Baseline Optical Coherence Tomography Parameters That May Influence 6 Months Treatment Outcome of Polypoidal Choroidal Vasculopathy Eyes with Combination Therapy: A Short-Term Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Treatment
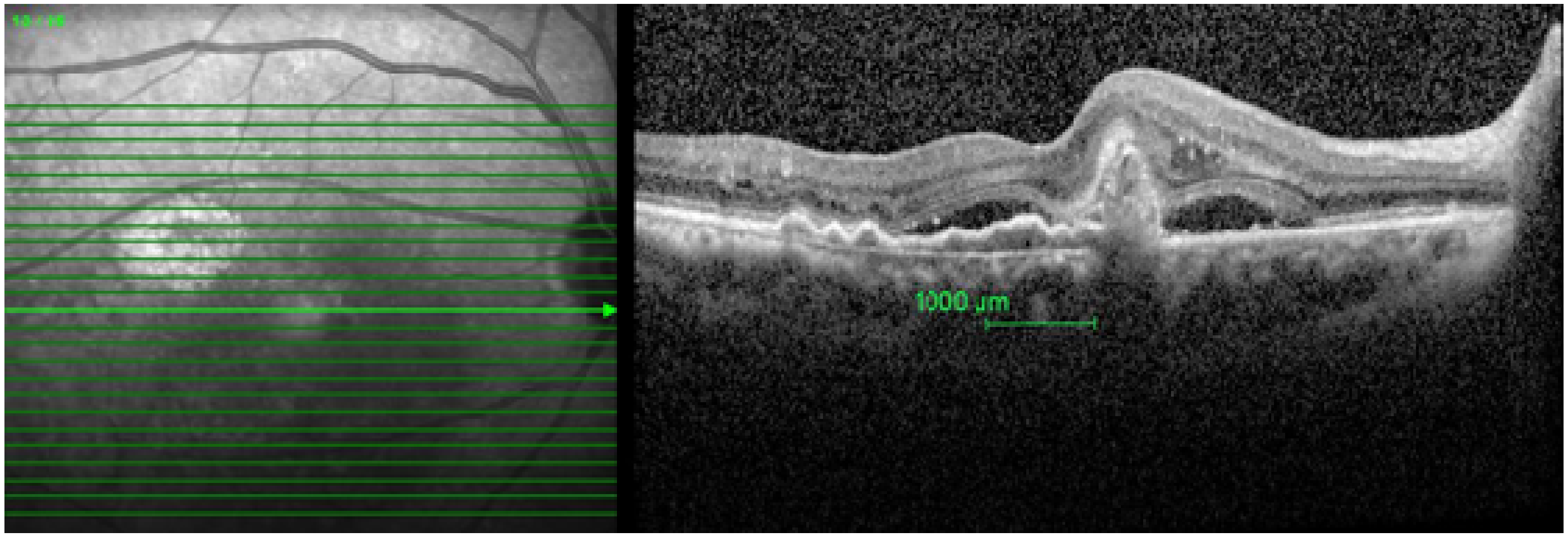
2.2. Image Analysis
- 1.
- Integrity of photoreceptors inner segment and outer segment (IS-OS) junction, external limiting membrane (ELM), and retinal pigment epithelium-Bruch’s membrane (RPE-BM) complex were graded as intact or disrupted. When a 75 percent or more part of ELM, IS-OS junction, and RPE-BM complex 1 mm center of the fovea was present, it was graded as intact. When less than 75 percent of ELM, IS-OS junction, and RPE-BM complex was present, it was graded as disrupted.
- 2.
- Presence of the following pathologies was recorded 1000 microns (1 mm) within the fovea:
- Retinal pigment epithelium detachment (PED)—It is the separation of retinal pigment epithelium (RPE) from the RPE-BM complex.
- Sub-retinal fluid (SRF)—It is seen from the outer border of the RPE to the outer segment of the photoreceptor.
- Polyp—It is the hypo reflective lumen attached to the posterior surface of PED.
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wojtkowski, M. High-speed optical coherence tomography: Basics and applications. Appl. Opt. 2010, 49, D30–D61. [Google Scholar] [CrossRef] [PubMed]
- Keane, P.A.; Sadda, S.R. Predicting visual outcomes for macular disease using optical coherence tomography. Saudi J. Ophthalmol. 2011, 25, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Sharanjeet-Kaur, S.; Ghoshal, R.; Fadzil, N.; Ghosh, S.; Aziz, R.A.B.A.; Ngah, N.F.; Mutalib, H.A. Visual functions and retinal morphology in patients with polypoidal choroidal vasculopathy seen in an age-related macular degeneration referral centre of Malaysia. Malays. J. Public Health Med. 2018, 1, 124–134. [Google Scholar]
- Ghoshal, R.; Sharanjeet-Kaur, S.; Fadzil, N.; Mutalib, H.A.; Ghosh, S.; Ngah, N.F.; Aziz, R.A.B.A. Correlation between Visual Functions and Retinal Morphology in Eyes with Early and Intermediate Age-Related Macular Degeneration. Int. J. Environ. Res. Public Health 2020, 17, 6379. [Google Scholar] [CrossRef]
- Ghoshal, R.; Sharanjeet-Kaur, S.; Fadzil, N.; Ghosh, S.; Ngah, N.F.; Aziz, R.A.B.A. Quality of life in patients with neovascularage related macular degeneration (n-AMD) seen in a public hospital of Malaysia. Sains Malays. 2018, 47, 2447–2454. [Google Scholar] [CrossRef]
- Nazima, S.A.; Hanisah, A.H.; Rona, A.N.; Wong, H.S.; Amin, A.; Bastion, M.L.C.; Mushawiahti, M.; Hazlita, M.I. Patterns of Polypoidal Choroidal Vasculopathy among a multiracial Population in a Malaysia Hospital. Med. Health 2016, 11, 245–256. [Google Scholar]
- Ghoshal, R.; Sharanjeet-Kaur, S.; Fadzil, N.; Ghosh, S.; Ngah, N.F.; Aziz, R.A.B.A. Visual Parameters and Retinal Morphology for Polypoidal Choroidal Vasculopathy Pre- and Post-Intravitreal Ranibizumab with or without Photodynamic Therapy: A Short-Term Prospective Study. Int. J. Environ. Res. Public Health 2021, 18, 2581. [Google Scholar] [CrossRef]
- Ghoshal, R.; Sharanjeet-Kaur, S.; Fadzil, N.M.; Ghosh, S.; Ngah, N.F.; Aziz, R.A.A. Patient-Perceived Benefit of Treatment in Polypoidal Choroidal Vasculopathy: A Pilot Study. Int. J. Environ. Res. Public Health 2020, 17, 6378. [Google Scholar] [CrossRef]
- Mori, R.; Yuzawa, M.; Lee, Z.; Haruyama, M.; Akaza, E. Factors influencing visual outcome of polypoidal choroidal vasculopathy 1 year after photodynamic therapy. Graefe’s Arch. Clin. Exp. Ophthalmol. 2010, 248, 1233–1239. [Google Scholar] [CrossRef]
- Kikushima, W.; Sakurada, Y.; Sugiyama, A.; Yoneyama, S.; Tanabe, N.; Matsubara, M.; Mabuchi, F.; Iijima, H. Comparison of two-year outcomes after photodynamic therapy with ranibizumab or aflibercept for polypoidal choroidal vasculopathy. Sci. Rep. 2017, 7, 16461. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yao, J.; Wang, X.H.; Zhao, L.; Wang, L.J.; Wang, J.M.; Zhou, A.Y. Sensitivity and specificity of optical coherence tomography in diagnosing polypoidal choroidal vasculopathy. Nan Fang Yi Ke Da Xue Xue Bao 2016, 37, 165–171. [Google Scholar]
- Koh, A.; Lai, T.Y.Y.; Takahashi, K.; Wong, T.Y.; Chen, L.J.; Ruamviboonsuk, P.; Tan, C.S.; Feller, C.; Margaron, P.; Lim, T.H.; et al. EVEREST II study group. Efficacy and Safety of Ranibizumab with or Without Verteporfin Photodynamic Therapy for Polypoidal Choroidal Vasculopathy: A Randomized Clinical Trial. JAMA Ophthalmol. 2017, 135, 1206–1213. [Google Scholar]
- Buari, N.H.; Chen, A.H.; Musa, N. Comparison of reading speed with3 different log-scaled reading charts. J. Optom. 2014, 7, 210–216. [Google Scholar] [CrossRef]
- Ho, M.; Lo, E.C.F.; Young, A.L.; Liu, D.T.L. Outcome of polypoidal choroidal vasculopathy at 1 year by combined therapy of photodynamic therapy with ranibizumab and predictive factors governing the outcome. Eye 2014, 28, 1469–1476. [Google Scholar] [CrossRef] [PubMed]
- Waldstein, S.M.; Wright, J.; Warburton, J.; Margaron, P.; Simader, C.; Schmidt-Erfurth, U. Predictive Value of Retinal Morphology forVisual Acuity Outcomes of Different Ranibizumab Treatment Regimens for Neovascular AMD. Ophthalmology 2016, 123, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Grossniklaus, H.E.; Green, W.R. Choroidal neovascularization. Am. J. Ophthalmol. 2004, 137, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.; Souka, A.; Adelman, R.A. Age-related macular degeneration: Using morphological predictors to modify current treatment protocols. Acta Ophthalmol. 2018, 96, 120–133. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Erfurth, U.; Waldstein, S.M.; Deak, G.G.; Kundi, M.; Simader, C. Pigment epithelial detachment followed by retinal cystoid degeneration leads to vision loss in treatment of neovascular age-related macular degeneration. Ophthalmology 2015, 122, 822–832. [Google Scholar] [CrossRef] [PubMed]
- Cheung, C.M.G.; Lai, T.Y.Y.; Ruamviboonsuk, P.; Chen, S.J.; Chen, Y.; Freund, K.B.; Gomi, F.; Koh, A.H.; Lee, W.K.; Wong, T.Y. Polypoidal Choroidal Vasculopathy: Definition, Pathogenesis, Diagnosis, and Management. Ophthalmology 2018, 125, 708–724. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, M.; Yuzawa, M.; Shimada, H.; Mori, R. Correlation between indocyanine green angiographic findings and histopathology of polypoidal choroidal vasculopathy. Jpn. J. Ophthalmol. 2004, 48, 249–255. [Google Scholar] [CrossRef]
- Kuroiwa, S.; Tateiwa, H.; Hisatomi, T.; Ishibashi, T.; Yoshimura, N. Pathological features of surgically excised polypoidal choroidal vasculopathy membranes. Clin. Exp. Ophthalmol. 2004, 32, 297–302. [Google Scholar] [CrossRef]
- Nakashizuka, H.; Mitsumata, M.; Okisaka, S.; Shimada, H.; Kawamura, A.; Mori, R.; Yuzawa, M. Clinicopathologic findings in polypoidal choroidal vasculopathy. Investig. Ophthalmol. Vis. Sci. 2008, 49, 4729–4737. [Google Scholar] [CrossRef] [PubMed]
- Keane, P.A.; Patel, P.J.; Ouyang, Y.; Chen, F.K.; Ikeji, F.; Walsh, A.C.; Tufail, A.; Sadda, S.R. Effects of retinal morphology on contrast sensitivity and reading ability in neovascular age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2010, 51, 5431–5437. [Google Scholar] [CrossRef] [PubMed]
- Spaide, R.F.; Curcio, C.A. Anatomical correlates to the bands seen in the outer retina by optical coherence tomography: Literature review and model. Retina 2011, 31, 1609–1619. [Google Scholar] [CrossRef] [PubMed]
- Newman, E.; Reichenbach, A. The Müller cell: A functional element of the retina. Trends Neurosci. 1996, 19, 307–312. [Google Scholar] [CrossRef]
- Oishi, A.; Hata, M.; Shimozono, M.; Mandai, M.; Nishida, A.; Kurimoto, Y. The Significance of External Limiting Membrane Status for Visual Acuity in Age-Related Macular Degeneration. Am. J. Ophthalmol. 2010, 150, 27–32. [Google Scholar] [CrossRef]
- Wakabayashi, T.; Fujiwara, M.; Sakaguchi, H.; Kusaka, S.; Oshima, Y. Foveal microstructure and visual acuity in surgically closed macular holes: Spectral-domain optical coherence tomographic analysis. Ophthalmology 2010, 117, 1815–1824. [Google Scholar] [CrossRef] [PubMed]
- Landa, G.; Gentile, R.C.; Garcia, P.M.; Muldoon, T.O.; Rosen, R.B. External limiting membrane and visual outcome in macular hole repair: Spectral domain OCT analysis. Eye 2012, 26, 61–69. [Google Scholar] [CrossRef]
- Marco, A.B.F.; Witkin, A.J. Outer Retinal Layers as Predictors of Vision Loss. Rev. Ophthalmol. 2015, XXII, 78–83. [Google Scholar]
- Wakabayashi, T.; Oshima, Y.; Fujimoto, H.; Murakami, Y.; Sakaguchi, H.; Kusaka, S.; Tano, Y. Foveal microstructure and visual acuity after retinal detachment repair: Imaging analysis by Fourier-domain optical coherence tomography. Ophthalmology 2009, 116, 519–528. [Google Scholar] [CrossRef]
- Chhablani, J.; Kim, J.S.; Freeman, W.R.; Kozak, I.; Wang, H.Y.; Cheng, L. Predictors of visual outcome in eyes with choroidal neovascularization secondary to age related macular degeneration treated with intravitreal bevacizumab monotherapy. Int. J. Ophthalmol. 2013, 6, 62–66. [Google Scholar] [PubMed]
- Roberts, P.; Mittermueller, T.J.; Montuoro, A.; Sulzbacher, F.; Munk, M.; Sacu, S.; Schmidt-Erfurth, U. A quantitative approach to identify morphological features relevant for visual function in ranibizumab therapy of neovascular AMD. Investig. Ophthalmol. Vis. Sci. 2015, 55, 6623–6630. [Google Scholar] [CrossRef] [PubMed]
- Saxena, S.; Srivastav, K.; Cheung, C.M.; Ng, J.Y.W.; Lai, T.T.Y. Photoreceptor inner segment ellipsoid band integrity on spectral domain optical coherence tomography. Clin. Ophthalmol. 2014, 8, 2507–2522. [Google Scholar] [PubMed]
- Oishi, A.; Shimozono, M.; Mandai, M.; Nishida, A.; Kurimoto, Y. Recovery of photoreceptor outer segments after anti-VEGF therapy for age-related macular degeneration. Graefe’s Arch. Clin. Exp. Ophthalmol. 2013, 251, 435–440. [Google Scholar] [CrossRef]
| Variable | Mean Distance Visual Acuity (logMAR) | p | Mean Near Visual Acuity (logMAR) | p | Mean Contrast Sensitivity (log Contrast Sensitivity) | p |
|---|---|---|---|---|---|---|
| Foveal Sub -Retinal Fluid | ||||||
| Present (n = 20) | 0.50 ± 0.07 | 0.043 | 0.46 ± 0.26 | 0.202 | 0.98 ± 0.29 | 0.040 |
| Absent (n = 6) | 0.25 ± 0.27 | 0.30 ± 0.28 | 1.19 ± 0.21 | |||
| Foveal Polyp | ||||||
| Present (n = 8) | 0.64 ± 0.39 | 0.011 | 0.58 ± 0.30 | 0.032 | 1.26 ± 0.19 | 0.005 |
| Absent (n = 18) | 0.36 ± 0.14 | 0.35 ± 0.22 | 0.99 ± 0.24 | |||
| Foveal Pigment Epithelium Detachment | ||||||
| Present (n = 19) | 0.46 ± 0.30 | 0.662 | 0.40 ± 0.28 | 0.477 | 1.12 ± 0.20 | 0.898 |
| Absent (n = 7) | 0.41 ± 0.15 | 0.48 ± 0.22 | 1.13 ± 0.32 | |||
| External Limiting Membrane | ||||||
| Intact (n = 13) | 0.34 ± 0.14 | 0.32 ± 0.14 | 0.053 | 1.25 ± 0.20 | 0.005 | |
| Disrupt (n = 13) | 0.54 ± 0.34 | 0.044 | 0.52 ± 0.34 | 0.99 ± 0.24 | ||
| Photoreceptors Inner segment and outer segment Junction | ||||||
| Intact (n = 13) | 0.34 ± 0.13 | 0.040 | 0.31 ± 0.19 | 0.024 | 1.23 ± 0.20 | 0.023 |
| Disrupt (n = 13) | 0.55 ± 0.32 | 0.53 ± 0.28 | 1.01 ± 0.26 | |||
| Retinal Pigment Epithelium-Bruch membrane complex | ||||||
| Intact (n = 8) | 0.48 ± 0.12 | 0.662 | 0.55 ± 0.27 | 0.106 | 1.12 ± 0.34 | 0.662 |
| Disrupt (n = 18) | 0.42 ± 0.15 | 0.37 ± 0.24 | 1.24 ± 0.25 | |||
| External Limiting Membrane + Photoreceptors Inner Segment and Outer Segment Junction | ||||||
| Both intact | 0.32 ± 0.13 | 0.086 | 0.27 ± 0.19 | 0.040 | 1.27 ± 0.19 | 0.029 |
| Either intact | 0.47 ± 0.90 | 0.55 ± 0.17 | 1.12 ± 0.15 | |||
| Both disrupted | 0.57 ± 0.35 | 0.52 ± 0.29 | 0.98 ± 0.26 |
| Variable | Average Retinal Thickness | Average Retinal Volume | Central Thickness | Maximum Thickness of Central 1 mm | Minimum Thickness of Central 1 mm | Baseline Distance Visual Acuity/Baseline Near Visual Acuity/Baseline Contrast Sensitivity/Baseline Reading Speed |
|---|---|---|---|---|---|---|
| 6 months distance visual acuity | r = 0.219 CI (0 .63 to −0.19) p = 0.282 | r = 0.213 CI (0.64 to −0.19) p = 0.295 | r = 0.339 CI (0.74 to −0.05) p = 0.90 | r = 0.215 CI (0.62 to −0.19) p = 0.292 | r = 0.040 CI (0.46 to −0.38) p = 0.848 | r = 0.53 CI (0.88 to 0.17) p = 0.006 |
| 6 months near visual acuity | r = 0.031 CI (0.45 to −0.39) p = 0.880 | r = −0.247 CI (0.16 to −0.65) p = 0.223 | r = 0.145 CI (0.56 to −0.27) p = 0.480 | r = 0.086 CI (0.56 to −0.34) p = 0.676 | r = −0.133 CI (0.28 to −0.55) p = 0.517 | r = 0.40 CI (0.78 to 0.01) p = 0.042 |
| 6 months contrast sensitivity | r = −0.131 CI (0.28 to −0.55) p = 0.523 | r = 0.302 CI (0.70 to −0.10) p = 0.134 | r = −0.206 CI (0.20 to −0.61) p = 0.312 | r = 0.004 CI (0.42 to −0.41) p = 0.984 | r = −0.002 CI (0.42 to −0.42) p = 0.994 | r = 0.41 CI (0.79 to 0.02) p = 0.037 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghoshal, R.; Sharanjeet-Kaur, S.; Fadzil, N.M.; Ghosh, S.; Ngah, N.; Aziz, R.A.A. Baseline Optical Coherence Tomography Parameters That May Influence 6 Months Treatment Outcome of Polypoidal Choroidal Vasculopathy Eyes with Combination Therapy: A Short-Term Pilot Study. Int. J. Environ. Res. Public Health 2021, 18, 5378. https://doi.org/10.3390/ijerph18105378
Ghoshal R, Sharanjeet-Kaur S, Fadzil NM, Ghosh S, Ngah N, Aziz RAA. Baseline Optical Coherence Tomography Parameters That May Influence 6 Months Treatment Outcome of Polypoidal Choroidal Vasculopathy Eyes with Combination Therapy: A Short-Term Pilot Study. International Journal of Environmental Research and Public Health. 2021; 18(10):5378. https://doi.org/10.3390/ijerph18105378
Chicago/Turabian StyleGhoshal, Rituparna, Sharanjeet Sharanjeet-Kaur, Norliza Mohamad Fadzil, Somnath Ghosh, NorFariza Ngah, and Roslin Azni Abd Aziz. 2021. "Baseline Optical Coherence Tomography Parameters That May Influence 6 Months Treatment Outcome of Polypoidal Choroidal Vasculopathy Eyes with Combination Therapy: A Short-Term Pilot Study" International Journal of Environmental Research and Public Health 18, no. 10: 5378. https://doi.org/10.3390/ijerph18105378
APA StyleGhoshal, R., Sharanjeet-Kaur, S., Fadzil, N. M., Ghosh, S., Ngah, N., & Aziz, R. A. A. (2021). Baseline Optical Coherence Tomography Parameters That May Influence 6 Months Treatment Outcome of Polypoidal Choroidal Vasculopathy Eyes with Combination Therapy: A Short-Term Pilot Study. International Journal of Environmental Research and Public Health, 18(10), 5378. https://doi.org/10.3390/ijerph18105378






