Associations between Physiological Biomarkers and Psychosocial Measures of Pregnancy-Specific Anxiety and Depression with Support Intervention
Abstract
:1. Introduction
2. Methods
2.1. Study Design
2.2. Participants
2.3. Data Collection
2.4. Immunologic Assays
2.5. Psychological Measures
2.6. Statistical Analysis
3. Results
4. Discussion
5. Strengths and Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gangestad, S.W.; Hooper AE, C.; Eaton, M.A. On the function of placental corticotropin-releasing hormone: A role in maternal-fetal conflicts over blood glucose concentrations. Biol. Rev. 2012, 87, 856–873. [Google Scholar] [CrossRef] [PubMed]
- Valsamakis, G.; Chrousos, G.; Mastorakos, G. Stress, female reproduction and pregnancy. Psychoneuroendocrinology 2019, 100, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Chau, A.; Markley, J.C.; Juang, J.; Tsen, L.C. Cytokines in the perinatal period—Part I. Int. J. Obstet. Anesth. 2016, 26, 39–47. [Google Scholar] [CrossRef]
- Denney, J.M.; Nelson, E.L.; Wadhwa, P.D.; Waters, T.P.; Mathew, L.; Chung, E.K.; Goldenberg, R.L.; Culhane, J.F. Longitudinal modulation of immune system cytokine profile during pregnancy. Cytokine 2011, 53, 170–177. [Google Scholar] [CrossRef] [Green Version]
- Moreli, J.B.; Ruocco, A.M.C.; Vernini, J.M.; Rudge, M.V.C.; Calderon, I.M.P. Interleukin 10 and tumor necrosis factor-alpha in pregnancy: Aspects of interest in clinical obstetrics. Int. Sch. Res. Netw. Obstet. Gynecol. 2012, 2012, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Beijers, R.; Buitelaar, J.K.; de Weerth, C. Mechanisms underlying the effects of prenatal psychosocial stress on child outcomes. Beyond the HPA axis. Eur. Child Adolesc. Psychiatry 2014, 23, 943–956. [Google Scholar] [CrossRef]
- Glover, V. Annual research review: Prenatal stress and the origins of psychopathology: An evolutionary perspective. J. Child Psychol. Psychiatry 2011, 52, 356–367. [Google Scholar] [CrossRef] [PubMed]
- Gelman, P.L.; Mancilla-Herrera, I.; Flores-Ramos, M.; Takashima, M.S.; Coronel, F.C.; Fuentes, C.C.; Molina, A.P.; Hernández-Ruiz, J.; Silva-Aguilera, F.S.; Farfan-Labonne, B.; et al. The cytokine profile of women with severe anxiety and depression during pregnancy. BMC Psychiatry 2019, 19, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Coussons-Read, M.E.; Okun, M.L.; Schmitt, M.P.; Giese, S. Prenatal stress alters cytokine levels in a manner that may endanger human pregnancy. Psychosom. Med. 2005, 67, 625–631. [Google Scholar] [CrossRef]
- Karlsson, L.; Nousiainen, N.; Scheinin, N.M.; Maksimow, M.; Salmi, M.; Lehto, S.M.; Tolvanen, M.; Lukkarinen, H.; Karlsson, H. Cytokine profile and maternal depression and anxiety symptoms in mid-pregnancy—the FinnBrain Birth Cohort Study. Arch. Women’s Ment. Health 2017, 20, 39–48. [Google Scholar] [CrossRef]
- La Sala, G.B.; Ardizzoni, A.; Capodanno, F.; Manca, L.; Baschieri, M.C.; Soncini, E.; Peppoloni, S.; Blasi, E. Protein microarrays on midtrimester amniotic fluids: A novel approach for the diagnosis of early intrauterine inflammation related to preterm delivery. Int. J. Immunopathol. Pharmacol. 2012, 25, 1029–1040. [Google Scholar] [CrossRef] [PubMed]
- Giurgescu, C.; Sanguanklin, N.; Engeland, C.G.; White-Traut, R.C.; Park, C.; Mathews, H.L.; Janusek, L.W. Relationships among psychosocial factors, biomarkers, preeclampsia, and preterm birth in African American women: A pilot. Appl. Nurs. Res. 2015, 28, 1–6. [Google Scholar] [CrossRef]
- Coussons-Read, M.E.; Okun, M.L.; Nettles, C.D. Psychosocial stress increased inflammatory markers and alters cytokine production across pregnancy. Brainbehaviorand Immun. 2007, 21, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Weis, K.L.; Lederman, R.P.; Walker, K.C.; Chan, W. Mentors offering maternal support reduces prenatal, pregnancy-specific anxiety in a sample of military women. J. Obstet. Gynecol. Neonatal Nurs. 2017, 46, 669–685. [Google Scholar] [CrossRef]
- Weis, K.L.; Ryan, T.W. Mentors offering maternal support: A support intervention for military mothers. J. Obstet. Gynecol. Neonatal Nurs. 2012, 41, 303–313. [Google Scholar] [CrossRef]
- Weis, K.L.; Walker, K.C.; Chan, W.; Yuan, T.T.; Lederman, R.P. Risk of preterm birth and newborn low birthweight in military women with increased pregnancy-specific anxiety. Mil. Med. 2020, 185, 678–685. [Google Scholar] [CrossRef] [Green Version]
- Weis, K.L.; Lederman, R.P.; Lilly, A.E.; Schaffer, J. The relationship of military imposed marital separations on maternal acceptance of pregnancy. Res. Nurs. Health 2008, 31, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Lederman, R.; Weis, K. Psychosocial Adaptation Pregnancy: Seven Dimensions of Maternal Role Development, 3rd ed.; Springer: New York, NY, USA, 2009. [Google Scholar]
- Lederman, R.; Weis, K. Psychosocial Adaptation Pregnancy: Seven Dimensions of Maternal Development, 4th ed.; Springer: New York, NY, USA, 2020. [Google Scholar]
- Cox, J.L.; Chapman, G.; Murray, D.; Jones, P. Validation of the Edinburgh Postnatal Depression Scale (EPDS) in non-postnatal women. J. Affect. Disord. 1996, 39, 185–189. [Google Scholar] [CrossRef]
- Cox, J.; Holden, J.; Sagovsky, R. Detection of postnatal depression: Development of the 10-item Edinburgh postnatal depression scale. Br. J. Psychiatry 1987, 150, 782–786. [Google Scholar] [CrossRef] [Green Version]
- Henshaw, C.; Ericksen, J. How to use the EPDS and maximize its usefulness in the consultation process. In Identifying Perinatal Depression and Anxiety; Milgrom, J., Gemmill, A.W., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2015. [Google Scholar]
- Chow SS, W.; Craig, M.E.; Jones, C.A.; Hall, B.; Catteau, J.; Lloyd, A.R.; Rawlinson, W.D. Differences in amniotic fluid and maternal serum cytokine levels in early midtrimester women without evidence of infection. Cytokine 2008, 44, 78–84. [Google Scholar] [CrossRef]
- Burns, C.; Hall, S.T.; Smith, R.; Blackwell, C. Cytokine levels in late pregnancy: Are female infants better protected against inflammation? Front. Immunol. 2015, 6, 2–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barrat, F.; Lesourd, B.; Boulouis, H.J.; Thibault, D.; Vincent-Naulleau, S.; Gjata, B.; Louise, A.; Neway, T.; Pilet, C. Sex and parity modulate cytokine production during murine ageing. Clin. Exp. Immunol. 1997, 109, 562–568. [Google Scholar] [CrossRef]
- Chollet-Hinton, L.S.; Stuebe, A.M.; Casbas-Hernandez, P.; Chetwynd, E.; Troester, M.A. Temporal trends in the inflammatory cytokine profile of human breastmilk. Breastfeed. Med. 2014, 9, 530–537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomes, J.; Au, F.; Basak, A.; Cakmak, S.; Vincent, R.; Kumarathasan, P. Maternal blood biomarkers and adverse pregnancy outcomes: A systematic review and meta-analysis. Crit. Rev. Toxicol. 2019, 49, 461–478. [Google Scholar] [CrossRef] [PubMed]

| Variable Name | Total Sample (n = 57) | Intervention Group (n = 28) | Control Group (n = 29) |
|---|---|---|---|
| Age (years, mean (SD)) | 27.81 (4.50) | 26.29 (4.29) | 29.28 (4.26) |
| Age (frequency, %) | |||
| ≤25 | 16 (28.07) | 12 (42.86) | 4 (13.79) |
| >25 | 41 (71.93) | 16 (57.14) | 25 (86.21) |
| Race/ethnicity (%) | |||
| White, non-Hispanic | 32 (57.14) | 18 (64.29) | 14 (50) |
| Black, non-Hispanic | 8 (14.29) | 2 (7.14) | 6 (21.43) |
| Hispanic | 10 (17.86) | 5 (17.86) | 5 (17.86) |
| Others | 6 (10.71) | 3 (10.71) | 3 (10.71) |
| Prior deliveries (%) | |||
| 0 | 18 (31.58) | 9 (32.14) | 9 (31.03) |
| 1 to 2 | 35 (61.40) | 18 (64.29) | 17 (58.62) |
| 3 or more | 4 (7.02) | 1 (3.57) | 3 (10.34) |
| Marital status (%) | |||
| Married | 53 (92.98) | 25 (89.29) | 28 (96.55) |
| Not married | 4 (7.02) | 3 (10.71) | 1 (3.45) |
| Military branch 1 | |||
| Air Force | 42 (75) | 21 (75) | 21 (75) |
| Army | 7 (12.50) | 4 (14.29) | 3 (10.71) |
| Other | 7 (12.50) | 3 (10.71) | 4 (14.28) |
| Active-duty (%) | 20 (35.09) | 9 (32.14) | 11 (37.93) |
| Active-duty spouse (%) | 51 (91.07) | 24 (85.71) | 27 (96.43) |
| Trimester 1 | Trimester 2 | Trimester 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Total N = 57 | MOMS™ n = 28 | Control n = 29 | Total N = 57 | MOMS™ n = 28 | Control n = 29 | Total N = 57 | MOMS™ n = 28 | Control n = 29 |
| IL2 | 3.40 | 3.40 | 3.40 | 3.39 | 3.36 | 3.42 | 3.46 | 3.47 | 3.45 |
| (0.62) | (0.73) | (0.50) | (0.47) | (0.48) | (0.47) | (0.51) | (0.57) | (0.45) | |
| IL6 | 0.34 | 0.36 | 0.33 | 0.35 | 0.34 | 0.35 | 0.38 | 0.38 | 0.39 |
| (0.07) | (0.08) | (0.07) | (0.09) | (0.09) | (0.09) | (0.10) | (0.11) | (0.08) | |
| IL10 | 2.68 | 4.25 | 1.16 | 2.74 | 3.99 | 1.53 | 2.36 | 3.31 | 1.41 |
| (5.19) | (7.07) | (0.93) | (4.26) | (5.73) | (1.23) | (3.19) | (4.03) | (1.62) | |
| IL1b | 4.09 | 4.72 | 3.49 | 3.35 | 3.59 | 3.12 | 3.71 | 3.99 | 3.44 |
| (4.21) | (5.44) | (2.47) | (1.43) | (1.89) | (0.72) | (2.45) | (2.68) | (2.20) | |
| TNF-α | 23.97 | 25.31 | 22.68 | 18.38 | 17.25 | 19.46 | 19.29 | 17.98 | 20.60 |
| (20.76) | (23.71) | (17.77) | (13.27) | (11.91) | (14.59) | (17.44) | (14.52) | (20.13) | |
| TNF-α/IL10 | 22.00 | 15.53 | 28.24 | 15.65 | 12.36 | 18.82 | 21.52 | 12.85 | 30.18 |
| (28.01) | (19.77) | (33.31) | (15.90) | (13.60) | (17.48) | (37.44) | (13.38) | (50.20) | |
| IL6/IL10 | 0.32 | 0.24 | 0.40 | 0.30 | 0.25 | 0.35 | 0.42 | 0.29 | 0.55 |
| (0.21) | (0.20) | (0.19) | (0.22) | (0.22) | (0.21) | (0.39) | (0.27) | (0.44) | |
| CRP | 2.55 | 3.05 | 2.04 | 5.23 | 5.93 | 4.49 | 5.53 | 6.15 | 4.85 |
| (3.02) | (3.35) | (2.60) | (2.95) | (3.17) | (2.56) | (2.72) | (2.54) | (2.80) | |
| CORT | 9.26 | 9.39 | 9.13 | 13.79 | 13.28 | 14.28 | 20.43 | 20.65 | 20.22 |
| (2.92) | (1.90) | (3.67) | (4.80) | (4.40) | (5.18) | (8.04) | (7.08) | (8.99) | |
| CRH | 66.65 | 77.73 | 55.96 | 258.60 | 266.29 | 251.16 | 404.16 | 414.96 | 393.74 |
| (53.13) | (60.61) | (43.15) | (110.47) | (104.42) | (117.38) | (115.75) | (101.63) | (128.87) | |
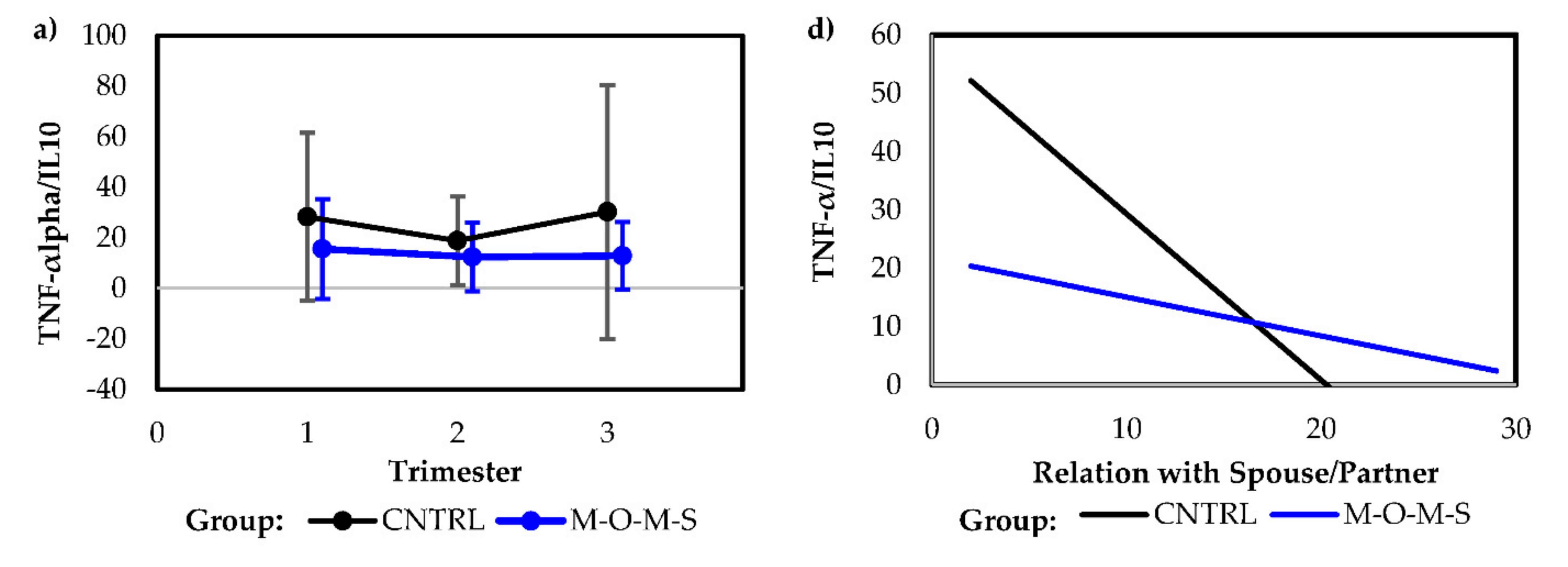
| Model Type | Predictor | Intercept | Coefficient | p-Value |
|---|---|---|---|---|
| Univariate model | Preparation for Labor | 52.47 (12.70) | −2.08 (0.94) | 0.03 |
| Univariate model | Relationship with Spouse/Partner | 57.81 (13.04) | −2.85 (1.10) | 0.01 |
| Combined model | 73.14 (15.76) | |||
| Preparation for Labor | −1.61 (0.95) | 0.10 | ||
| Relationship with Spouse/Partner | −2.42 (1.12) | 0.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weis, K.L.; Yuan, T.T.; Walker, K.C.; Gibbons, T.F.; Chan, W. Associations between Physiological Biomarkers and Psychosocial Measures of Pregnancy-Specific Anxiety and Depression with Support Intervention. Int. J. Environ. Res. Public Health 2021, 18, 8043. https://doi.org/10.3390/ijerph18158043
Weis KL, Yuan TT, Walker KC, Gibbons TF, Chan W. Associations between Physiological Biomarkers and Psychosocial Measures of Pregnancy-Specific Anxiety and Depression with Support Intervention. International Journal of Environmental Research and Public Health. 2021; 18(15):8043. https://doi.org/10.3390/ijerph18158043
Chicago/Turabian StyleWeis, Karen L., Tony T. Yuan, Katherine C. Walker, Thomas F. Gibbons, and Wenyaw Chan. 2021. "Associations between Physiological Biomarkers and Psychosocial Measures of Pregnancy-Specific Anxiety and Depression with Support Intervention" International Journal of Environmental Research and Public Health 18, no. 15: 8043. https://doi.org/10.3390/ijerph18158043
APA StyleWeis, K. L., Yuan, T. T., Walker, K. C., Gibbons, T. F., & Chan, W. (2021). Associations between Physiological Biomarkers and Psychosocial Measures of Pregnancy-Specific Anxiety and Depression with Support Intervention. International Journal of Environmental Research and Public Health, 18(15), 8043. https://doi.org/10.3390/ijerph18158043






