Association of Short-Term Particulate Matter Exposure among 5-Year Cancer Survivors with Incident Cardiovascular Disease: A Time-Stratified Case-Crossover Study
Abstract
:1. Introduction
2. Materials and Methods
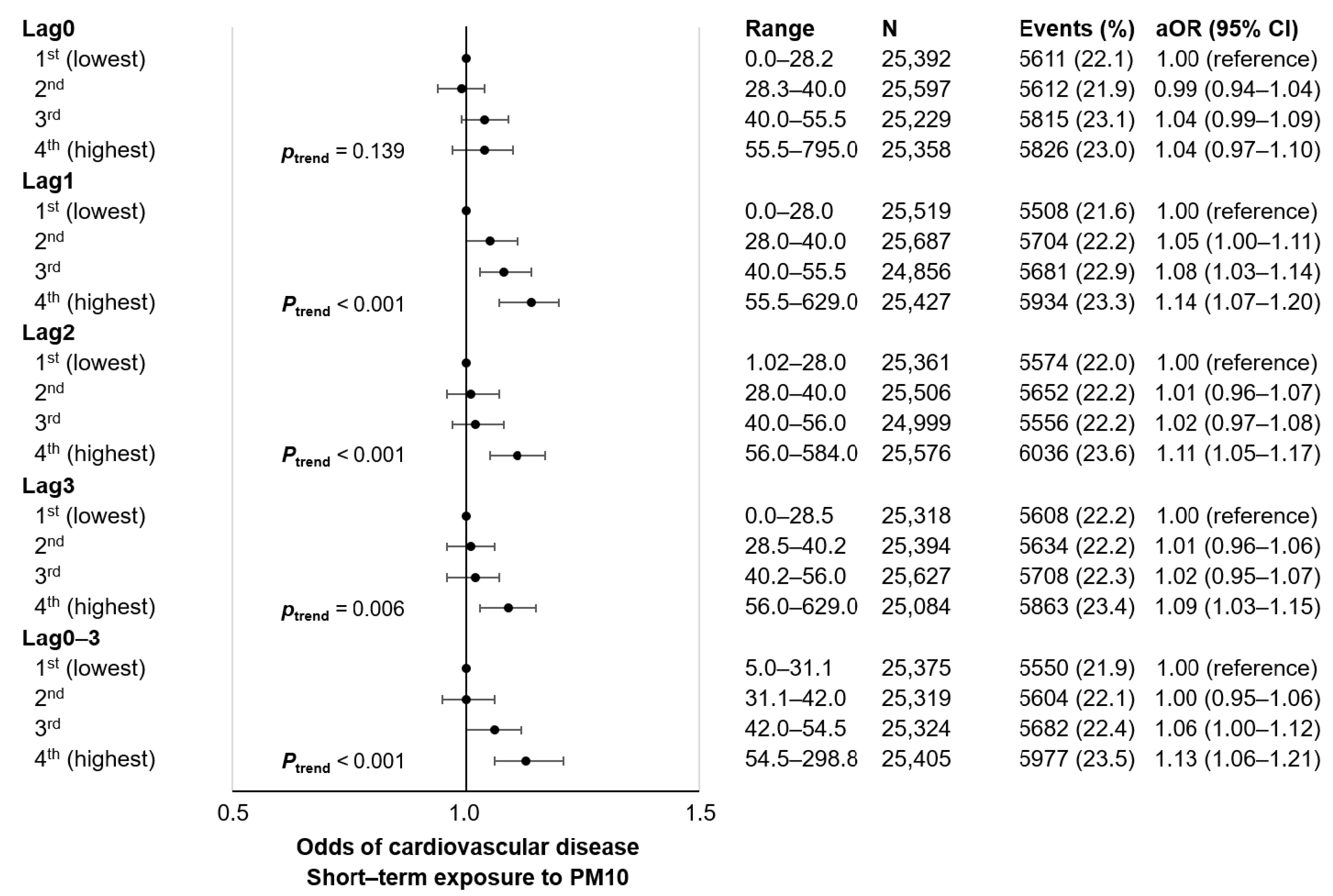
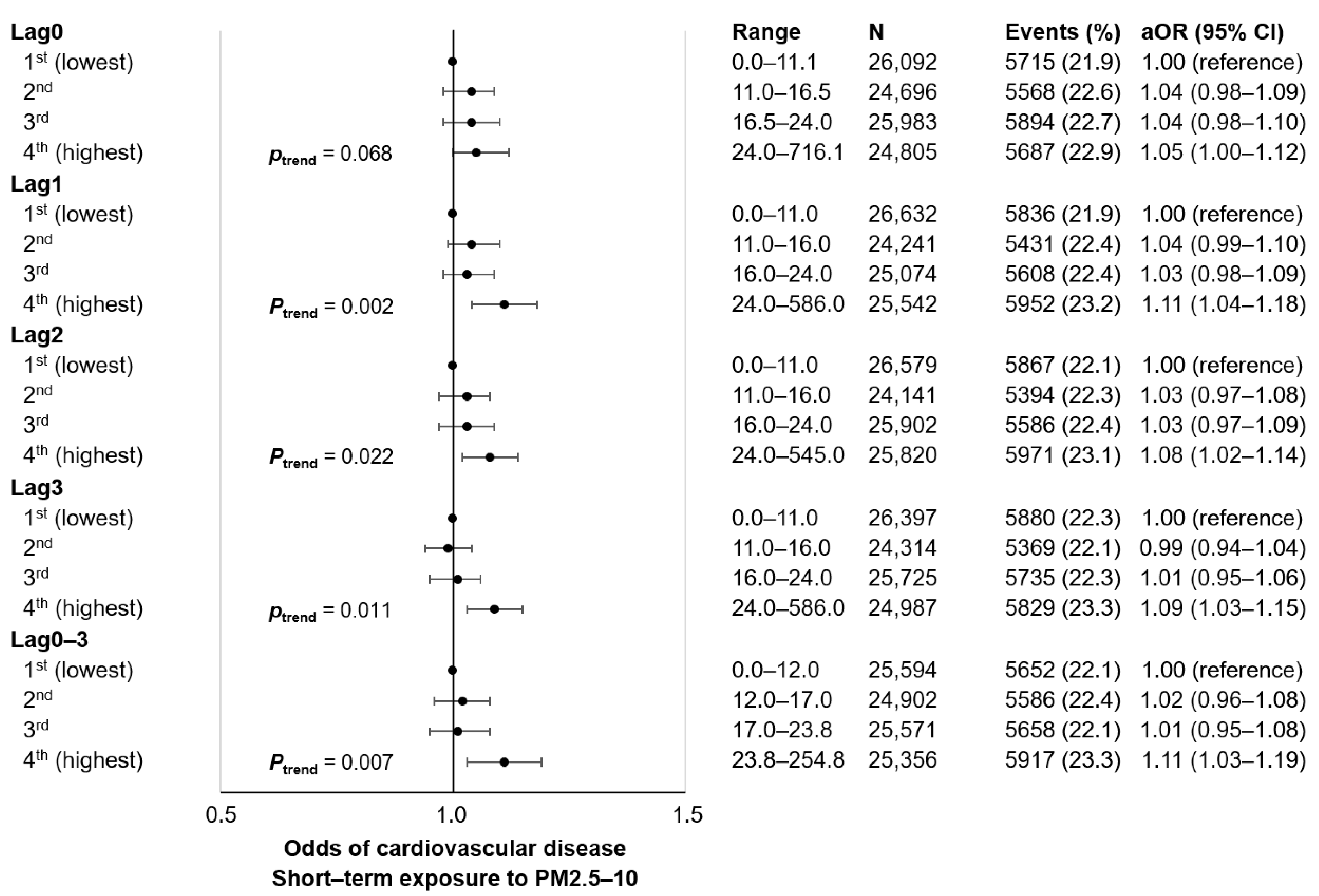
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, K.H.; Kabir, E.; Kabir, S. A review on the human health impact of airborne particulate matter. Environ. Int. 2015, 74, 136–143. [Google Scholar] [CrossRef]
- Loomis, D.; Grosse, Y.; Lauby-Secretan, B.; El Ghissassi, F.; Bouvard, V.; Benbrahim-Tallaa, L.; Guha, N.; Baan, R.; Mattock, H.; Straif, K.; et al. The carcinogenicity of outdoor air pollution. Lancet Oncol. 2013, 14, 1262–1263. [Google Scholar] [CrossRef]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Outdoor Air Pollution: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC: Lyon, France, 2016; Volume 109, pp. 9–444. [Google Scholar]
- Hamra, G.B.; Guha, N.; Cohen, A.; Laden, F.; Raaschou-Nielsen, O.; Samet, J.M.; Vineis, P.; Forastiere, F.; Saldiva, P.; Yorifuji, T.; et al. Outdoor particulate matter exposure and lung cancer: A systematic review and meta-analysis. Environ. Health Perspect. 2014, 122, 906–911. [Google Scholar] [CrossRef] [Green Version]
- Brook, R.D.; Rajagopalan, S.; Pope, C.A., 3rd; Brook, J.R.; Bhatnagar, A.; Diez-Roux, A.V.; Holguin, F.; Hong, Y.; Luepker, R.V.; Mittleman, M.A.; et al. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation 2010, 121, 2331–2378. [Google Scholar] [CrossRef] [Green Version]
- Sacks, J.D.; Stanek, L.W.; Luben, T.J.; Johns, D.O.; Buckley, B.J.; Brown, J.S.; Ross, M. Particulate matter-induced health effects: Who is susceptible? Environ. Health Perspect. 2011, 119, 446–454. [Google Scholar] [CrossRef]
- Strongman, H.; Gadd, S.; Matthews, A.; Mansfield, K.E.; Stanway, S.; Lyon, A.R.; Dos-Santos-Silva, I.; Smeeth, L.; Bhaskaran, K. Medium and long-term risks of specific cardiovascular diseases in survivors of 20 adult cancers: A population-based cohort study using multiple linked UK electronic health records databases. Lancet 2019, 394, 1041–1054. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.; Kim, K.H.; Kim, K.; Chang, J.; Kim, S.M.; Kim, S.R.; Cho, Y.; Lee, G.; Son, J.S.; Park, S.M. Association between Post-Diagnosis Particulate Matter Exposure among 5-Year Cancer Survivors and Cardiovascular Disease Risk in Three Metropolitan Areas from South Korea. Int. J. Environ. Res. Public Health 2020, 17, 2841. [Google Scholar] [CrossRef]
- Coleman, N.C.; Ezzati, M.; Marshall, J.D.; Robinson, A.L.; Burnett, R.T.; Pope, C.A., 3rd. Fine Particulate Matter Air Pollution and Mortality Risk Among US Cancer Patients and Survivors. JNCI Cancer Spectr. 2021, 5, pkab001. [Google Scholar] [CrossRef]
- Cheol Seong, S.; Kim, Y.Y.; Khang, Y.H.; Heon Park, J.; Kang, H.J.; Lee, H.; Do, C.H.; Song, J.S.; Hyon Bang, J.; Ha, S.; et al. Data Resource Profile: The National Health Information Database of the National Health Insurance Service in South Korea. Int. J. Epidemiol. 2017, 46, 799–800. [Google Scholar] [CrossRef] [Green Version]
- Son, J.S.; Choi, S.; Kim, K.; Kim, S.M.; Choi, D.; Lee, G.; Jeong, S.M.; Park, S.Y.; Kim, Y.Y.; Yun, J.M.; et al. Association of Blood Pressure Classification in Korean Young Adults According to the 2017 American College of Cardiology/American Heart Association Guidelines With Subsequent Cardiovascular Disease Events. JAMA 2018, 320, 1783–1792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seo, S.; Choi, S.; Kim, K.; Kim, S.M.; Park, S.M. Association between urban green space and the risk of cardiovascular disease: A longitudinal study in seven Korean metropolitan areas. Environ. Int. 2019, 125, 51–57. [Google Scholar] [CrossRef]
- Kim, S.R.; Choi, S.; Kim, K.; Chang, J.; Kim, S.M.; Cho, Y.; Oh, Y.H.; Lee, G.; Son, J.S.; Kim, K.H.; et al. Association of the combined effects of air pollution and changes in physical activity with cardiovascular disease in young adults. Eur. Heart J. 2021, 42, 2487–2497. [Google Scholar] [CrossRef]
- Janes, H.; Sheppard, L.; Lumley, T. Case-crossover analyses of air pollution exposure data: Referent selection strategies and their implications for bias. Epidemiology 2005, 16, 717–726. [Google Scholar] [CrossRef] [PubMed]
- Carracedo-Martinez, E.; Taracido, M.; Tobias, A.; Saez, M.; Figueiras, A. Case-crossover analysis of air pollution health effects: A systematic review of methodology and application. Environ. Health Perspect. 2010, 118, 1173–1182. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J.A.; Puett, R.C.; Laden, F.; Wellenius, G.A.; Sapkota, A.; Liao, D.; Yanosky, J.D.; Carter-Pokras, O.; He, X.; Hart, J.E. Case-crossover analysis of short-term particulate matter exposures and stroke in the health professionals follow-up study. Environ. Int. 2019, 124, 153–160. [Google Scholar] [CrossRef]
- Virani, S.S.; Alonso, A.; Aparicio, H.J.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics-2021 Update: A Report From the American Heart Association. Circulation 2021, 143, e254–e743. [Google Scholar] [CrossRef]
- Kim, K.H.; Choi, S.; Kim, K.; Chang, J.; Kim, S.M.; Kim, S.R.; Cho, Y.; Oh, Y.H.; Lee, G.; Son, J.S.; et al. Association between physical activity and subsequent cardiovascular disease among 5-year breast cancer survivors. Breast Cancer Res. Treat. 2021, 188, 203–214. [Google Scholar] [CrossRef]
- Parker, J.D.; Kravets, N.; Vaidyanathan, A. Particulate Matter Air Pollution Exposure and Heart Disease Mortality Risks by Race and Ethnicity in the United States: 1997 to 2009 National Health Interview Survey With Mortality Follow-Up Through 2011. Circulation 2018, 137, 1688–1697. [Google Scholar] [CrossRef]
- Cui, P.; Huang, Y.; Han, J.; Song, F.; Chen, K. Ambient particulate matter and lung cancer incidence and mortality: A meta-analysis of prospective studies. Eur. J. Public Health 2015, 25, 324–329. [Google Scholar] [CrossRef]
- Cohen, G.; Levy, I.; Yuval; Kark, J.D.; Levin, N.; Broday, D.M.; Steinberg, D.M.; Gerber, Y. Long-term exposure to traffic-related air pollution and cancer among survivors of myocardial infarction: A 20-year follow-up study. Eur. J. Prev. Cardiol. 2017, 24, 92–102. [Google Scholar] [CrossRef]
- Peel, J.L.; Metzger, K.B.; Klein, M.; Flanders, W.D.; Mulholland, J.A.; Tolbert, P.E. Ambient air pollution and cardiovascular emergency department visits in potentially sensitive groups. Am. J. Epidemiol. 2007, 165, 625–633. [Google Scholar] [CrossRef] [Green Version]
- Host, S.; Larrieu, S.; Pascal, L.; Blanchard, M.; Declercq, C.; Fabre, P.; Jusot, J.F.; Chardon, B.; Le Tertre, A.; Wagner, V.; et al. Short-term associations between fine and coarse particles and hospital admissions for cardiorespiratory diseases in six French cities. Occup. Environ. Med. 2008, 65, 544–551. [Google Scholar] [CrossRef]
- Kan, H.; London, S.J.; Chen, G.; Zhang, Y.; Song, G.; Zhao, N.; Jiang, L.; Chen, B. Season, sex, age, and education as modifiers of the effects of outdoor air pollution on daily mortality in Shanghai, China: The Public Health and Air Pollution in Asia (PAPA) Study. Environ. Health Perspect. 2008, 116, 1183–1188. [Google Scholar] [CrossRef]
- Park, S.K.; O’Neill, M.S.; Vokonas, P.S.; Sparrow, D.; Schwartz, J. Effects of air pollution on heart rate variability: The VA normative aging study. Environ. Health Perspect. 2005, 113, 304–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, N.; Wang, M.; Bramble, L.A.; Schmitz, D.A.; Schauer, J.J.; Sioutas, C.; Harkema, J.R.; Nel, A.E. The adjuvant effect of ambient particulate matter is closely reflected by the particulate oxidant potential. Environ. Health Perspect. 2009, 117, 1116–1123. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, M.S.; Burnett, R.T.; Yale, J.F.; Valois, M.F.; Brook, J.R. Associations between ambient air pollution and daily mortality among persons with diabetes and cardiovascular disease. Environ. Res. 2006, 100, 255–267. [Google Scholar] [CrossRef]
- Park, S.M.; Yun, Y.H.; Kim, Y.A.; Jo, M.; Won, Y.J.; Back, J.H.; Lee, E.S. Prediagnosis Body Mass Index and Risk of Secondary Primary Cancer in Male Cancer Survivors: A Large Cohort Study. J. Clin. Oncol. 2016, 34, 4116–4124. [Google Scholar] [CrossRef] [Green Version]
- Pope, C.A., 3rd; Coleman, N.; Pond, Z.A.; Burnett, R.T. Fine particulate air pollution and human mortality: 25+ years of cohort studies. Environ. Res. 2020, 183, 108924. [Google Scholar] [CrossRef]
- Hamanaka, R.B.; Mutlu, G.M. Particulate Matter Air Pollution: Effects on the Cardiovascular System. Front. Endocrinol. (Lausanne) 2018, 9, 680. [Google Scholar] [CrossRef] [Green Version]
- Riediker, M.; Cascio, W.E.; Griggs, T.R.; Herbst, M.C.; Bromberg, P.A.; Neas, L.; Williams, R.W.; Devlin, R.B. Particulate matter exposure in cars is associated with cardiovascular effects in healthy young men. Am. J. Respir. Crit. Care Med. 2004, 169, 934–940. [Google Scholar] [CrossRef]
- Soberanes, S.; Urich, D.; Baker, C.M.; Burgess, Z.; Chiarella, S.E.; Bell, E.L.; Ghio, A.J.; De Vizcaya-Ruiz, A.; Liu, J.; Ridge, K.M.; et al. Mitochondrial complex III-generated oxidants activate ASK1 and JNK to induce alveolar epithelial cell death following exposure to particulate matter air pollution. J. Biol. Chem. 2009, 284, 2176–2186. [Google Scholar] [CrossRef] [Green Version]
- Mutlu, E.A.; Comba, I.Y.; Cho, T.; Engen, P.A.; Yazici, C.; Soberanes, S.; Hamanaka, R.B.; Nigdelioglu, R.; Meliton, A.Y.; Ghio, A.J.; et al. Inhalational exposure to particulate matter air pollution alters the composition of the gut microbiome. Environ. Pollut. 2018, 240, 817–830. [Google Scholar] [CrossRef]
- Block, M.L.; Calderon-Garciduenas, L. Air pollution: Mechanisms of neuroinflammation and CNS disease. Trends Neurosci. 2009, 32, 506–516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Cai, J.; Chen, R.; Zhao, Z.; Ying, Z.; Wang, L.; Chen, J.; Hao, K.; Kinney, P.L.; Chen, H.; et al. Particulate Matter Exposure and Stress Hormone Levels: A Randomized, Double-Blind, Crossover Trial of Air Purification. Circulation 2017, 136, 618–627. [Google Scholar] [CrossRef]
- Chiarella, S.E.; Soberanes, S.; Urich, D.; Morales-Nebreda, L.; Nigdelioglu, R.; Green, D.; Young, J.B.; Gonzalez, A.; Rosario, C.; Misharin, A.V.; et al. β2-Adrenergic agonists augment air pollution-induced IL-6 release and thrombosis. J. Clin. Investig. 2014, 124, 2935–2946. [Google Scholar] [CrossRef]
- Cosselman, K.E.; Krishnan, R.M.; Oron, A.P.; Jansen, K.; Peretz, A.; Sullivan, J.H.; Larson, T.V.; Kaufman, J.D. Blood pressure response to controlled diesel exhaust exposure in human subjects. Hypertension 2012, 59, 943–948. [Google Scholar] [CrossRef] [PubMed]
- Folino, F.; Buja, G.; Zanotto, G.; Marras, E.; Allocca, G.; Vaccari, D.; Gasparini, G.; Bertaglia, E.; Zoppo, F.; Calzolari, V.; et al. Association between air pollution and ventricular arrhythmias in high-risk patients (ARIA study): A multicentre longitudinal study. Lancet Planet. Health 2017, 1, e58–e64. [Google Scholar] [CrossRef]
- Qiu, H.; Yu, I.T.S.; Wang, X.R.; Tian, L.W.; Tse, L.A.; Wong, T.W. Season and humidity dependence of the effects of air pollution on COPD hospitalizations in Hong Kong. Atmos. Environ. 2013, 76, 74–80. [Google Scholar] [CrossRef]
- Turner, M.C.; Krewski, D.; Diver, W.R.; Pope, C.A., 3rd; Burnett, R.T.; Jerrett, M.; Marshall, J.D.; Gapstur, S.M. Ambient Air Pollution and Cancer Mortality in the Cancer Prevention Study II. Environ. Health Perspect. 2017, 125, 087013. [Google Scholar] [CrossRef] [Green Version]
- Wong, C.M.; Tsang, H.; Lai, H.K.; Thomas, G.N.; Lam, K.B.; Chan, K.P.; Zheng, Q.; Ayres, J.G.; Lee, S.Y.; Lam, T.H.; et al. Cancer Mortality Risks from Long-term Exposure to Ambient Fine Particle. Cancer Epidemiol. Biomark. Prev. 2016, 25, 839–845. [Google Scholar] [CrossRef] [Green Version]

| Cardiovascular Disease | Coronary Heart Disease | Stroke | |
|---|---|---|---|
| Number of participants | 22,864 | 8306 | 14,558 |
| Lag0–3 PM10, μg/m3 | |||
| Mean (SD) | 44.9 (25.3) | 44.6 (24.9) | 45.1 (25.4) |
| Range (minimum-maximum) | |||
| 1st quartile | 5.0–31.1 | 5.0–31.1 | 5.0–31.1 |
| 2nd quartile | 31.1–42.0 | 31.1–42.0 | 31.1–42.0 |
| 3rd quartile | 42.0–54.5 | 42.0–54.5 | 42.0–54.5 |
| 4th quartile | 54.5–298.8 | 54.5–289.9 | 54.5–298.8 |
| Lag0–3 PM2.5, μg/m3 | |||
| Mean (SD) | 25.1 (14.2) | 25.0 (14.4) | 25.2 (14.0) |
| Range (minimum-maximum) | |||
| 1st quartile | 0.0–16.9 | 0.0–16.9 | 0.0–16.9 |
| 2nd quartile | 16.9–23.5 | 16.9–23.5 | 16.9–23.5 |
| 3rd quartile | 23.5–31.3 | 23.5–31.3 | 23.5–31.3 |
| 4th quartile | 31.3–113.3 | 31.3–113.3 | 31.3–109.5 |
| Lag0–3 PM2.5–10, μg/m3 | |||
| Mean (SD) | 19.8 (17.1) | 19.6 (16.4) | 20.0 (17.6) |
| Range (minimum-maximum) | |||
| 1st quartile | 0.0–12.0 | 0.0–12.0 | 0.0–12.0 |
| 2nd quartile | 12.0–17.0 | 12.0–17.0 | 12.0–17.0 |
| 3rd quartile | 17.0–23.8 | 17.0–23.8 | 17.0–23.8 |
| 4th quartile | 23.8–254.8 | 23.8–245.9 | 23.8–254.8 |
| Temperature, °C, mean (SD) | 12.9 (10.3) | 12.8 (10.3) | 12.9 (10.3) |
| Age, years, mean (SD) | 71.7 (10.8) | 70.5 (10.3) | 72.5 (11.1) |
| Sex, N (%) | |||
| Men | 12,750 (55.8) | 5178 (62.3) | 7572 (52.0) |
| Women | 10,114 (44.2) | 3128 (37.7) | 6986 (48.0) |
| Household income, quartiles, N (%) | |||
| 1st (highest) | 9564 (41.8) | 3437 (41.4) | 6127 (42.1) |
| 2nd | 4033 (17.6) | 1519 (18.3) | 2514 (17.3) |
| 3rd | 2796 (12.2) | 1015 (12.2) | 1781 (12.2) |
| 4th (lowest) | 6471 (28.3) | 2335 (28.1) | 4136 (28.4) |
| Adjusted Odds Ratio, 95% Confidence Interval | |||||
|---|---|---|---|---|---|
| PM, Quartiles | |||||
| 1st (Lowest) | 2nd | 3rd | 4th (Highest) | p for Trend | |
| PM10, lag 0–3 | |||||
| Age, years | |||||
| <65 | 1.00 (reference) | 1.01 (0.91–1.13) | 1.04 (0.92–1.17) | 1.09 (0.96–1.24) | 0.173 |
| ≥65 | 1.00 (reference) | 1.00 (0.94–1.06) | 1.06 (0.99–1.13) | 1.15 (1.06–1.24) | <0.001 |
| Sex | |||||
| Men | 1.00 (reference) | 1.03 (0.95–1.10) | 1.07 (0.99–1.16) | 1.14 (1.04–1.24) | 0.003 |
| Women | 1.00 (reference) | 0.97 (0.89–1.05) | 1.05 (0.95–1.14) | 1.13 (1.03–1.25) | 0.004 |
| Household income | |||||
| Upper half | 1.00 (reference) | 1.01 (0.94–1.08) | 1.08 (1.00–1.16) | 1.14 (1.05–1.24) | <0.001 |
| Lower half | 1.00 (reference) | 0.99 (0.91–1.08) | 1.02 (0.93–1.12) | 1.12 (1.01–1.25) | 0.015 |
| PM2.5, lag 0–3 | |||||
| Age, years | |||||
| <65 | 1.00 (reference) | 1.10 (0.98–1.21) | 1.17 (1.04–1.30) | 1.15 (1.01–1.29) | 0.025 |
| ≥65 | 1.00 (reference) | 1.05 (0.99–1.12) | 1.02 (0.96–1.09) | 1.11 (1.04–1.19) | 0.014 |
| Sex | |||||
| Men | 1.00 (reference) | 1.07 (1.00–1.15) | 1.03 (0.95–1.11) | 1.09 (1.00–1.18) | 0.122 |
| Women | 1.00 (reference) | 1.06 (0.98–1.15) | 1.09 (1.00–1.20) | 1.16 (1.06–1.27) | 0.002 |
| Household income | |||||
| Upper half | 1.00 (reference) | 1.06 (0.99–1.13) | 1.04 (0.97–1.12) | 1.12 (1.04–1.22) | 0.010 |
| Lower half | 1.00 (reference) | 1.08 (0.99–1.16) | 1.08 (0.99–1.18) | 1.11 (1.01–1.23) | 0.044 |
| PM2.5–10, lag 0–3 | |||||
| Age, years | |||||
| <65 | 1.00 (reference) | 1.04 (0.93–1.16) | 1.02 (0.90–1.16) | 1.13 (0.98–1.28) | 0.119 |
| ≥65 | 1.00 (reference) | 1.01 (0.95–1.08) | 1.01 (0.94–1.08) | 1.10 (1.02–1.19) | 0.026 |
| Sex | |||||
| Men | 1.00 (reference) | 1.04 (0.97–1.12) | 1.10 (1.01–1.19) | 1.13 (1.02–1.26) | 0.005 |
| Women | 1.00 (reference) | 0.98 (0.90–1.07) | 0.91 (0.82–1.00) | 1.07 (0.96–1.19) | 0.386 |
| Household income | |||||
| Upper half | 1.00 (reference) | 1.02 (0.95–1.10) | 1.01 (0.93–1.10) | 1.11 (1.02–1.20) | 0.042 |
| Lower half | 1.00 (reference) | 1.01 (0.93–1.10) | 1.01 (0.92–1.12) | 1.12 (1.00–1.24) | 0.065 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, S.; Kim, K.H.; Choi, D.; Jeong, S.; Kim, K.; Chang, J.; Kim, S.M.; Kim, S.R.; Cho, Y.; Lee, G.; et al. Association of Short-Term Particulate Matter Exposure among 5-Year Cancer Survivors with Incident Cardiovascular Disease: A Time-Stratified Case-Crossover Study. Int. J. Environ. Res. Public Health 2021, 18, 7996. https://doi.org/10.3390/ijerph18157996
Choi S, Kim KH, Choi D, Jeong S, Kim K, Chang J, Kim SM, Kim SR, Cho Y, Lee G, et al. Association of Short-Term Particulate Matter Exposure among 5-Year Cancer Survivors with Incident Cardiovascular Disease: A Time-Stratified Case-Crossover Study. International Journal of Environmental Research and Public Health. 2021; 18(15):7996. https://doi.org/10.3390/ijerph18157996
Chicago/Turabian StyleChoi, Seulggie, Kyae Hyung Kim, Daein Choi, Seogsong Jeong, Kyuwoong Kim, Jooyoung Chang, Sung Min Kim, Seong Rae Kim, Yoosun Cho, Gyeongsil Lee, and et al. 2021. "Association of Short-Term Particulate Matter Exposure among 5-Year Cancer Survivors with Incident Cardiovascular Disease: A Time-Stratified Case-Crossover Study" International Journal of Environmental Research and Public Health 18, no. 15: 7996. https://doi.org/10.3390/ijerph18157996
APA StyleChoi, S., Kim, K. H., Choi, D., Jeong, S., Kim, K., Chang, J., Kim, S. M., Kim, S. R., Cho, Y., Lee, G., Son, J. S., & Park, S. M. (2021). Association of Short-Term Particulate Matter Exposure among 5-Year Cancer Survivors with Incident Cardiovascular Disease: A Time-Stratified Case-Crossover Study. International Journal of Environmental Research and Public Health, 18(15), 7996. https://doi.org/10.3390/ijerph18157996






