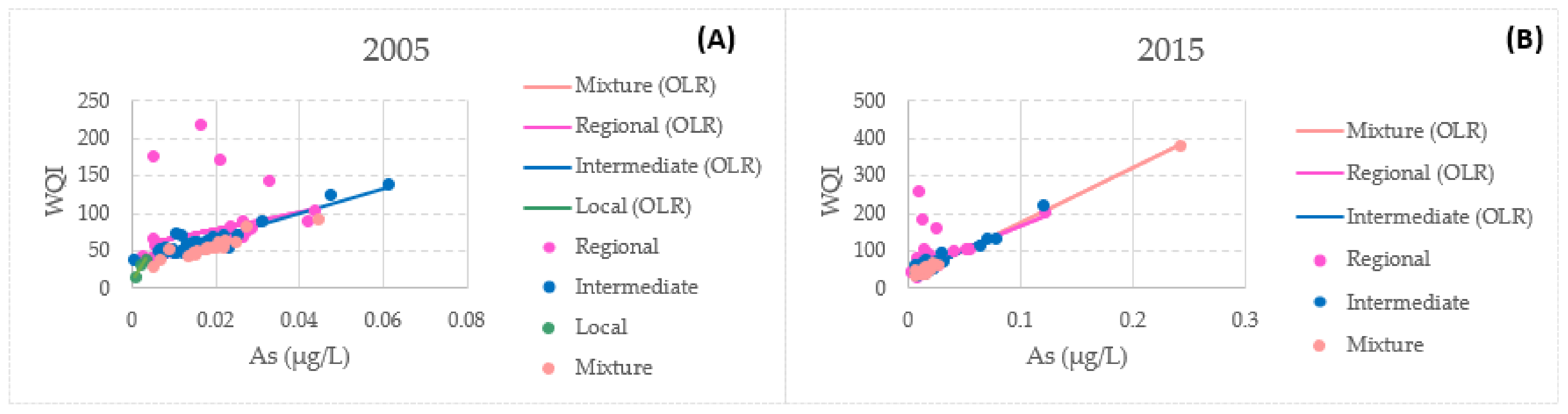
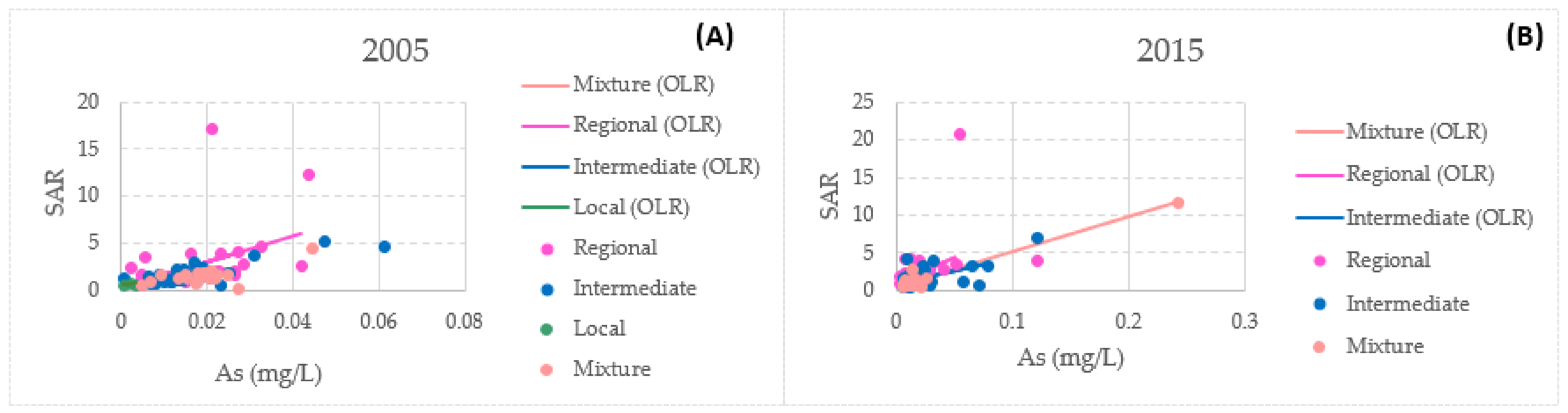
3.4. Bivariate Data Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kalhor, K.; Ghasemizadeh, R.; Rajic, L.; Alshawabkeh, A. Assessment of groundwater quality and remediation in karst aquifers: A review. Groundw. Sustain. Dev. 2019, 8, 104–121. [Google Scholar] [CrossRef] [PubMed]
- Fazi, S.; Amalfitano, S.; Casentini, B.; Davolos, D.; Pietrangeli, B.; Crognale, S.; Lotti, F.; Rossetti, S. Arsenic removal from naturally contaminated waters: A review of methods combining chemical and biological treatments. Rend. Lincei 2016, 27, 51–58. [Google Scholar] [CrossRef]
- Carranza-Álvarez, C.; Medellín-Castillo, N.A.; Maldonado-Miranda, J.J.; Alfaro-de-la-Torre, M.C. Water Quality Management in San Luis Potosi. Available online: https://www.researchgate.net/publication/336129506_Water_Quality_Management_in_San_Luis_Potosi_Mexico (accessed on 24 July 2021).
- Villeneuve, S.; Cook, P.G.; Shanafield, M.; Wood, C.; White, N. Groundwater recharge via infiltration through an ephemeral riverbed, central Australia. J. Arid Environ. 2015, 117, 47–58. [Google Scholar] [CrossRef]
- Adam, A.B.; Appiah-Adjei, E.K. Groundwater potential for irrigation in the Nabogo basin, Northern Region of Ghana. Groundw. Sustain. Dev. 2019, 9, 100274. [Google Scholar] [CrossRef]
- Peña-Guzmán, C.; Ulloa-Sánchez, S.; Mora, K.; Helena-Bustos, R.; Lopez-Barrera, E.; Alvarez, J.; Rodriguez-Pinzón, M. Emerging pollutants in the urban water cycle in Latin America: A review of the current literature. J. Environ. Manag. 2019, 237, 408–423. [Google Scholar] [CrossRef]
- Lu, S.; Zhang, X.; Bao, H.; Skitmore, M. Review of social water cycle research in a changing environment. Renew. Sustain. Energy Rev. 2016, 63, 132–140. [Google Scholar] [CrossRef] [Green Version]
- Pujades, E.; Jurado, A. Groundwater-related aspects during the development of deep excavations below the water table: A short review. Undergr. Sp. 2019, 1–11. [Google Scholar] [CrossRef]
- Winston, R.B.; Ayotte, J.D. Performance Assessments of a Novel Well Design for Reducing Exposure to Bedrock-Derived Arsenic. Groundwater 2018, 56, 762–769. [Google Scholar] [CrossRef]
- Schmidt, S.I.; Hahn, H.J. What is groundwater and what does this mean to fauna?—An opinion. Limnologica 2012, 42, 1–6. [Google Scholar] [CrossRef]
- Gevaert, A.; Hoogesteger, J.; Stoof, C. Suitability of using Groundwater Temperature and Geology to Predict Arsenic Contamination in Drinking Water—A Case Study in Central Mexico. Available online: https://ecommons.cornell.edu/handle/1813/29590 (accessed on 24 July 2021).
- Reyes-Toscano, C.A.; Alfaro-Cuevas-Villanueva, R.; Cortés-Martínez, R.; Morton-Bermea, O.; Hernández-Álvarez, E.; Buenrostro-Delgado, O.; Ávila-Olivera, J.A. Hydrogeochemical characteristics and assessment of drinkingwater quality in the Urban Area of Zamora, Mexico. Water 2020, 12, 556. [Google Scholar] [CrossRef] [Green Version]
- Navarro, O.; González, J.; Júnez-Ferreira, H.E.; Bautista, C.-F.; Cardona, A. Correlation of Arsenic and Fluoride in the Groundwater for Human Consumption in a Semiarid Region of Mexico. Procedia Eng. 2017, 186, 333–340. [Google Scholar] [CrossRef]
- Smedley, P.L.; Kinniburgh, D.G. A review of the source, behaviour and distribution of arsenic in natural waters. Appl. Geochem. 2002, 17, 517–568. [Google Scholar] [CrossRef] [Green Version]
- Karakuş, C.B. Evaluation of groundwater quality in Sivas province (Turkey) using water quality index and GIS-based analytic hierarchy process. Int. J. Environ. Health Res. 2019, 29, 500–519. [Google Scholar] [CrossRef]
- Dehbandi, R.; Abbasnejad, A.; Karimi, Z.; Herath, I.; Bundschuh, J. Hydrogeochemical controls on arsenic mobility in an arid inland basin, Southeast of Iran: The role of alkaline conditions and salt water intrusion. Environ. Pollut. 2019, 249, 910–922. [Google Scholar] [CrossRef]
- Zhang, L.; Qin, X.; Tang, J.; Liu, W.; Yang, H. Review of arsenic geochemical characteristics and its significance on arsenic pollution studies in karst groundwater, Southwest China. Appl. Geochem. 2017, 77, 80–88. [Google Scholar] [CrossRef]
- Sarabia Meléndez, I.F.; Cisneros Almazán, R.; Aceves De Alba, J.; Durán García, H.M.; Castro Larragoitia, J. Calidad del Agua de Riego en Suelos Agrícolas y Cultivos Del Valle de San Luis Potosí, México. Rev. Int. Contam. Ambient. 2011, 27, 103–113. Available online: http://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S0188-49992011000200002 (accessed on 27 July 2021).
- Rahman, M.A.; Rahman, A.; Khan, M.Z.K.; Renzaho, A.M.N. Human health risks and socio-economic perspectives of arsenic exposure in Bangladesh: A scoping review. Ecotoxicol. Environ. Saf. 2018, 150, 335–343. [Google Scholar] [CrossRef]
- Uppal, J.S.; Zheng, Q.; Le, X.C. Arsenic in drinking water—Recent examples and updates from Southeast Asia. Curr. Opin. Environ. Sci. Health 2019, 7, 126–135. [Google Scholar] [CrossRef]
- Sarkar, A.; Paul, B. The global menace of arsenic and its conventional remediation—A critical review. Chemosphere 2016, 158, 37–49. [Google Scholar] [CrossRef]
- Powers, M.; Yracheta, J.; Harvey, D.; O’Leary, M.; Best, L.G.; Black Bear, A.; MacDonald, L.; Susan, J.; Hasan, K.; Thomas, E.; et al. Arsenic in groundwater in private wells in rural North Dakota and South Dakota: Water quality assessment for an intervention trial. Environ. Res. 2019, 168, 41–47. [Google Scholar] [CrossRef]
- Ersbøll, A.K.; Monrad, M.; Sørensen, M.; Baastrup, R.; Hansen, B.; Bach, F.W.; Tjønneland, A.; Overvad, K.; Raaschou-Nielsen, O. Low-level exposure to arsenic in drinking water and incidence rate of stroke: A cohort study in Denmark. Environ. Int. 2018, 120, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Stern, E.A. Global Arsenic Contamination: Living with the Poison Nectar. Environ. Sci. Policy Sustain. Dev. 2017, 59, 24–28. [Google Scholar] [CrossRef]
- Zhao, C.; Yang, J.; Zheng, Y.; Yang, J.; Guo, G.; Wang, J.; Chen, T. Effects of environmental governance in mining areas: The trend of arsenic concentration in the environmental media of a typical mining area in 25 years. Chemosphere 2019, 235, 849–857. [Google Scholar] [CrossRef] [PubMed]
- Mar Wai, K.; Umezaki, M.; Mar, O.; Umemura, M.; Watanabe, C. Arsenic exposure through drinking Water and oxidative stress Status: A cross-sectional study in the Ayeyarwady region, Myanmar. J. Trace Elem. Med. Biol. 2019, 54, 103–109. [Google Scholar] [CrossRef]
- Malyan, S.K.; Singh, R.; Rawat, M.; Kumar, M.; Pugazhendhi, A.; Kumar, A.; Kumar, V.; Kumar, S.S. An overview of carcinogenic pollutants in groundwater of India. Biocatal. Agric. Biotechnol. 2019, 21, 101288. [Google Scholar] [CrossRef]
- Liu, Q.; Lu, X.; Peng, H.; Popowich, A.; Tao, J.; Uppal, J.S.; Yan, X.; Boe, D.; Le, X.C. Speciation of arsenic—A review of phenylarsenicals and related arsenic metabolites. TrAC Trends Anal. Chem. 2018, 104, 171–182. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency. 2010 U.S. Environmental Protection Agency (EPA) Decontamination Research and Development Conference; EPA/600/R-11/052; U.S. Environmental Protection Agency: Washington, DC, USA, 2011. Available online: https://cfpub.epa.gov/si/si_public_record_report.cfm?Lab=NHSRC&dirEntryId=231764 (accessed on 24 July 2021).
- McClintock, T.R.; Chen, Y.; Bundschuh, J.; Oliver, J.T.; Navoni, J.; Olmos, V.; Lepori, E.V.; Ahsan, H.; Parvez, F. Arsenic exposure in Latin America: Biomarkers, risk assessments and related health effects. Sci. Total Environ. 2012, 429, 76–91. [Google Scholar] [CrossRef] [Green Version]
- WHO (World Health Organization). Guidelines for Drinking-Water Quality, 4th ed.; WHO: Geneva, Switzerland, 2011; ISBN 978-92-4-154995-0. Available online: https://www.who.int/publications/i/item/9789241549950 (accessed on 27 July 2021).
- Ahmad, A.; van der Wens, P.; Baken, K.; de Waal, L.; Bhattacharya, P.; Stuyfzand, P. Arsenic reduction to <1 µg/L in Dutch drinking water. Environ. Int. 2020, 134, 105253. [Google Scholar] [CrossRef]
- Flores, E. Tratamiento de Agua para Consumo Humano con Alto Contenido de Arsénico: Estudio de un Caso en Zimapán Hidalgo-México. Inf. Tecnol. 2009, 20, 85–93. [Google Scholar] [CrossRef]
- Padilla-Reyes, D.; Núñez-Peña, E.; Escalona, F.; Bluhm-Gutiérrez, J. Calidad Del Agua del Acuífero Guadalupe-Bañuelos, Estado de Zacatecas, México. GEOS 2012, 32, 367–384. Available online: https://www.ugm.org.mx/publicaciones/geos/pdf/geos12-2/padilla-32-2.pdf (accessed on 27 July 2021).
- Hoogesteger, J.; Wester, P. Regulating groundwater use: The challenges of policy implementation in Guanajuato, Central Mexico. Environ. Sci. Policy 2017, 77, 107–113. [Google Scholar] [CrossRef]
- Anuard, P.-G.; Julián, G.-T.; Hugo, J.-F.; Carlos, B.-C.; Arturo, H.-A.; Edith, O.-T.; Claudia, Á.-S. Integration of Isotopic (2H and 18O) and Geophysical Applications to Define a Groundwater Conceptual Model in Semiarid Regions. Water 2019, 11, 488. [Google Scholar] [CrossRef] [Green Version]
- Comisión Nacional del Agua Actualización de la Disponibilidad Media Anual de Agua en el Acuífero Chupaderos (3226), Estado de Zacatecas. D. Of. Fed. 2018, 1–37. Available online: https://sigagis.conagua.gob.mx/gas1/Edos_Acuiferos_18/zacatecas/DR_3226.pdf (accessed on 27 July 2021).
- INEGI. Síntesis Geográfica de Zacatecas. 1981, pp. 1–18. Available online: http://internet.contenidos.inegi.org.mx/contenidos/productos/prod_serv/contenidos/espanol/bvinegi/productos/historicos/2104/702825220686/702825220686_1.pdf (accessed on 27 July 2021).
- Carta Geológica de Zacatecas F13-6. Zacatecas. pp. 1–26. Available online: http://mapserver.sgm.gob.mx/Cartas_Online/geologia/63_F13-6_GM.pdf (accessed on 27 July 2021).
- Julio, I.; Lopez, V.; Cuevas, A.; Ramón, C.; Montiel, M.; Hernández Pérez, I. Consejo de Recursos Minerales Carta Magnética “Zacatecas” F13-6 Escala 1: 250 000 Secretaría de Economía Coordinación General de Minería. 2001, pp. 1–26. Available online: http://www.sgm.gob.mx/pdfs/textos_guia/63_F13-6_GF_INF.pdf (accessed on 27 July 2021).
- Lenore, S.; Clesceri, A.; Greenberg, E.; Trussel, R.R. Standard Methods for the Examination of Water and Wastewater, Prepared and Published Jointly by American Public Health Association, 20th ed.; American Public Health Association: Washington, DC, USA, 2006. [Google Scholar]
- Tóth, J. A theoretical analysis of groundwater flow in small drainage basins. J. Geophys. Res. 1963, 68, 4795–4812. [Google Scholar] [CrossRef]
- Avila-Sandoval, C.; Júnez-Ferreira, H.; González-Trinidad, J.; Bautista-Capetillo, C.; Pacheco-Guerrero, A.; Olmos-Trujillo, E. Spatio-temporal analysis of natural and anthropogenic arsenic sources in groundwater flow systems. Int. J. Environ. Res. Public Health 2018, 15, 2374. [Google Scholar] [CrossRef] [Green Version]
- DOF Diario Oficial de la Federación, Secretaría de Salud, Modificación a la Norma Oficial Mexicana NOM-127-SSA1-1994. “Salud Ambiental, Agua Para Uso y Consumo Humano-Límites Permisibles de Calidad y Tratamientos a Que Debe Someterse el Agua Para su Potabiliz. 2000, pp. 1–21. Available online: http://dof.gob.mx/nota_detalle.php?codigo=2063863&fecha=31/12/1969 (accessed on 27 July 2021).
- Bhavan, M.; Shah, B.; Marg, Z.; Bureau of Indian Standards (BIS). Guidelines for Drinking-water Quality, Indian Standard. 2012; pp. 1–16. Available online: http://cgwb.gov.in/Documents/WQ-standards.pdf (accessed on 27 July 2021).
- Brown, R.M.; McClelland, N.I.; Deininger, R.A.; Tozer, R.G. A water quality index—Do we dare? Water Sew. Works 1970, 117, 339–343. [Google Scholar]
- Sadat-Noori, S.M.; Ebrahimi, K.; Liaghat, A.M. Groundwater quality assessment using the Water Quality Index and GIS in Saveh-Nobaran aquifer, Iran. Environ. Earth Sci. 2014, 71, 3827–3843. [Google Scholar] [CrossRef]
- Omwene, P.I.; Öncel, M.S.; Çelen, M.; Kobya, M. Influence of arsenic and boron on the water quality index in mining stressed catchments of Emet and Orhaneli streams (Turkey). Environ. Monit. Assess. 2019, 191, 1–16. [Google Scholar] [CrossRef]
- Richards, L.A. Diagnosis and Improvement of Saline and Alkali Soil. Available online: https://www.ars.usda.gov/ARSUserFiles/20360500/hb60_pdf/hb60complete.pdf (accessed on 25 July 2021).
- Raju, N.J. Hydrogeochemical parameters for assessment of groundwater quality in the upper Gunjanaeru River basin, Cuddapah District, Andhra Pradesh, South India. Environ. Geol. 2007, 52, 1067–1074. [Google Scholar] [CrossRef]
- Szabolcs, I. The influence of irrigation water of high Sodium Carbonate content on soils. Agrokémia Talajt. 1964, 13, 237–246. [Google Scholar]
- Adimalla, N.; Qian, H. Groundwater quality evaluation using water quality index (WQI) for drinking purposes and human health risk (HHR) assessment in an agricultural region of Nanganur, south India. Ecotoxicol. Environ. Saf. 2019, 176, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Rosales-Rivera, M.; Díaz-González, L.; Verma, S.P. A new online computer program (BiDASys) for ordinary and uncertainty weighted least-squares linear regressions: Case studies from food chemistry. Rev. Mex. Ing. Quim. 2018, 17, 507–522. [Google Scholar] [CrossRef]
- Chebotarev, I.I. Metamorphism of natural waters in the crust of weathering-2. Geochim. Cosmochim. Acta 1955, 8, 137–170. [Google Scholar] [CrossRef]
- Bravo, H.R.; Jiang, F.; Hunt, R.J. Using groundwater temperature data to constrain parameter estimation in a groundwater flow model of a wetland system. Water Resour. Res. 2002, 38, 28-1–28-14. [Google Scholar] [CrossRef]
- Piper, A.M. A graphic procedure in the geochemical interpretation of water-analyses. Eos Trans. Am. Geophys. Union 1944, 25, 914–928. [Google Scholar] [CrossRef]
- Liu, C.W.; Wu, M.Z. Geochemical, mineralogical and statistical characteristics of arsenic in groundwater of the Lanyang Plain, Taiwan. J. Hydrol. 2019, 577, 123975. [Google Scholar] [CrossRef]
- Aullón Alcaine, A.; Schulz, C.; Bundschuh, J.; Jacks, G.; Thunvik, R.; Gustafsson, J.P.; Mörth, C.M.; Sracek, O.; Ahmad, A.; Bhattacharya, P. Hydrogeochemical controls on the mobility of arsenic, fluoride and other geogenic co-contaminants in the shallow aquifers of northeastern La Pampa Province in Argentina. Sci. Total Environ. 2020, 715, 136671. [Google Scholar] [CrossRef]
- Giggenbach, W.F. Geothermal solute equilibria. Derivation of Na-K-Mg-Ca geoindicators. Geochim. Cosmochim. Acta 1988, 52, 2749–2765. [Google Scholar] [CrossRef]
- Martínez-Florentino, A.K.; Esteller, M.V.; Domínguez-Mariani, E.; Expósito, J.L.; Paredes, J. Hydrogeochemistry, isotopes and geothermometry of Ixtapan de la Sal–Tonatico hot springs, Mexico. Environ. Earth Sci. 2019, 78, 600. [Google Scholar] [CrossRef]
- Abiye, T.A.; Bhattacharya, P. Arsenic concentration in groundwater: Archetypal study from South Africa. Groundw. Sustain. Dev. 2019, 9, 1–7. [Google Scholar] [CrossRef]
- Abbasnia, A.; Radfard, M.; Mahvi, A.H.; Nabizadeh, R.; Yousefi, M.; Soleimani, H.; Alimohammadi, M. Groundwater quality assessment for irrigation purposes based on irrigation water quality index and its zoning with GIS in the villages of Chabahar, Sistan and Baluchistan, Iran. Data Br. 2018, 19, 623–631. [Google Scholar] [CrossRef]
- Zhao, X.; Guo, H.; Wang, Y.; Wang, G.; Wang, H.; Zang, X.; Zhu, J. Groundwater hydrogeochemical characteristics and quality suitability assessment for irrigation and drinking purposes in an agricultural region of the North China plain. Environ. Earth Sci. 2021, 80, 162. [Google Scholar] [CrossRef]
- Adimalla, N.; Li, P.; Venkatayogi, S. Hydrogeochemical Evaluation of Groundwater Quality for Drinking and Irrigation Purposes and Integrated Interpretation with Water Quality Index Studies. Environ. Process. 2018, 5, 363–383. [Google Scholar] [CrossRef]
- Dorjderem, B.; Torres-Martínez, J.A.; Mahlknecht, J. Intensive long-term pumping in the Principal-Lagunera Region aquifer (Mexico) causing heavy impact on groundwater quality. Proc. Energy Rep. 2020, 6, 862–867. [Google Scholar] [CrossRef]
- Polya, D.A.; Sparrenbom, C.; Datta, S.; Guo, H. Groundwater arsenic biogeochemistry—Key questions and use of tracers to understand arsenic-prone groundwater systems. Geosci. Front. 2019, 10, 1635–1641. [Google Scholar] [CrossRef]
- Saeedi, M.; Abessi, O.; Sharifi, F.; Meraji, H. Development of groundwater quality index. Envin. Monit Assess 2010, 163, 327–335. [Google Scholar] [CrossRef]
- Ram, A.; Tiwari, S.K.; Pandey, H.K.; Chaurasia, A.K.; Singh, S.; Singh, Y.V. Groundwater quality assessment using water quality index (WQI) under GIS framework. Appl. Water Sci. 2021, 11, 46. [Google Scholar] [CrossRef]
- Wilcox, L.V. Classification and Use of Irrigation Water, USDA Circular 969, 19p. 1955. Available online: http://www.sciepub.com/reference/82326 (accessed on 16 March 2021).
- Hwang, J.; Park, S.; Kim, H.; Kim, M.; Jo, H.; Kim, J.; Lee, G.; Shin, I.; Kim, T. Hydrochemistry for the Assessment of Groundwater Quality in Korea. J. Agric. Chem. Environ. 2017, 6, 1–29. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.K.; Tjell, J.C.; Sloth, J.J.; Holm, P.E. Review of arsenic contamination, exposure through water and food and low cost mitigation options for rural areas. Appl. Geochem. 2014, 41, 11–33. [Google Scholar] [CrossRef]
- García-Rico, L.; Valenzuela-Rodríguez, M.P.; Meza-Montenegro, M.M.; Lopez-Duarte, A.L. Arsenic in rice and rice products in Northwestern Mexico and health risk assessment. Food Addit. Contam. Part B Surveill. 2019, 13, 25–33. [Google Scholar] [CrossRef]
- Pearson, K. VII. Mathematical contributions to the theory of evolution—III. Regression, heredity, and panmixia. Philos. Trans. R. Soc. London. Ser. A Contain. Pap. Math. Phys. Character 1896, 187, 253–318. [Google Scholar] [CrossRef] [Green Version]
- Kumar, M.; Goswami, R.; Patel, A.K.; Srivastava, M.; Das, N. Scenario, Perspectives and Mechanism of Arsenic and Fluoride Co-occurrence in the Groundwater: A Review. Chemosphere 2020, 126126. [Google Scholar] [CrossRef] [PubMed]
- Alarcón-Herrera, M.T.; Martin-Alarcon, D.A.; Gutiérrez, M.; Reynoso-Cuevas, L.; Martín-Domínguez, A.; Olmos-Márquez, M.A.; Bundschuh, J. Co-occurrence, possible origin, and health-risk assessment of arsenic and fluoride in drinking water sources in Mexico: Geographical data visualization. Sci. Total Environ. 2020, 698, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Alarcón-Herrera, M.T.; Bundschuh, J.; Nath, B.; Nicolli, H.B.; Gutierrez, M.; Reyes-Gomez, V.M.; Nuñez, D.; Martín-Dominguez, I.R.; Sracek, O. Co-occurrence of arsenic and fluoride in groundwater of semi-arid regions in Latin America: Genesis, mobility and remediation. J. Hazard. Mater. 2013, 262, 960–969. [Google Scholar] [CrossRef]
| Parameters | WHO Standard | US EPA | Mexican Official Norm NOM-127-SSA1-1994 | Bureau of Indian Standards (BIS) |
|---|---|---|---|---|
| pH | 8.5 | 6.5–8.4 | 6.5–8.5 | 6.5–8.6 |
| TDS | 500 | 500 | 500 | 2000 |
| Cl− | 250 | 250 | 250 | 1000 |
| SO4−2 | 250 | 250 | 250 | 400 |
| Total hardness | 180 | 180 | 500 | 600 |
| F− | 1.5 | 4 | 1.5 | 1.5 |
| As | 0.01 | 0.01 | 0.025 | 0.05 |
| Parameter | Wi |
|---|---|
| As | 5 |
| pH | 3 |
| TDS | 5 |
| Cl− | 5 |
| SO4−2 | 5 |
| Na+ | 4 |
| K+ | 2 |
| HCO3− | 1 |
| Ca2+ | 3 |
| Mg2+ | 3 |
| F− | 5 |
| Permissible Limits for Agricultural Irrigation from Groundwater | ||
|---|---|---|
| Index | Range | Classification |
| EC (µs/cm) | <250 | Excellent |
| 250–750 | Good | |
| 750–2250 | Doubtful | |
| >2250 | Unsuitable | |
| SAR (meq/L) | <10 | Excellent |
| 10–18 | Good | |
| 18–26 | Doubtful | |
| >26 | Unsuitable | |
| %Na | <20 | Excellent |
| 20–40 | Good | |
| 40–60 | Permissible | |
| 60–80 | Doubtful | |
| >80 | Unsuitable | |
| MH (%) | <50 | Desirable |
| >50 | Undesirable | |
| PI (%) | <25 | Unsuitable |
| 25–75 | Moderately suitable | |
| >75 | Suitable | |
| Flow | Statistics Data | T°C | pH | TDS | HCO3− | Cl− | SO42− | F− | Na+ | K+ | Ca2+ | Mg2+ | As (µg/L) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Regional | Min | 20.00 | 6.36 | 152.00 | 121.51 | 5.42 | 10.00 | 0.00 | 23.8 | 2.70 | 10.30 | 0.30 | 2.49 |
| Max | 29.00 | 8.55 | 1260.00 | 1439.21 | 223.38 | 1080.00 | 0.10 | 612.50 | 26.00 | 237.00 | 108.00 | 43.70 | |
| Mean | 25.34 | 7.75 | 271.90 | 285.47 | 20.40 | 94.20 | 0.84 | 79.43 | 10.38 | 52.96 | 14.18 | 17.85 | |
| Variance | 4.82 | 0.30 | 73,168.85 | 50,657.01 | 1536.48 | 38,658.80 | 0.35 | 12,831.81 | 23.30 | 1458.32 | 475.41 | 101.45 | |
| Desv | 2.19 | 0.54 | 197.49 | 225.84 | 39.20 | 196.62 | 0.54 | 113.27 | 3.58 | 38.18 | 21.80 | 10.07 | |
| N | 32.00 | 32.00 | 32.00 | 32.00 | 32.00 | 32.00 | 32.00 | 32.00 | 32.00 | 32.00 | 32.00 | 32.00 | |
| Intermediate | Min | 16.20 | 6.09 | 168.00 | 72.90 | 0.92 | 12.00 | 0.03 | 9.00 | 2.70 | 15.50 | 1.00 | 4.40 |
| Max | 30.00 | 8.40 | 427.00 | 473.16 | 81.23 | 167.50 | 1.82 | 145.00 | 27.70 | 92.60 | 54.00 | 61.40 | |
| Mean | 25.02 | 7.84 | 64.63 | 74.85 | 12.07 | 45.77 | 0.42 | 45.94 | 10.50 | 50.53 | 13.74 | 15.36 | |
| Variance | 4.69 | 0.19 | 3592.14 | 3348.85 | 127.75 | 804.73 | 0.20 | 683.30 | 20.67 | 157.20 | 139.42 | 109.25 | |
| Desv | 2.14 | 0.44 | 249.15 | 257.55 | 11.30 | 27.95 | 0.62 | 57.46 | 26.35 | 4.60 | 13.22 | 10.33 | |
| N | 44.00 | 44.00 | 44.00 | 44.00 | 44.00 | 44.00 | 44.00 | 44.00 | 44.00 | 44.00 | 44.00 | 44.00 | |
| Local | Min | 17.40 | 7.80 | 61.00 | 36.45 | 3.51 | 8.00 | 0.00 | 8.20 | 4.70 | 9.60 | 1.10 | 0.49 |
| Max | 19.70 | 8.28 | 110.00 | 374.59 | 10.60 | 28 | 0.00 | 25.00 | 8.20 | 50.30 | 32.60 | 3.29 | |
| Mean | 18.30 | 8.11 | 144.66 | 199.40 | 6.97 | 21 | 0.00 | 18.40 | 5.90 | 29.23 | 15.10 | 1.93 | |
| Variance | 1.46 | 0.07 | 14,562.33 | 14,398.54 | 12.58 | 127.00 | 0.00 | 80.28 | 3.97 | 415.63 | 253.26 | 1.96 | |
| Desv | 1.20 | 0.26 | 120.67 | 119.90 | 3.54 | 11.26 | 0.00 | 8.95 | 1.99 | 20.38 | 15.91 | 1.40 | |
| N | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 | 5.00 | |
| Mixture | Min | 21.10 | 6.48 | 139.00 | 197.15 | 4.06 | 9.00 | 0.10 | 14.60 | 5.70 | 28.20 | 2.50 | 4.70 |
| Max | 29.20 | 8.13 | 391.00 | 473.16 | 18.28 | 110.00 | 1.86 | 98.00 | 13.20 | 117.60 | 25.20 | 44.50 | |
| Mean | 23.81 | 7.18 | 241.15 | 251.08 | 10.33 | 35.65 | 1.03 | 44.25 | 9.99 | 46.06 | 10.61 | 18.57 | |
| Variance | 3.81 | 0.23 | 3092.45 | 3385.65 | 10.87 | 503.61 | 0.17 | 300.59 | 4.67 | 326.98 | 50.75 | 69.13 | |
| Desv | 1.95 | 0.47 | 55.60 | 58.18 | 3.30 | 22.44 | 0.35 | 17.33 | 2.16 | 18.08 | 7.12 | 8.31 | |
| N | 20.00 | 20.00 | 20.00 | 20.00 | 20.00 | 20.00 | 20.00 | 20.00 | 20.00 | 20.00 | 20.00 | 20.00 |
| Flow | Statistics Data | T°C | pH | TDS | HCO3− | Cl− | SO42− | F− | Na+ | K+ | Ca2+ | Mg+2 | As (µg/L) | Depth (m) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Regional | Min | 22.50 | 6.71 | 137.20 | 134.69 | 8.44 | 2.00 | 0.44 | 17.64 | 1.06 | 1.85 | 0.10 | 3.64 | 10.00 |
| Max | 37.00 | 8.89 | 347.90 | 363.07 | 35.73 | 82.00 | 5.40 | 106.73 | 17.62 | 63.97 | 0.095 | 1219.00 | 274.00 | |
| Mean | 27.68 | 7.39 | 319.18 | 227.25 | 50.68 | 82.12 | 1.33 | 61.34 | 11.03 | 4905 | 29.27 | 20.51 | 159.57 | |
| Variance | 10.44 | 0.29 | 93,344.49 | 2708.69 | 28.41 | 289.58 | 0.85 | 509.76 | 28.08 | 122.99 | 64.78 | 382.10 | 3616.61 | |
| Desv | 3.22 | 0.46 | 48.06 | 52.55 | 5.41 | 17.02 | 0.92 | 22.82 | 3.47 | 11.02 | 7.99 | 19.55 | 60.14 | |
| N | 47.00 | 47.00 | 47.00 | 47.00 | 47.00 | 47.00 | 47.00 | 47.00 | 47.00 | 47.00 | 47.00 | 47.00 | 47.00 | |
| Intermediate | Min | 19.10 | 6.43 | 58.80 | 133.71 | 8.44 | 6.00 | 0.39 | 12.41 | 2.03 | 5.78 | 1.15 | 6.25 | 80.00 |
| Max | 40.10 | 8.19 | 721.77 | 816.18 | 268.00 | 230.00 | 4.25 | 181.30 | 23.18 | 198.92 | 50.18 | 241.30 | 300.00 | |
| Mean | 26.04 | 7.32 | 253.46 | 227.08 | 36.39 | 35.10 | 1.04 | 47.58 | 10.20 | 40.93 | 16.26 | 21.70 | 177.49 | |
| Variance | 11.22 | 0.27 | 8927.19 | 3415.82 | 1308.79 | 1228.18 | 0.38 | 700.10 | 17.57 | 281.29 | 129.29 | 468.89 | 2859.45 | |
| Desv | 3.36 | 0.49 | 121.09 | 104.52 | 55.42 | 35.05 | 0.61 | 35.18 | 4.61 | 29.48 | 11.97 | 20.99 | 53.47 | |
| N | 49.00 | 49.00 | 49.00 | 49.00 | 49.00 | 49.00 | 49.00 | 49.00 | 49.00 | 49.00 | 49.00 | 49.00 | 49.00 | |
| Mixture | Min | 21.70 | 6.59 | 98.00 | 132.74 | 8.93 | 2.00 | 0.39 | 18.13 | 3.99 | 9.41 | 0.83 | 5.19 | 87.00 |
| Max | 30.20 | 8.34 | 392.00 | 420.90 | 31.76 | 72.00 | 3.60 | 166.13 | 13.38 | 47.24 | 40.76 | 120.65 | 210.00 | |
| Mean | 25.99 | 7.68 | 217.43 | 213.76 | 14.91 | 26.35 | 1.02 | 39.58 | 8.93 | 29.23 | 13.39 | 25.30 | 168.79 | |
| Variance | 5.39 | 0.18 | 5076.45 | 4559.01 | 37.10 | 337.82 | 0.08 | 8.25 | 456.87 | 114.11 | 136.97 | 2615.75 | 1950.18 | |
| Desv | 2.21 | 0.38 | 72.02 | 66.74 | 5.66 | 16.97 | 0.67 | 33.12 | 2.95 | 9.77 | 11.12 | 51.21 | 45.00 | |
| N | 20.00 | 20.00 | 20.00 | 20.00 | 20.00 | 20.00 | 20.00 | 20.00 | 20.00 | 20.00 | 20.00 | 20.00 | 20.00 |
| 2005 | ||||||||||||||||||||
| Parameters | WHO standard | US EPA | Mexican Official norm NOM-127-SSA1-1994 | Bureau of Indian Standards (BIS) | ||||||||||||||||
| Mean values | ||||||||||||||||||||
| Reg. | Int. | Loc. | Mix. | Reg. | Int. | Loc. | Mix. | Reg. | Int. | Loc. | Mix. | Reg. | Int. | Loc. | Mix. | Reg. | Int. | Loc. | Mix. | |
| pH | 7.75 | 7.84 | 8.11 | 7.18 | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ |
| TDS | 316.90 | 246.76 | 144.66 | 241.15 | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ |
| Cl− | 20.40 | 12.07 | 6.97 | 10.33 | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ |
| SO42− | 94.20 | 45.77 | 21.00 | 35.65 | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ |
| NO3− | 0.70 | 0.63 | 0.74 | 0.76 | X | X | X | X | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ |
| F | 0.85 | 0.61 | 0.00 | 0.98 | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ |
| As | 0.01 | 0.01 | 0.00 | 0.02 | X | X | ✔ | X | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ |
| 2015 | ||||||||||||||||||||
| Parameters | WHO standard | US EPA | Mexican Official norm NOM-127-SSA1-1994 | Bureau of Indian Standards (BIS) | ||||||||||||||||
| Mean values | ||||||||||||||||||||
| Reg. | Int. | Loc. | Mix. | Reg. | Int. | Loc. | Mix. | Reg. | Int. | Loc. | Mix. | Reg. | Int. | Loc. | Mix. | Reg. | Int. | Loc. | Mix. | |
| pH | 7.39 | 7.38 | - | 7.72 | ✔ | ✔ | - | ✔ | ✔ | ✔ | - | ✔ | ✔ | ✔ | - | ✔ | ✔ | ✔ | - | ✔ |
| TDS | 319.18 | 235.64 | - | 223.85 | ✔ | ✔ | - | ✔ | ✔ | ✔ | - | ✔ | ✔ | ✔ | - | ✔ | ✔ | ✔ | - | ✔ |
| Cl− | 50.68 | 27.08 | - | 15.34 | ✔ | ✔ | - | ✔ | ✔ | ✔ | - | ✔ | ✔ | ✔ | - | ✔ | ✔ | ✔ | - | ✔ |
| SO42− | 82.12 | 35.08 | - | 26.35 | ✔ | ✔ | - | ✔ | ✔ | ✔ | - | ✔ | ✔ | ✔ | - | ✔ | ✔ | ✔ | - | ✔ |
| NO3− | 2.92 | 2.90 | - | 2.93 | X | X | - | X | X | X | - | X | ✔ | ✔ | - | ✔ | ✔ | ✔ | - | ✔ |
| F | 1.33 | 1.02 | - | 0.83 | ✔ | ✔ | - | ✔ | ✔ | ✔ | - | ✔ | ✔ | ✔ | - | ✔ | ✔ | ✔ | - | ✔ |
| As | 0.02 | 0.02 | - | 0.03 | X | X | - | X | X | X | - | X | ✔ | ✔ | - | X | ✔ | ✔ | - | ✔ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortiz-Letechipia, J.; González-Trinidad, J.; Júnez-Ferreira, H.E.; Bautista-Capetillo, C.; Dávila-Hernández, S. Evaluation of Groundwater Quality for Human Consumption and Irrigation in Relation to Arsenic Concentration in Flow Systems in a Semi-Arid Mexican Region. Int. J. Environ. Res. Public Health 2021, 18, 8045. https://doi.org/10.3390/ijerph18158045
Ortiz-Letechipia J, González-Trinidad J, Júnez-Ferreira HE, Bautista-Capetillo C, Dávila-Hernández S. Evaluation of Groundwater Quality for Human Consumption and Irrigation in Relation to Arsenic Concentration in Flow Systems in a Semi-Arid Mexican Region. International Journal of Environmental Research and Public Health. 2021; 18(15):8045. https://doi.org/10.3390/ijerph18158045
Chicago/Turabian StyleOrtiz-Letechipia, Jennifer, Julián González-Trinidad, Hugo Enrique Júnez-Ferreira, Carlos Bautista-Capetillo, and Sandra Dávila-Hernández. 2021. "Evaluation of Groundwater Quality for Human Consumption and Irrigation in Relation to Arsenic Concentration in Flow Systems in a Semi-Arid Mexican Region" International Journal of Environmental Research and Public Health 18, no. 15: 8045. https://doi.org/10.3390/ijerph18158045
APA StyleOrtiz-Letechipia, J., González-Trinidad, J., Júnez-Ferreira, H. E., Bautista-Capetillo, C., & Dávila-Hernández, S. (2021). Evaluation of Groundwater Quality for Human Consumption and Irrigation in Relation to Arsenic Concentration in Flow Systems in a Semi-Arid Mexican Region. International Journal of Environmental Research and Public Health, 18(15), 8045. https://doi.org/10.3390/ijerph18158045