Clinical Factors Associated with Asymptomatic Women Having Inconclusive Screening Mammography Results: Experiences from a Single Medical Center in Taiwan
Abstract
1. Introduction
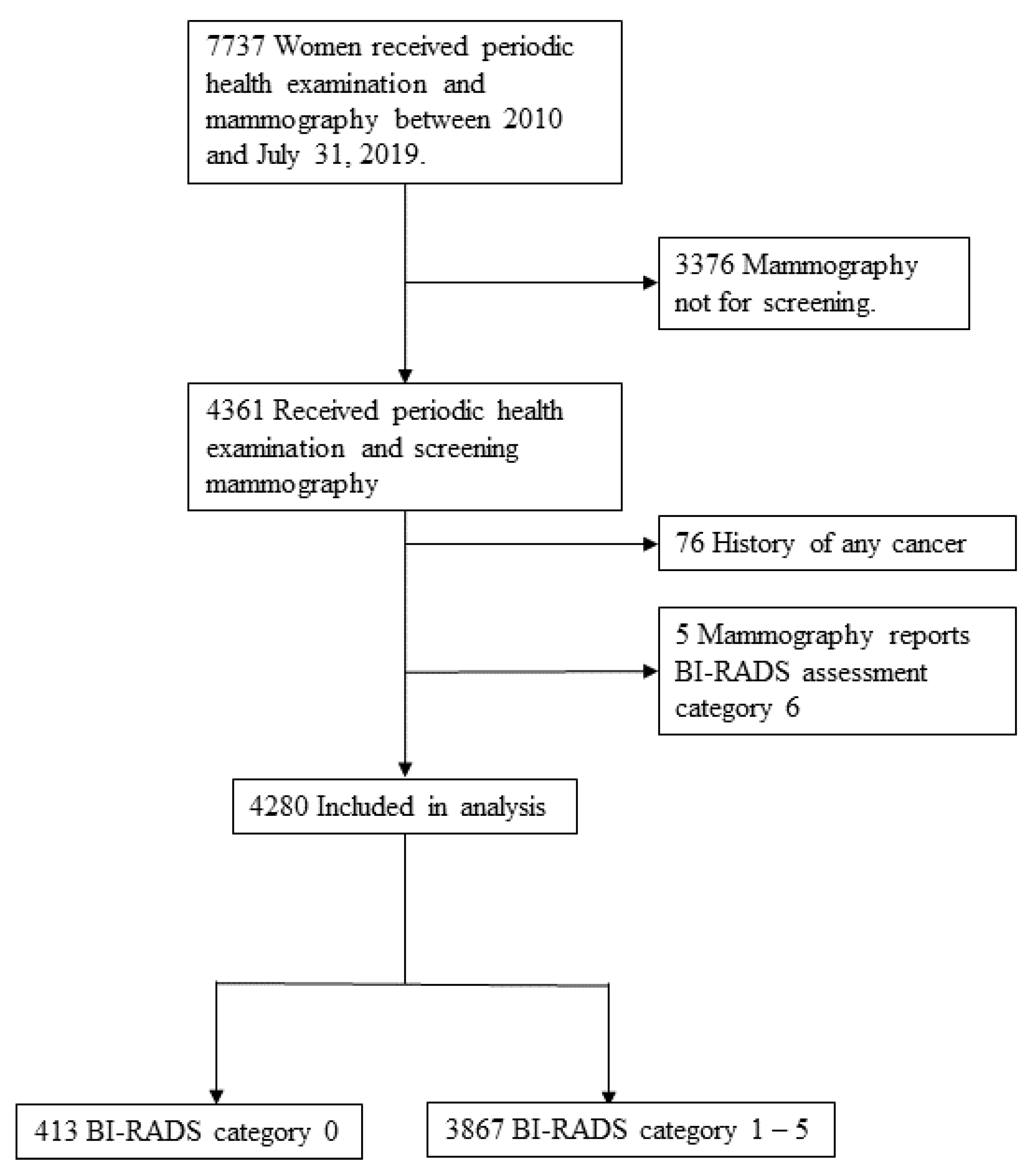
2. Materials and Methods
2.1. Study Population and Design
2.2. Data Collection
2.3. Mammographic Assessment and Management Recommendations
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ministry of Health and Welfare. Causes of Deaths Statistics. 2019. Available online: https://www.mohw.gov.tw/np-128-2.html (accessed on 14 September 2020).
- US Preventive Services Task Force. Screening for breast cancer: U.S. Preventive Services Task Force recommendation statement. Ann. Intern. Med. 2016, 164, 279–296. [Google Scholar] [CrossRef] [PubMed]
- D’Orsi, C.J.; Sickles, E.A.; Mendelson, E.B.; Morris, E.A. ACR BI-RADS Atlas, Breast Imaging Reporting and Data System; American College of Radiology: Reston, VA, USA, 2003. [Google Scholar]
- D’Orsi, C.J.; Sickles, E.A.; Mendelson, E.B.; Morris, E.A. ACR BI-RADS Atlas, Breast Imaging Reporting and Data System; American College of Radiology: Reston, VA, USA, 2013. [Google Scholar]
- Eklund, G.W.; Cardenosa, G.; Parsons, W. Assessing adequacy of mammographic image quality. Radiology 1994, 190, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Justice, T.D.; Stiff, J.H.; Myers, J.A.; Milam, M.R. Prediction of Incomplete Screening Mammograms Based on Age and Race. J. Am. Board Fam. Med. 2012, 25, 128–130. [Google Scholar] [CrossRef] [PubMed]
- Boyd, N.F.; Lockwood, G.A.; Byng, J.W.; Little, L.E.; Yaffe, M.J.; Tritchler, D.L. The relationship of anthropometric measures to radio-logical features of the breast in premenopausal women. Br. J. Cancer 1998, 78, 1233–1238. [Google Scholar] [CrossRef]
- Tehranifar, P.; Protacio, A.; Schmitt, K.M.; Desperito, E.; Oskar, S.; Potter, A.J.; Engmann, N.J.; Terry, M.B. The metabolic syndrome and mammographic breast density in a racially diverse and predominantly immigrant sample of women. Cancer Causes Control 2015, 26, 1393–1403. [Google Scholar] [CrossRef] [PubMed]
- Lund Håheim, L.; Wisløff, T.F.; Holme, I.; Nafstad, P. Metabolic syndrome predicts prostate cancer in a cohort of middle-aged Norwegian men followed for 27 years. Am. J. Epidemiol. 2006, 64, 769–774. [Google Scholar] [CrossRef] [PubMed]
- Laukkanen, J.A.; Laaksonen, D.E.; Niskanen, L.; Pukkala, E.; Hakkarainen, A.; Salonen, J.T. Metabolic syndrome and the risk of pros-tate cancer in Finnish men: A population-based study. Cancer Epidemiol. Biomark. Prev. 2004, 13, 1646–1650. [Google Scholar]
- Ahmed, R.L.; Schmitz, K.H.; Anderson, K.E.; Rosamond, W.D.; Folsom, A.R. The metabolic syndrome and risk of incident colorectal cancer. Cancer 2006, 107, 28–36. [Google Scholar] [CrossRef]
- Kim, J.H.; Lim, Y.J.; Kim, Y.-H.; Sung, I.-K.; Shim, S.G.; Oh, S.-O.; Park, S.-S.; Yang, S.; Son, H.J.; Rhee, P.-L.; et al. Is Metabolic Syndrome A Risk Factor for Colorectal Adenoma? Cancer Epidemiol. Biomark. Prev. 2007, 16, 1543–1546. [Google Scholar] [CrossRef]
- Kabat, G.C.; Kim, M.; Chlebowski, R.T.; Khandekar, J.; Ko, M.G.; McTiernan, A.; Neuhouser, M.L.; Parker, D.R.; Shikany, J.M.; Stefanick, M.L.; et al. A Longitudinal Study of the Metabolic Syndrome and Risk of Postmenopausal Breast Cancer. Cancer Epidemiol. Biomark. Prev. 2009, 18, 2046–2053. [Google Scholar] [CrossRef]
- Guo, M.; Liu, T.; Li, P.; Wang, T.; Zeng, C.; Yang, M.; Li, G.; Han, J.; Wu, W.; Zhang, R. Association Between Metabolic Syndrome and Breast Cancer Risk: An Updated Meta-Analysis of Follow-Up Studies. Front. Oncol. 2019, 9, 1290. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M.; Cleeman, J.I.; Merz, C.N.; Brewer, H.B., Jr.; Clark, L.T.; Hunninghake, D.B.; Pasternak, R.C.; Smith, S.C.; Stone, N.J.; Coordinating Committee of the National Cholesterol Education. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation 2004, 110, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Ramani, S.K.; Rastogi, A.; Mahajan, A.; Nair, N.; Shet, T.; Thakur, M.H. Imaging of the treated breast post breast conservation sur-gery/oncoplasty: Pictorial review. World J. Radiol. 2017, 9, 321–329. [Google Scholar] [CrossRef]
- Esen, G.; Olgun, D.C. Ultrasonography of the Postsurgical Breast Including Implants. Ultrasound Clin. 2008, 3, 295–329. [Google Scholar] [CrossRef]
- Devolli-Disha, E.; Manxhuka-Kërliu, S.; Ymeri, H.; Kutllovci, A. Comparative Accuracy of Mammography and Ultrasound in Women with Breast Symptoms According to Age and Breast Density. Bosn. J. Basic Med. Sci. 2009, 9, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Houssami, N.; Irwig, L.; Simpson, J.M.; McKessar, M.; Blome, S.; Noakes, J. The Influence of Knowledge of Mammography Findings on the Accuracy of Breast Ultrasound in Symptomatic Women. Breast J. 2005, 11, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Saarenmaa, I.; Salminen, T.; Geiger, U.; Heikkinen, P.; Hyvärinen, S.; Isola, J.; Kataja, V.; Kokko, M.-L.; Kokko, R.; Kumpulainen, E.; et al. The effect of age and density of the breast on the sensitivity of breast cancer diagnostic by mammography and ultrasonography. Breast Cancer Res. Treat 2001, 67, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Sibbering, D.; Burrell, H.; Evans, A.; Yeoman, L.; Wilson, A.; Robertson, J.; Blarney, R. Mammographic sensitivity in women under 50 years presenting symptomatically with breast cancer. Breast 1995, 4, 127–129. [Google Scholar] [CrossRef]
- Ciatto, S.; Del Turco, M.R.; Catarzi, S.; Morrone, D. The contribution of ultrasonography to the differential diagnosis of breast cancer. Neoplasma 1994, 41, 341–345. [Google Scholar]
- Sung, H.; Ren, J.; Li, J.; Pfeiffer, R.M.; Wang, Y.; Guida, J.L.; Fang, Y.; Shi, J.; Zhang, K.; Li, N.; et al. Breast cancer risk factors and mammographic density among high-risk women in urban China. NPJ Breast Cancer 2018, 4, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Park, B. Association between changes in mammographic density category and the risk of breast cancer: A nationwide cohort study in East-Asian women. Int. J. Cancer 2021, 148, 2674–2684. [Google Scholar] [CrossRef] [PubMed]
- McTiernan, A.; Martin, C.F.; Peck, J.D.; Aragaki, A.K.; Chlebowski, R.T.; Pisano, E.D.; Wang, C.Y.; Brunner, R.L.; Johnson, K.C.; Manson, J.E.; et al. Women’s Health Initiative Mammogram Density Study Investigators. Estrogen-plus-progestin use and mammographic density in postmenopausal women: Women’s Health Initiative randomized trial. J. Natl. Cancer Inst. 2005, 97, 1366–1376. [Google Scholar] [CrossRef]
- Topal, N.B.; Ayhan, S.; Topal, U.; Bilgin, T. Effects of hormone replacement therapy regimens on mammographic breast density: The role of progestins. J. Obstet. Gynaecol. Res. 2006, 32, 305–308. [Google Scholar] [CrossRef] [PubMed]
- Greendale, G.A.; Reboussin, B.A.; Sie, A.; Singh, H.R.; Olson, L.K.; Gatewood, O.; Bassett, L.W.; Wasilauskas, C.; Bush, T. Effects of estrogen and estrogen-progestin on mammographic parenchymal density: Postmenopausal Estrogen/Progestin Interventions (PEPI) Investigators. Ann. Intern. Med. 1999, 130, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Spicer, D.V.; Ursin, G.; Parisky, Y.R.; Pearce, J.G.; Shoupe, D.; Pike, A.; Pike, M.C. Changes in mammographic densities induced by a hor-monal contraceptive designed to reduce breast cancer risk. J. Natl. Cancer Inst. 1994, 86, 431–436. [Google Scholar] [CrossRef]
- Boyd, N.F.; Melnichouk, O.; Martin, L.J.; Hislop, G.; Chiarelli, A.M.; Yaffe, M.J.; Minkin, S. Mammographic Density, Response to Hormones, and Breast Cancer Risk. J. Clin. Oncol. 2011, 29, 2985–2992. [Google Scholar] [CrossRef]
- Greendale, G.A.; Reboussin, B.; Slone, S.; Wasilauskas, C.; Pike, M.C.; Ursin, G. Postmenopausal hormone therapy and change in mammographic density. J. Natl. Cancer Inst. 2003, 95, 30–37. [Google Scholar] [CrossRef]
- Marshall, K.G. Prevention. How much harm? How much benefit? 2. Ten potential pitfalls in determining the clinical signifi-cance of benefits. CMAJ 1996, 154, 1837–1843. [Google Scholar]
- Cole, P.; Morrison, A.S. Basic Issues in Population Screening for Cancer. J. Natl. Cancer Inst. 1980, 64, 1263–1272. [Google Scholar] [CrossRef]
- Ohuchi, N.; Suzuki, A.; Sobue, T.; Kawai, M.; Yamamoto, S.; Zheng, Y.F.; Shiono, Y.N.; Saito, H.; Kuriyama, S.; Tohno, E.; et al. Sensitivity and specificity of mammography and ad-junctive ultrasonography to screen for breast cancer in the Japan Strategic Anti-cancer Randomized Trial (J-START): A ran-domised controlled trial. Lancet 2016, 387, 341–348. [Google Scholar] [CrossRef]
- Kolb, T.M.; Lichy, J.; Newhouse, J.H. Comparison of the Performance of Screening Mammography, Physical Examination, and Breast US and Evaluation of Factors that Influence Them: An Analysis of 27,825 Patient Evaluations. Radiology 2002, 225, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Corsetti, V.; Houssami, N.; Ghirardi, M.; Ferrari, A.; Speziani, M.; Bellarosa, S.; Remida, G.; Gasparotti, C.; Galligioni, E.; Ciatto, S. Evidence of the effect of adjunct ultrasound screening in women with mammography-negative dense breasts: Interval breast cancers at 1year follow-up. Eur. J. Cancer 2011, 47, 1021–1026. [Google Scholar] [CrossRef] [PubMed]
- Corsetti, V.; Houssami, N.; Ferrari, A.; Ghirardi, M.; Bellarosa, S.; Angelini, O.; Bani, C.; Sardo, P.; Remida, G.; Galligioni, E.; et al. Breast screening with ultrasound in women with mammography-negative dense breasts: Evidence on incremental cancer detection and false positives, and associated cost. Eur. J. Cancer 2008, 44, 539–544. [Google Scholar] [CrossRef]
- Sirous, M.; Shahnani, P.S.; Sirous, A. Investigation of Frequency Distribution of Breast Imaging Reporting and Data System (BI-RADS) Classification and Epidemiological Factors Related to Breast Cancer in Iran: A 7-year Study (2010–2016). Adv. Biomed. Res. 2018, 7, 56. [Google Scholar] [PubMed]
| BI-RADS Category 1–5 (n = 3867) | BI-RADS Category 0 (n = 413) | p Value | |||
|---|---|---|---|---|---|
| Age | 56.92 | ±7.20 | 56.30 | ±7.51 | 0.124 |
| Metabolic syndrome | 0.978 | ||||
| No | 2985 | (77.19%) | 318 | (77.00%) | |
| Yes | 882 | (22.81%) | 95 | (23.00%) | |
| WC ≥ 80 cm (n = 4270) | 1571 | (40.72%) | 168 | (40.78%) | 1.000 |
| TG ≥ 150 mg/dL (n = 4278) | 3745 | (96.90%) | 401 | (97.09%) | 0.942 |
| HDL < 50 mg/dL (n = 4270) | 736 | (19.08%) | 79 | (19.17%) | 1.000 |
| fasting glucose ≥ 100 mg/dL/diabetes (n = 4278) a | 3836 | (99.25%) | 411 | (99.52%) | 0.763 |
| SBP ≥ 130 mmHg/DBP ≥ 85 mmHg/hypertension (n = 4278) a | 3860 | (99.87%) | 413 | (100%) | 1.000 |
| BMI (n = 4276) | 0.669 | ||||
| <18.5 | 110 | (2.85%) | 12 | (2.91%) | |
| 18.5–23.9 | 2040 | (52.80%) | 208 | (50.49%) | |
| ≥24 | 1714 | (44.36%) | 192 | (46.60%) | |
| Current smoker | 77 | (1.99%) | 10 | (2.42%) | 0.685 |
| Alcohol drinking | 379 | (9.80%) | 45 | (10.90%) | 0.534 |
| Habit of exercise | 0.368 | ||||
| No | 1475 | (38.14%) | 147 | (35.59%) | |
| <2.5 hr/week | 1383 | (35.76%) | 162 | (39.23%) | |
| ≥2.5 hr/week | 1009 | (26.09%) | 104 | (25.18%) | |
| History of benign breast disease | 714 | (18.46%) | 82 | (19.85%) | 0.533 |
| Menopause status | 0.010 * | ||||
| Premenopausal | 822 | (21.26%) | 111 | (26.88%) | |
| Postmenopausal | 3045 | (78.74%) | 302 | (73.12%) | |
| History of breast surgery | 210 | (5.43%) | 35 | (8.47%) | 0.016 * |
| Family history of breast cancer | 278 | (7.19%) | 27 | (6.54%) | 0.698 |
| Age at menarche, years | 0.500 | ||||
| <12 | 104 | (2.69%) | 15 | (3.63%) | |
| 12–13 | 1342 | (34.70%) | 146 | (35.35%) | |
| ≥14 | 2421 | (62.61%) | 252 | (61.02%) | |
| Age at first live birth, years (n = 3896) | 0.428 | ||||
| ≤24 | 1168 | (33.20%) | 125 | (33.07%) | |
| 25–29 | 1680 | (47.75%) | 171 | (45.24%) | |
| ≥30 | 670 | (19.04%) | 82 | (21.69%) | |
| Abortion | 0.039 * | ||||
| 0–1 | 3008 | (77.79%) | 340 | (82.32%) | |
| ≥2 | 859 | (22.21%) | 73 | (17.68%) | |
| Parity | 0.708 | ||||
| nulliparous | 347 | (8.97%) | 35 | (8.47%) | |
| 1 | 419 | (10.84%) | 40 | (9.69%) | |
| ≥2 | 3101 | (80.19%) | 338 | (81.84%) | |
| Breast feeding | 2011 | (52.00%) | 233 | (56.42%) | 0.098 |
| Use of hormonal contraceptives | 0.048 * | ||||
| No | 3095 | (80.04%) | 343 | (83.05%) | |
| <5 years | 600 | (15.52%) | 62 | (15.01%) | |
| ≥5 years | 172 | (4.45%) | 8 | (1.94%) | |
| Breast composition BI-RADS | |||||
| A | 23 | (0.60%) | 0 | (0.00%) | 0.182 |
| B | 138 | (3.57%) | 9 | (2.18%) | |
| C | 3324 | (86.00%) | 365 | (88.38%) | |
| D | 380 | (9.83%) | 39 | (9.44%) | |
| Univariate | Multivariate | ||||||
|---|---|---|---|---|---|---|---|
| Num | OR | 95%CI | p Value | OR | 95%CI | p Value | |
| Metabolic syndrome | |||||||
| No | 318 | ref. | |||||
| Yes | 95 | 1.01 | (0.79–1.29) | 0.929 | |||
| Menopause status | |||||||
| Post | 302 | ref. | |||||
| Pre | 111 | 1.36 | (1.08–1.71) | 0.009 ** | 1.34 | (1.06–1.69) | 0.013 * |
| Abortion | |||||||
| ≥2 | 73 | ref. | |||||
| 0–1 | 340 | 1.33 | (1.02–1.73) | 0.034 * | |||
| History of breast surgery | |||||||
| No | 378 | ref. | |||||
| Yes | 35 | 1.61 | (1.11–2.34) | 0.012 * | 1.64 | (1.13–2.39) | 0.010 * |
| Use of hormonal contraceptives | |||||||
| No | 343 | ref. | ref. | ||||
| <5 years | 62 | 0.93 | (0.70–1.24) | 0.629 | 0.95 | (0.71–1.26) | 0.719 |
| ≥5 years | 8 | 0.42 | (0.20–0.86) | 0.018 * | 0.42 | (0.21–0.87) | 0.019 * |
| Breast composition BI–RADS | |||||||
| A | 0 | 0.00 | (0.00–0.00) | 0.998 | |||
| B | 9 | 0.64 | (0.30–1.35) | 0.236 | |||
| C | 365 | 1.07 | (0.76–1.51) | 0.703 | |||
| D | 39 | ref. | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.-L.; Hsu, P.-S.; Lin, C.-Y.; Yang, S.-F. Clinical Factors Associated with Asymptomatic Women Having Inconclusive Screening Mammography Results: Experiences from a Single Medical Center in Taiwan. Int. J. Environ. Res. Public Health 2021, 18, 5410. https://doi.org/10.3390/ijerph18105410
Wang C-L, Hsu P-S, Lin C-Y, Yang S-F. Clinical Factors Associated with Asymptomatic Women Having Inconclusive Screening Mammography Results: Experiences from a Single Medical Center in Taiwan. International Journal of Environmental Research and Public Health. 2021; 18(10):5410. https://doi.org/10.3390/ijerph18105410
Chicago/Turabian StyleWang, Chun-Li, Pi-Shan Hsu, Chia-Yen Lin, and Shun-Fa Yang. 2021. "Clinical Factors Associated with Asymptomatic Women Having Inconclusive Screening Mammography Results: Experiences from a Single Medical Center in Taiwan" International Journal of Environmental Research and Public Health 18, no. 10: 5410. https://doi.org/10.3390/ijerph18105410
APA StyleWang, C.-L., Hsu, P.-S., Lin, C.-Y., & Yang, S.-F. (2021). Clinical Factors Associated with Asymptomatic Women Having Inconclusive Screening Mammography Results: Experiences from a Single Medical Center in Taiwan. International Journal of Environmental Research and Public Health, 18(10), 5410. https://doi.org/10.3390/ijerph18105410







