Casomorphins and Gliadorphins Have Diverse Systemic Effects Spanning Gut, Brain and Internal Organs
Abstract
:1. Introduction
2. Methodology
3. The Role of Opioid Receptors
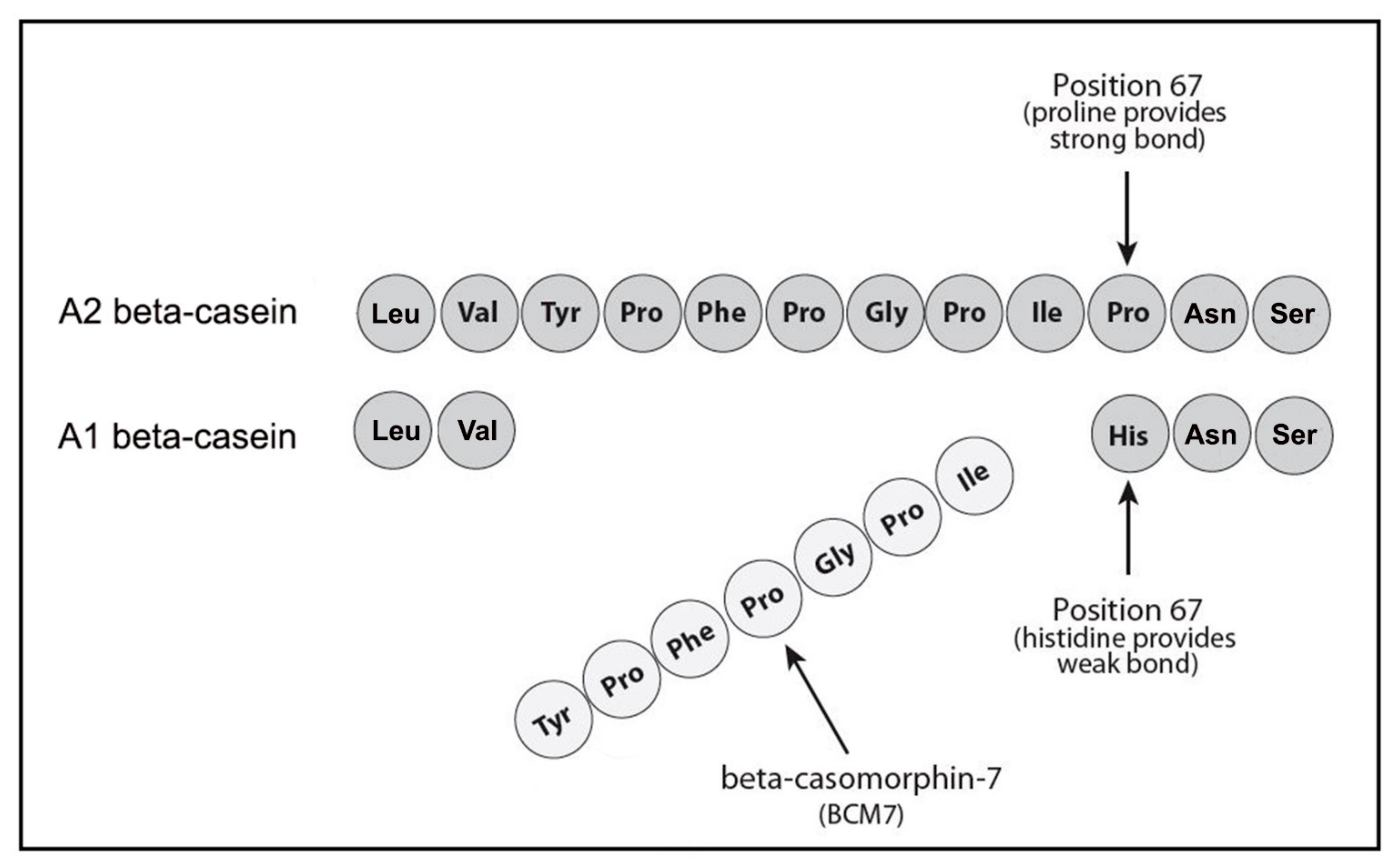
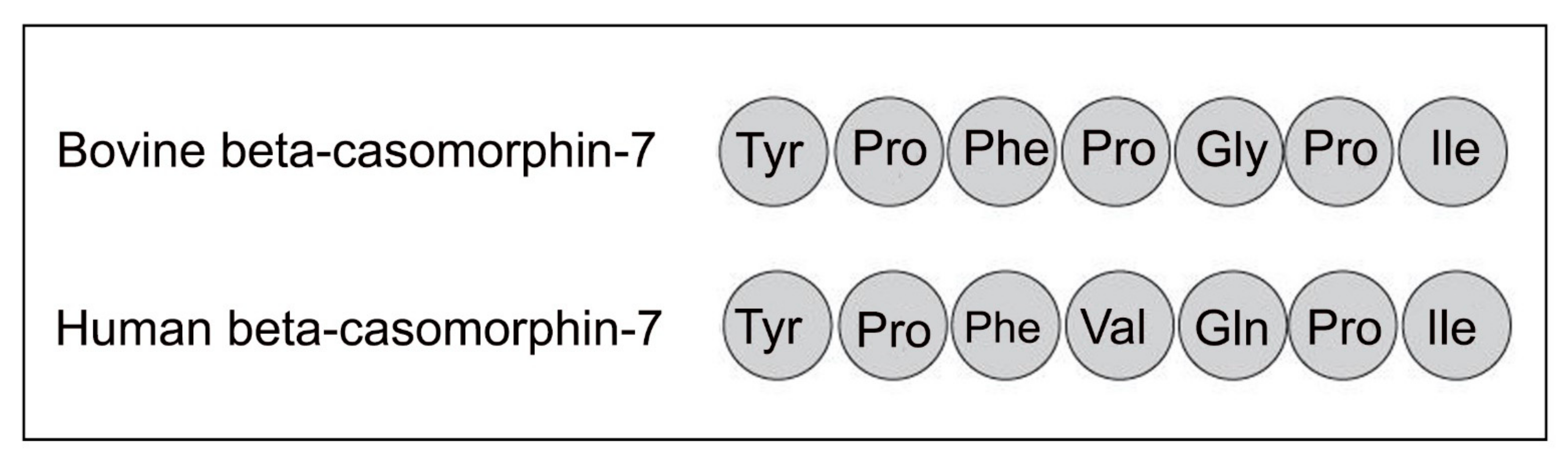
4. Casomorphins
5. Casomorphin Effects on Human Health
5.1. Type 1 Diabetes
5.2. Heart Disease
5.3. Links to the Brain
5.4. Gut Conditions
5.5. Other Conditions
6. Gliadin Peptides and Gliadorphin
7. Links to Microbiota
8. Conclusions
Funding
Conflicts of Interest
References
- Clemente, J.C.; Ursell, L.K.; Parfrey, L.W.; Knight, R. The impact of the gut microbiota on human health: An integrative view. Cell 2012, 148, 1258–1270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balakireva, A.V.; Zamyatnin, A.A. Properties of Gluten Intolerance: Gluten Structure, Evolution, Pathogenicity and Detoxification Capabilities. Nutrients 2016, 8, 644. [Google Scholar] [CrossRef] [Green Version]
- Hardy, M.Y.; Russell, A.K.; Pizzey, C.; Jones, C.M.; Watson, K.A.; La Gruta, N.L.; Cameron, D.J.; Tye-Din, J.A. Characterisation of clinical and immune reactivity to barley and rye ingestion in children with coeliac disease. Gut 2020, 69, 830–840. [Google Scholar] [CrossRef] [PubMed]
- Bromilow, S.; Gethings, L.A.; Buckley, M.; Bromley, M.; Shewry, P.; Langridge, J.I.; Mills, C.E.N. A curated gluten protein sequence database to support development of proteomics methods for determination of gluten in gluten-free foods. J. Proteom. 2017, 163, 67–75. [Google Scholar]
- Kaneko, K.; Iwasaki, M.; Yoshikawa, M.; Ohinata, K. Orally administered soymorphins, soy-derived opioid peptides, suppress feeding and intestinal transit via gut mu(1)-receptor coupled to 5-HT(1A), D(2), and GABA(B) systems. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 299, G799–G805. [Google Scholar] [CrossRef] [Green Version]
- Cassell, R.J.; Mores, K.L.; Zerfas, B.L.; Mahmoud, A.H.; Lill, M.A.; Trader, D.J.; van Rijn, R.M. Rubiscolins are naturally occurring G protein-biased delta opioid receptor peptides. Eur. Neuropsychopharmacol. 2019, 29, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Molberg, O.; Uhlen, A.K.; Jensen, T.; Flaete, S.L.; Fleckenstein, B.; Arentz-Hansen, M.; Raki, M.; Lundin, K.; Sollid, L. Mapping of gluten T-cell epitopes in the bread wheat ancestors: Implications for celiac disease. Gastroenterology 2005, 12, 393–401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konic-Ristic, A.; Dodig, D.; Krstic, R.; Jelic, S.; Stankovic, I.; Ninkovic, A.; Radic, J.; Besu, I.; Bonaci-Nikolic, B.; Jojic, N.; et al. Different levels of humoral immunoreactivity to different wheat cultivars gliadin are present in patients with celiac disease and in patients with multiple myeloma. BMC Immunol. 2009, 10, 32. [Google Scholar] [CrossRef] [Green Version]
- Henschen, A.; Lottspeich, F.; Brantl, V.; Teschemacher, H. Novel opioid peptides derived from casein (beta-casomorphins). II. Structure of active components from bovine casein peptone. Hoppe-Seyler’s Z. Physiol. Chem. 1979, 360, 1217–1224. [Google Scholar]
- Losowsky, M.S. A history of coeliac disease. Dig. Dis. 2008, 26, 112–120. [Google Scholar] [CrossRef]
- Graf, L.; Horvath, K.; Walcz, E.; Berzetei, I.; Burnier, J. Effect of two synthetic alpha-gliadin peptides on lymphocytes in celiac disease: Identification of a novel class of opioid receptors. Neuropeptides 1987, 9, 113–122. [Google Scholar] [CrossRef]
- Ul Haq, M.R.; Kapila, R.; Sharma, R.; Saliganti, V.; Kapila, S. Comparative evaluation of cow β-casein variants (A1/A2) consumption on Th2-mediated inflammatory response in mouse gut. Eur. J. Nutr. 2014, 53, 1039–1049. [Google Scholar] [CrossRef] [PubMed]
- Eidson, L.N.; Murphy, A.Z. Blockade of Toll-like receptor 4 attenuates morphine tolerance and facilitates the pain-relieving properties of morphine. J. Neurosci. 2013, 33, 15952–15963. [Google Scholar] [CrossRef] [Green Version]
- Sokolov, O.Y.; Pryanikova, N.A.; Kost, N.V.; Zolotarev, Y.A.; Ryukert’, E.N.; Zozulya, A.A. Reactions between beta-casomorphins-7 and 5-HT2-serotonin receptors. Bull. Exp. Biol. Med. 2005, 140, 582–584. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, M.; Takahashi, M.; Yang, S. Delta opioid peptides derived from plant proteins. Curr. Pharm. Des. 2003, 9, 1325–1330. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, A.; Daliri, E.B.-M.; Kwami Ofosu, F.; Yeon, S.-J.; Oh, D.-H. Food-Derived Opioid Peptides in Human Health: A Review. Int. J. Mol. Sci. 2020, 21, 8825. [Google Scholar] [CrossRef] [PubMed]
- Jordan, B.; Cvejic, S.; Devi, L. Opioids and Their Complicated Receptor Complexes. Neuropsychopharmacology 2000, 23, S5–S18. [Google Scholar] [CrossRef]
- Hughes, J.; Beaumont, A.; Fuentes, J.A.; Malfroy, B.; Unsworth, C. Opioid peptides: Aspects of their origin, release and metabolism. J. Exp. Biol. 1980, 89, 239–255. [Google Scholar] [CrossRef]
- Wittert, G.; Hope, P.; Pyle, D. Tissue distribution of opioid receptor gene expression in the rat. Biochem. Biophys. Res. Commun. 1996, 218, 877–881. [Google Scholar] [CrossRef]
- Al-Hasani, R.; Bruchas, M.R. Molecular Mechanisms of Opioid Receptor-dependent Signaling and Behavior. Anesthesiology 2011, 115, 1363–1381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pal, S.; Woodford, K.; Kukuljan, S.; Ho, S. Milk Intolerance, Beta-Casein and Lactose. Nutrients 2015, 7, 7285–7297. [Google Scholar] [CrossRef]
- Brooke-Taylor, S.; Dwyer, K.; Woodford, K.; Kost, N. Systematic Review of the Gastrointestinal Effects of A1 Compared with A2 β-Casein. Adv. Nutr. 2017, 8, 739–748. [Google Scholar] [CrossRef] [PubMed]
- Fiedorowicz, E.; Kaczmarski, M.; Cieślińska, A.; Sienkiewicz-Szłapka, E.; Jarmołowska, B.; Chwała, B.; Kostyra, E. β-casomorphin-7 alters μ-opioid receptor and dipeptidyl peptidase IV genes expression in children with atopic dermatitis. Peptides 2014, 62, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Brantl, V.; Teschemacher, H.; Blasig, J. ’ Henschen, A.; Lottspeich, F. Opioid activities of beta-casomorphins. Life Sci. 1981, 28, 1903–1909. [Google Scholar] [CrossRef]
- Jinsmaa, Y.; Yoshikawa, M. Enzymatic release of neocasomorphin and beta-casomorphin from bovine beta-casein. Peptides 1999, 20, 957–962. [Google Scholar] [CrossRef]
- Saito, T.; Nakamura, T.; Kitazawa, H.; Kawai, Y.; Itoh, T. Isolation and structural analysis of antihypertensive peptides that exist naturally in gouda cheese. J. Dairy Sci. 2000, 83, 1434–1440. [Google Scholar] [CrossRef]
- Deth, R.; Clarke, A.; Ni, J.; Trivedi, M. Clinical evaluation of glutathione concentrations after consumption of milk containing different subtypes of β-casein: Results from a randomized, cross-over clinical trial. Nutr. J. 2016, 15, 82. [Google Scholar] [CrossRef] [Green Version]
- Barnett, M.P.; McNabb, W.C.; Roy, N.C.; Woodford, K.B.; Clarke, A.J. Dietary A1 beta-casein affects gastrointestinal transit time, dipeptidyl peptidase-4 activity, and inflammatory status relative to A2 beta-casein in Wistar rats. Int. J. Food Sci. Nutr. 2014, 65, 720–727. [Google Scholar] [CrossRef]
- Ng-Kwai-Hang, K.F.; Grosclaude, F. Genetic Polymorphism of Milk Proteins. In Advanced Dairy Chemistry—1 Proteins; Springer: Boston, MA, USA, 2003. [Google Scholar]
- Farrell, H.M., Jr.; Jimenez-Flores, R.; Bleck, G.T.; Brown, E.M.; Butler, J.E.; Creamer, L.K.; Hicks, C.L.; Hollar, C.M.; Ng-Kwai-Hang, K.F.; Swaisgood, H.E. Nomenclature of proteins of cow’s milk-sixth revision. J. Dairy Sci. 2004, 87, 1641–1674. [Google Scholar]
- Woodford, K.B. Devil in the Milk; Chelsea Green: White River Junction, VT, USA, 2009. [Google Scholar]
- Koch, G.; Wiedemann, K.; Teschemacher, H. Opioid activities of human ßcasomorphins. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1985, 331, 351–354. [Google Scholar] [CrossRef] [PubMed]
- Jarmolowska, B.; Sidor, K.; Iwan, M.; Bielikowicz, K.; Kaczmarski, M.; Kostyra, E.; Kostyra, H. Changes of ß-casomorphin content in human milk during lactation. Peptides 2007, 28, 1982–1986. [Google Scholar] [CrossRef] [PubMed]
- Laugesen, M.; Elliott, R. Ischaemic heart disease, Type 1 diabetes, and cow milk A1 ß-casein. J. N. Zealand Med. Assoc. 2003, 116, 1168. [Google Scholar]
- Laugesen, M.; Elliott, R. The influence of consumption of A1 ß-casein on heart disease and Type 1 diabetes–the authors reply. J. N. Zealand Med. Assoc. 2003, 116, 1170. [Google Scholar]
- Woodford, K.B. ; Devil in the Milk; Craig Potton Publishing: Nelson, New Zealand, 2007. [Google Scholar]
- Gerstein, H.C. Cow’s Milk Exposure and Type I Diabetes Mellitus: A critical overview of the clinical literature. Diabetes Care 1994, 17, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Gerstein, H.C.; VanderMeulen, J. The relationship between cow’s milk exposure and type 1 diabetes. Diabet. Med. 1996, 13, 23–29. [Google Scholar] [CrossRef]
- Akerblom, H.K.; Vaarala, O. Cow milk proteins, autoimmunity and type 1 diabetes. Exp. Clin. Endocrinol. Diabetes 1997, 105, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Pozzilli, P. Beta-casein in cow’s milk: A major antigenic determinant for type 1 diabetes? J. Endocrinol. Investig. 1999, 22, 562–567. [Google Scholar] [CrossRef]
- Padberg, S.; Schumm-Draeger, P.M.; Petzoldt, R.; Becker, F.; Federlin, K. The significance of A1 and A2 antibodies against beta-casein in type-1 diabetes mellitus. Dtsch. Med. Wochenschr. 1999, 124, 1518–1521. [Google Scholar] [CrossRef]
- Vaarala, O.; Atkinson, M.A.; Neu, J. The “Perfect Storm” for Type 1 Diabetes. Diabetes 2008, 57, 2555–2562. [Google Scholar] [CrossRef] [Green Version]
- Olivares, M.; Schüppel, V.; Hassan, A.M.; Beaumont, M.; Neyrinck, A.M.; Bindels, L.B.; Benítez-Páez, A.; Sanz, Y.; Haller, D.; Holzer, P.; et al. The Potential Role of the Dipeptidyl Peptidase-4-Like Activity from the Gut Microbiota on the Host Health. Front. Microbiol. 2018, 9, 1900. [Google Scholar] [CrossRef]
- Sakurai, T.; Yamada, A.; Hashikura, N.; Odamaki, T.; Xiao, J.Z. Degradation of food-derived opioid peptides by bifidobacteria. Benef. Microbes 2018, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Laparra, J.M.; Sanz, Y. Bifidobacteria inhibit the inflammatory response induced by gliadins in intestinal epithelial cells via modifications of toxic peptide generation during digestion. J. Cell. Biochem. 2010, 109, 801–807. [Google Scholar] [CrossRef] [Green Version]
- Sokolov, O.; Kost, N.; Andreeva, O.; Korneeva, E.; Meshavkin, V.; Tarakanova, Y.; Dadayan, A.; Zolotarev, Y.; Grachev, S.; Mikheeva, I.; et al. Autistic children display elevated urine levels of bovine casomorphin-7 immunoreactivity. Peptides 2014, 56, 68–71. [Google Scholar] [CrossRef] [PubMed]
- Wasilewska, J.; Sienkiewicz-Szłapka, E.; Kuźbida, E.; Jarmołowska, B.; Kaczmarski, M.; Kostyra, E. The exogenous opioid peptides and DPPIV serum activity in infants with apnoea expressed as apparent life-threatening events (ALTE). Neuropeptides 2011, 45, 189–195. [Google Scholar] [CrossRef]
- Chia, J.S.J.; McRae, J.L.; Kukuljan, S.; Woodford, K.; Elliott, R.B.; Swinburn, B.; Dwyer, K.M. A1 beta-casein milk protein and other environmental pre-disposing factors for type 1 diabetes. Nutr. Diabetes 2017, 7, e274. [Google Scholar] [CrossRef] [Green Version]
- Chia, J.; McRae, J.L.; Enjapoori, A.K.; Lefèvre, C.M.; Kukuljan, S.; Dwyer, K.M. Dietary Cows’ Milk Protein A1 Beta-Casein Increases the Incidence of T1D in NOD Mice. Nutrients 2018, 10, 1291. [Google Scholar] [CrossRef] [Green Version]
- Beau, I.; Berger, A.; Servin, A.L. Rotavirus impairs the biosynthesis of brush-border-associated dipeptidyl peptidase IV in human enterocyte-like Caco-2/TC7 cells. Cell. Microbiol. 2007, 9, 779–789. [Google Scholar] [CrossRef] [PubMed]
- Claustre, J.; Toumi, F.; Trompette, A.; Jourdan, G.; Guignard, H.; Chayvialle, J.A.; Plaisancié, P. Effects of peptides derived from dietary proteins on mucus secretion in rat jejunum. Am. J. Physiol. Gastrointest. Liver Physiol. 2002, 283, G521–G528. [Google Scholar] [CrossRef] [Green Version]
- Trompette, A.; Claustre, J.; Caillon, F.; Jourdan, G.; Chayvialle, J.A.; Plaisancie, P. Milk bioactive peptides and beta-casomorphins induce mucus release in rat jejunum. J. Nutr. 2003, 133, 3499–3503. [Google Scholar] [CrossRef]
- Zoghbi, S.; Trompette, A.; Claustre, J.; El Homsi, M.; Garzon, J.; Jourdan, G.; Scoazec, J.Y.; Plaisancié, P. beta-Casomorphin-7 regulates the secretion and expression of gastrointestinal mucins through a mu-opioid pathway. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 290, G1105–G1113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gavin, P.G.; Hamilton-Williams, E.E. The gut microbiota in type 1 diabetes: Friend or foe? Curr. Opin. Endocrinol. Diabetes Obes. 2019, 26, 207–212. [Google Scholar] [CrossRef]
- Zhou, H.; Sun, L.; Zhang, S.; Zhao, X.; Gang, X.; Wang, G. Evaluating the Causal Role of Gut Microbiota in Type 1 Diabetes and Its Possible Pathogenic Mechanisms. Front. Endocrinol. 2020, 11, 125. [Google Scholar] [CrossRef] [PubMed]
- Ipp, E.; Dobbs, R.; Unger, R. Morphine and β-endorphin influence the secretion of the endocrine pancreas. Nature 1978, 276, 190–191. [Google Scholar] [CrossRef]
- Khawaja, X.Z.; Green, I.C.; Thorpe, J.R.; Titheradge, M.A. The occurrence and receptor specificity of endogenous opioid peptides within the pancreas and liver of the rat. Comparison with brain. Biochem. J. 1990, 267, 233–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, T.; Peng, B.; Pintar, J. The MOR-1 Opioid Receptor Regulates Glucose Homeostasis by Modulating Insulin Secretion. Mol. Endocrinol. 2009, 23, 671–678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tudurí, E.; Beiroa, D.; Stegbauer, J.; Jerno, J.; López, M.; Diéguez, C.; Nogueiras, R. Acute stimulation of brain mu opioid receptors inhibits glucose-stimulated insulin secretion via sympathetic innervation. Neuropharmacology 2016, 110, 322–332. [Google Scholar] [CrossRef]
- Monetini, L.; Cavallo, M.G.; Manfrini, S.; Stefanini, L.; Picarelli, A.; Di Tola, M.; Petrone, A.; Bianchi, M.; La Presa, M.; Di Giulio, C.; et al. Antibodies to bovine beta-casein in diabetes and other autoimmune diseases. Horm. Metab. Res. 2002, 34, 455–459. [Google Scholar] [CrossRef] [PubMed]
- McLachlan, C.N. beta-casein A1, ischaemic heart disease mortality, and other illnesses. Med. Hypotheses 2001, 56, 262–272. [Google Scholar] [CrossRef] [Green Version]
- Tailford, K.A.; Berry, C.L.; Thomas, A.C.; Campbell, J.H. A casein variant in cow’s milk is atherogenic. Atherosclerosis 2003, 170, 13–19. [Google Scholar] [CrossRef]
- Steinerova, A.; Korotvicka, M.; Racek, J.; Rajdl, D.; Trefil, L.; Stozicky, F.; Rokyta, A. Significant increase in antibodies against oxidized LDL particles (IgoxLDL) in three-month old infants who received milk formula. Atherosclerosis 2004, 173, 147–148. [Google Scholar]
- Steinerova, A.; Racek, J.; Korotvicka, M.; Stozicky, F. Beta casein A1 is a possible risk factor for atherosclerosis. Atheroscler. Suppl. 2009, 10, e1464. [Google Scholar] [CrossRef]
- Steinerova, A.; Stozicky, F.; Korotvicka, M.; Racek, J.; Tatzber, F. Does artificial suckling nutrition pose a risk of atherosclerosis at old age. Cesko Slov. Pediatr. 2006, 61, 519–523. [Google Scholar]
- Torreilles, J.; Guérin, M.C. Des peptides issus de la caséine peuvent provoquer l’oxydation des LDL humaines par un processus dépendant des peroxydases et indépendant des métaux [Casein-derived peptides can promote human LDL oxidation by a peroxidase-dependent and metal-independent process]. Comptes Rendus Seances Soc. Biol. Fil. 1995, 189, 933–942. [Google Scholar]
- Briggs, R.D.; Rubenberg, M.L.; O’Neal, R.M.; Thomas, W.A.; Hartroft, W.S. Myocardial infarction in patients treated with Sippy and other high-milk diets: An autopsy study of fifteen hospitals in the U.S.A. and Great Britain. Circulation 1960, 21, 538–542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sobanski, P.; Krajnik, M.; Shaqura, M.; Bloch-Boguslawska, E.; Schäfer, M.; Mousa, S.A. The presence of mu-, delta-, and kappa-opioid receptors in human heart tissue. Heart Vessel. 2014, 29, 855–863. [Google Scholar] [CrossRef] [PubMed]
- Barron, A. Opioid peptides and the heart. Cardiovasc. Res. 1999, 43, 13–16. [Google Scholar] [CrossRef]
- Muhamed, B.; Parks, T.; Sliwa, K. Genetics of rheumatic fever and rheumatic heart disease. Nat. Rev. Cardiol. 2020, 17, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Zhang, Z.; Wang, X.; Cade, R.; Elmir, Z.; Fregly, M. Relation of beta-casomorphin to apnea in sudden infant death syndrome. Peptides 2003, 24, 937–943. [Google Scholar] [CrossRef]
- Hedner, J.; Hedner, T. beta-Casomorphins induce apnea and irregular breathing in adult rats and newborn rabbits. Life Sci. 1987, 41, 2303–2312. [Google Scholar] [CrossRef]
- Pasi, A.; Mahler, H.; Lansel, N.; Bernasconi, C.; Messiha, F.S. beta-Casomorphin-immunoreactivity in the brain stem of the human infant. Res. Commun. Chem. Pathol. Pharm. 1993, 80, 305–322. [Google Scholar]
- Kost, N.V.; Sokolov, O.Y.; Kurasova, O.B.; Dmitriev, A.D.; Tarakanova, J.N.; Gabaeva, M.V.; Zolotarev, Y.A.; Dadayan, A.K.; Grachev, S.A.; Korneeva, E.V.; et al. Beta-casomorphins-7 in infants on different type of feeding and different levels of psychomotor development. Peptides 2009, 30, 1854–1860. [Google Scholar] [CrossRef]
- Cade, J.R.; Privette, M.R.; Fregly, M.; Rowland, N.; Sun, Z.; Zele, V. Autism and Schizophrenia: Intestinal Disorders. Nutr. Neurosci. 2000, 3, 57–72. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Cade, J.R. A Peptide Found in Schizophrenia and Autism Causes Behavioral Changes in Rats. Autism 1999, 3, 85–95. [Google Scholar] [CrossRef]
- Knivsberg, A.M.; Reichelt, K.L.; Hoien, T.; Nodland, M. A randomised, controlled study of dietary intervention in autistic syndromes. Nutr. Neurosci. 2002, 5, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Whiteley, P.; Haracopos, D.; Knivsberg, A.M.; Reichelt, K.L.; Parlar, S.; Jacobsen, J.; Seim, A.; Pedersen, L.; Schondel, M.; Shattock, P. The ScanBrit randomised, controlled, single-blind study of a gluten- and casein-free dietary intervention for children with autism spectrum disorders. Nutr. Neurosci. 2010, 13, 87–100. [Google Scholar] [CrossRef]
- Jarmołowska, B.; Bukało, M.; Fiedorowicz, E.; Cieślińska, A.; Kordulewska, N.K.; Moszyńska, M.; Świątecki, A.; Kostyra, E. Role of Milk-Derived Opioid Peptides and Proline Dipeptidyl Peptidase-4 in Autism Spectrum Disorders. Nutrients 2019, 11, 87. [Google Scholar] [CrossRef] [Green Version]
- Jianqin, S.; Leiming, X.; Lu, X.; Yelland, G.W.; Ni, J.; Clarke, A.J. Effects of milk containing only A2 beta casein versus milk containing both A1 and A2 beta casein proteins on gastrointestinal physiology, symptoms of discomfort, and cognitive behavior of people with self-reported intolerance to traditional cows’ milk. Nutr. J. 2015, 15, 35. [Google Scholar] [CrossRef] [Green Version]
- Sheng, X.; Li, Z.; Ni, J.; Yelland, G. Effects of Conventional Milk Versus Milk Containing Only A2 β-Casein on Digestion in Chinese Children: A Randomized Study. J. Pediatr. Gastroenterol. Nutr. 2019, 69, 375–382. [Google Scholar] [CrossRef]
- Trivedi, M.S.; Shah, J.S.; Al-Mughairy, S.; Hodgson, N.W.; Simms, B.; Trooskens, G.A.; Van Criekinge, W.; Deth, R.C. Food-derived opioid peptides inhibit cysteine uptake with redox and epigenetic consequences. J. Nutr. Biochem. 2014, 25, 1011–1018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trivedi, M.; Shah, J.; Hodgson, N.; Byun, H.M.; Deth, R. Morphine induces redox-based changes in global DNA methylation and retrotransposon transcription by inhibition of excitatory amino acid transporter type 3-mediated cysteine uptake. Mol. Pharm. 2014, 85, 747–757. [Google Scholar] [CrossRef] [Green Version]
- Trivedi, M.; Zhang, Y.; Lopez-Toledano, M.; Clarke, A.; Deth, R. Differential neurogenic effects of casein-derived opioid peptides on neuronal stem cells: Implications for redox-based epigenetic changes. J. Nutr. Biochem. 2016, 37, 39–46. [Google Scholar] [CrossRef] [Green Version]
- Ho, S.; Woodford, K.; Kukuljan, S.; Pal, S. Comparative effects of A1 versus A2 beta-casein on gastrointestinal measures: A blinded randomised cross-over pilot study. Eur. J. Clin. Nutr. 2014, 68, 994–1000. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Sun, J.; Jiang, Z.Q.; Yang, Y.X. Effects of cow’s milk beta-casein variants on symptoms of milk intolerance in Chinese adults: A multicentre, randomised controlled study. Nutr. J. 2017, 16, 72. [Google Scholar] [CrossRef] [Green Version]
- Ramakrishnan, M.; Eaton, T.K.; Sermet, O.M.; Savaiano, D.A. Milk Containing A2 β-Casein ONLY, as a Single Meal, Causes Fewer Symptoms of Lactose Intolerance than Milk Containing A1 and A2 β-Caseins in Subjects with Lactose Maldigestion and Intolerance: A Randomized, Double-Blind, Crossover Trial. Nutrients 2020, 12, 3855. [Google Scholar] [CrossRef]
- Raynes, J.K.; Day, L.; Augustin, M.A.; Carver, J.A. Structural differences between bovine A(1) and A(2) β-casein alter micelle self-assembly and influence molecular chaperone activity. J. Dairy Sci. 2015, 98, 2172–2182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yadav, S.; Yadav, N.D.S.; Gheware, A.; Kulshreshtha, A.; Sharma, P.; Singh, V.P. Oral Feeding of Cow Milk Containing A1 Variant of β Casein Induces Pulmonary Inflammation in Male Balb/c Mice. Sci. Rep. 2020, 10, 8053. [Google Scholar] [CrossRef]
- Aslam, H.; Ruusunen, A.; Berk, M.; Loughman, A.; Rivera, L.; Pasco, J.A.; Jacka, F.N. Unravelled facets of milk derived opioid peptides: A focus on gut physiology, fractures and obesity. Int. J. Food Sci. Nutr. 2020, 71, 36–49. [Google Scholar] [CrossRef]
- Jiang, W.; Ju, C.; Jiang, H.; Zhang, D. Dairy foods intake and risk of Parkinson’s disease: A dose-response meta-analysis of prospective cohort studies. Eur. J. Epidemiol. 2014, 29, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Malosse, D.; Perron, H.; Sasco, A.; Seigneurin, J.M. Correlation between milk and dairy product consumption and multiple sclerosis prevalence: A worldwide study. Neuroepidemiology 1992, 11, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Malosse, D.; Perron, H. Correlation analysis between bovine populations, other farm animals, house pets, and multiple sclerosis prevalence. Neuroepidemiology 1993, 12, 15–27. [Google Scholar] [CrossRef]
- Bidlack, J.M. Detection and function of opioid receptors on cells from the immune system. Clin. Diagn. Lab. Immunol. 2000, 7, 719–723. [Google Scholar] [CrossRef] [Green Version]
- Eisenstein, T.K. The Role of Opioid Receptors in Immune System Function. Front. Immunol. 2019, 10, 2904. [Google Scholar] [CrossRef] [Green Version]
- Kapas, S.; Purbrick, A.; Hinson, J.P. Action of opioid peptides on the rat adrenal cortex: Stimulation of steroid secretion through a specific μ opioid receptor. J. Endocrinol. 1995, 144, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Rhodin, A.; Stridsberg, M.; Gordh, T. Opioid endocrinopathy: A clinical problem in patients with chronic pain and long-term oral opioid treatment. Clin. J. Pain 2010, 26, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Bergasa, N.V.; Rothman, R.B.; Mukerjee, E.; Vergalla, J.; Jones, E.A. Up-regulation of central mu-opioid receptors in a model of hepatic encephalopathy: A potential mechanism for increased sensitivity to morphine in liver failure. Life Sci. 2002, 70, 1701–1708. [Google Scholar] [CrossRef]
- Ebrahimkhani, M.R.; Kiani, S.; Oakley, F.; Kendall, T.; Shariftabrizi, A.; Tavangar, S.M.; Moezi, L.; Payabvash, S.; Karoon, A.; Hoseininik, H.; et al. Naltrexone, an opioid receptor antagonist, attenuates liver fibrosis in bile duct ligated rats. Gut 2006, 551, 606–1616. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Miao, J.; Zhang, Y. Protective effect of β-casomorphin-7 on type 1 diabetes rats induced with streptozotocin. Peptides 2010, 31, 1725–1729. [Google Scholar] [CrossRef]
- Yin, H.; Miao, J.; Ma, C.; Sun, G.; Zhang, Y. β-Casomorphin-7 cause decreasing in oxidative stress and inhibiting NF-κB-iNOS-NO signal pathway in pancreas of diabetes rats. J. Food Sci. 2012, 77, 278–282. [Google Scholar] [CrossRef]
- Zhang, W.; Miao, J.; Ma, C.; Han, D.; Zhang, Y. β-Casomorphin-7 attenuates the development of nephropathy in type 1 diabetes via inhibition of epithelial-mesenchymal transition of renal tubular epithelial cells. Peptides 2012, 36, 186–191. [Google Scholar] [CrossRef]
- Fasano, A.; Sapone, A.; Zevallos, V.; Schuppan, D. Nonceliac Gluten Sensitivity. Gastroenterology 2015, 148, 1195–1204. [Google Scholar] [CrossRef]
- Barnett, J.A.; Gibson, D.L. Separating the Empirical Wheat from the Pseudoscientific Chaff: A Critical Review of the Literature Surrounding Glyphosate, Dysbiosis and Wheat-Sensitivity. Front. Microbiol. 2020, 11, 556729. [Google Scholar] [CrossRef]
- Volta, U.; Tovoli, F.; Cicola, R.; Parisi, C.; Fabbri, A.; Piscaglia, M.; Fiorini, E.; Caio, G. Serological Tests in Gluten Sensitivity (Nonceliac Gluten Intolerance). J. Clin. Gastroenterol. 2012, 46, 680–685. [Google Scholar] [CrossRef]
- Sun, Z.; Cade, R. Findings in normal rats following administration of gliadorphin-7 (GD-7). Peptides 2003, 24, 321–323. [Google Scholar] [CrossRef]
- Norris, J.M.; Barriga, K.; Klingensmith, G.; Hoffman, M.; Eisenbarth, G.S.; Erlich, H.A.; Rewers, M. Timing of initial cereal exposure in infancy and risk of islet autoimmunity. JAMA 2003, 290, 1713–1720. [Google Scholar] [CrossRef]
- Scott, F.W. Food induced type 1 diabetes in the BB rat. Diabetes Metab. Rev. 1996, 12, 341–359. [Google Scholar] [CrossRef]
- Makhlouf, S.; Messelmani, M.; Zaouali, J.; Mrissa, R. Cognitive impairment in celiac disease and non-celiac gluten sensitivity: Review of literature on the main cognitive impairments, the imaging and the effect of gluten free diet. Acta Neurol. Belg. 2018, 118, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Koszarny, A.; Majdan, M.; Suszek, D.; Dryglewska, M.; Tabarkiewicz, J. Autoantibodies against gliadin in rheumatoid arthritis and primary Sjögren’s syndrome patients. Wiad. Lek. 2015, 68, 242–247. [Google Scholar] [PubMed]
- Yang, Y.; Deshpande, P.; Krishna, K.; Ranganathan, V.; Jayaraman, V.; Wang, T.; Bei, K.; Rajasekaran, J.; Krishnamurthy, H. Overlap of Characteristic Serological Antibodies in Rheumatoid Arthritis and Wheat-Related Disorders. Dis. Markers 2019, 4089178. [Google Scholar] [CrossRef] [Green Version]
- Mayer, E.; Tillisch, K.; Gupta, A. Gut/brain axis and the microbiota. J. Clin. Investig. 2015, 125, 926–938. [Google Scholar] [CrossRef]
- Martin, C.R.; Osadchiy, V.; Kalani, A.; Mayer, E.A. The Brain-Gut-Microbiome Axis. Cell. Mol. Gastroenterol. Hepatol. 2018, 6, 133–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids from Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. (Lausanne) 2020, 11, 25. [Google Scholar] [CrossRef] [Green Version]
- Rueda-Ruzafa, L.; Cruz, F.; Cardona, D.; Hone, A.J.; Molina-Torres, G.; Sánchez-Labraca, N.; Roman, P. Opioid system influences gut-brain axis: Dysbiosis and related alterations. Pharmacol. Res. 2020, 159, 104928. [Google Scholar] [CrossRef] [PubMed]
- Guantario, B.; Giribaldi, M.; Devirgiliis, C.; Finamore, A.; Colombino, E.; Capucchio, M.T.; Evangelista, R.; Motta, V.; Zinno, P.; Cirrincione, S.; et al. A Comprehensive Evaluation of the Impact of Bovine Milk Containing Different Beta-Casein Profiles on Gut Health of Ageing Mice. Nutrients 2020, 12, 2147. [Google Scholar] [CrossRef] [PubMed]
- Hansen, L.B.S.; Roager, H.M.; Søndertoft, N.B.; Gøbel, R.J.; Kristensen, M.; Vallès-Colomer, M.; Vieira-Silva, S.; Ibrügger, S.; Lind, M.V.; Mærkedahl, R.B.; et al. A low-gluten diet induces changes in the intestinal microbiome of healthy Danish adults. Nat. Commun. 2018, 9, 4630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dieterich, W.; Schuppan, D.; Schink, M.; Schwappacher, R.; Wirtz, R.; Abbas Agaimy, A.; Neurath, M.F.; Zopf, Y. Influence of low FODMAP and gluten-free diets on disease activity and intestinal microbiota in patients with non-celiac gluten sensitivity. Clin. Nutr. 2019, 38, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Haro, C.; Villatoro, M.; Vaquero, L.; Pastor, J.; Giménez, M.J.; Ozuna, C.V.; Sánchez-León, S.; García-Molina, M.D.; Segura, V.; Comino, I.; et al. The Dietary Intervention of Transgenic Low-Gliadin Wheat Bread in Patients with Non-Celiac Gluten Sensitivity (NCGS) Showed No Differences with Gluten Free Diet (GFD) but Provides Better Gut Microbiota Profile. Nutrients 2018, 10, 1964. [Google Scholar]
- Caio, G.; Lungaro, L.; Segata, N.; Guarino, M.; Zoli, G.; Volta, U.; De Giorgio, R. Effect of Gluten-Free Diet on Gut Microbiota Composition in Patients with Celiac Disease and Non-Celiac Gluten/Wheat Sensitivity. Nutrients 2020, 12, 1832. [Google Scholar] [CrossRef]
- Garcia-Mazcorro, J.F.; Rivera-Gutierrez, X.; Cobos-Quevedo, O.D.J.; Grube-Pagola, P.; Meixueiro-Daza, A.; Hernandez-Flores, K.; Cabrera-Jorge, F.J.; Vivanco-Cid, H.; Dowd, S.E.; Remes-Troche, J.M. First Insights into the Gut Microbiota of Mexican Patients with Celiac Disease and Non-Celiac Gluten Sensitivity. Nutrients 2018, 10, 1641. [Google Scholar] [CrossRef] [Green Version]
- Janer, C.; Arigoni, F.; Lee, B.H.; Peláez, C.; Requena, T. Enzymatic ability of Bifidobacterium animalis subsp. lactis to hydrolyze milk proteins: Identification and characterization of endopeptidase O. Appl. Environ. Microbiol. 2005, 71, 8460–8465. [Google Scholar] [CrossRef] [Green Version]
- Jin, Y.; Yu, Y.; Qi, Y.; Wang, F.; Yan, J.; Zou, H. Peptide profiling and the bioactivity character of yogurt in the simulated gastrointestinal digestion. J. Proteom. 2016, 141, 24–46. [Google Scholar] [CrossRef]
- Zhang, L.; Andersen, D.; Roager, H.; Bahl, M.L.; Hansen, C.H.F.; Danneskiold-Samsøe, N.B.; Kristiansen, K.; Radulescu, I.D.; Sina, C.; Frandsen, H.L.; et al. Effects of Gliadin consumption on the Intestinal Microbiota and Metabolic Homeostasis in Mice Fed a High-fat Diet. Sci. Rep. 2017, 7, 44613. [Google Scholar] [PubMed] [Green Version]
- Peng, J.; Xiao, X.; Hu, M.; Zhang, X. Interaction between gut microbiome and cardiovascular disease. Life Sci. 2018, 214, 153–157. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Woodford, K.B. Casomorphins and Gliadorphins Have Diverse Systemic Effects Spanning Gut, Brain and Internal Organs. Int. J. Environ. Res. Public Health 2021, 18, 7911. https://doi.org/10.3390/ijerph18157911
Woodford KB. Casomorphins and Gliadorphins Have Diverse Systemic Effects Spanning Gut, Brain and Internal Organs. International Journal of Environmental Research and Public Health. 2021; 18(15):7911. https://doi.org/10.3390/ijerph18157911
Chicago/Turabian StyleWoodford, Keith Bernard. 2021. "Casomorphins and Gliadorphins Have Diverse Systemic Effects Spanning Gut, Brain and Internal Organs" International Journal of Environmental Research and Public Health 18, no. 15: 7911. https://doi.org/10.3390/ijerph18157911
APA StyleWoodford, K. B. (2021). Casomorphins and Gliadorphins Have Diverse Systemic Effects Spanning Gut, Brain and Internal Organs. International Journal of Environmental Research and Public Health, 18(15), 7911. https://doi.org/10.3390/ijerph18157911




