The Acute Effect of Magnesium Supplementation on Endothelial Function: A Randomized Cross-Over Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Volunteers
2.2. Trial Protocol
2.3. Flow Mediated Dilatation (FMD)
2.4. Serum Magnesium
2.5. Statistics
3. Results
3.1. Dietary Intake
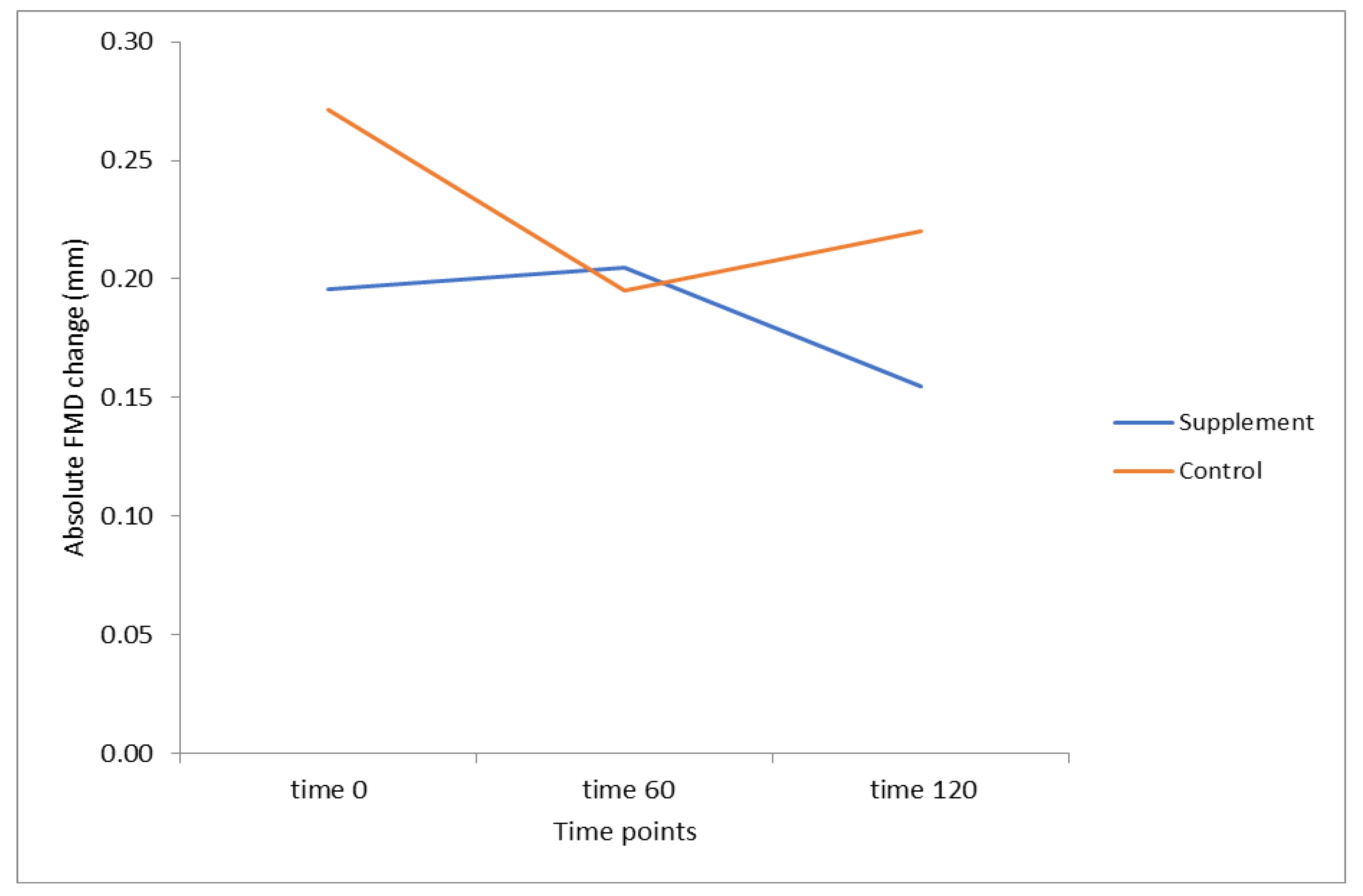
3.2. Flow-Mediated Dilatation
3.3. Blood Pressure and Mean Arterial Pressure
3.4. Serum Magnesium
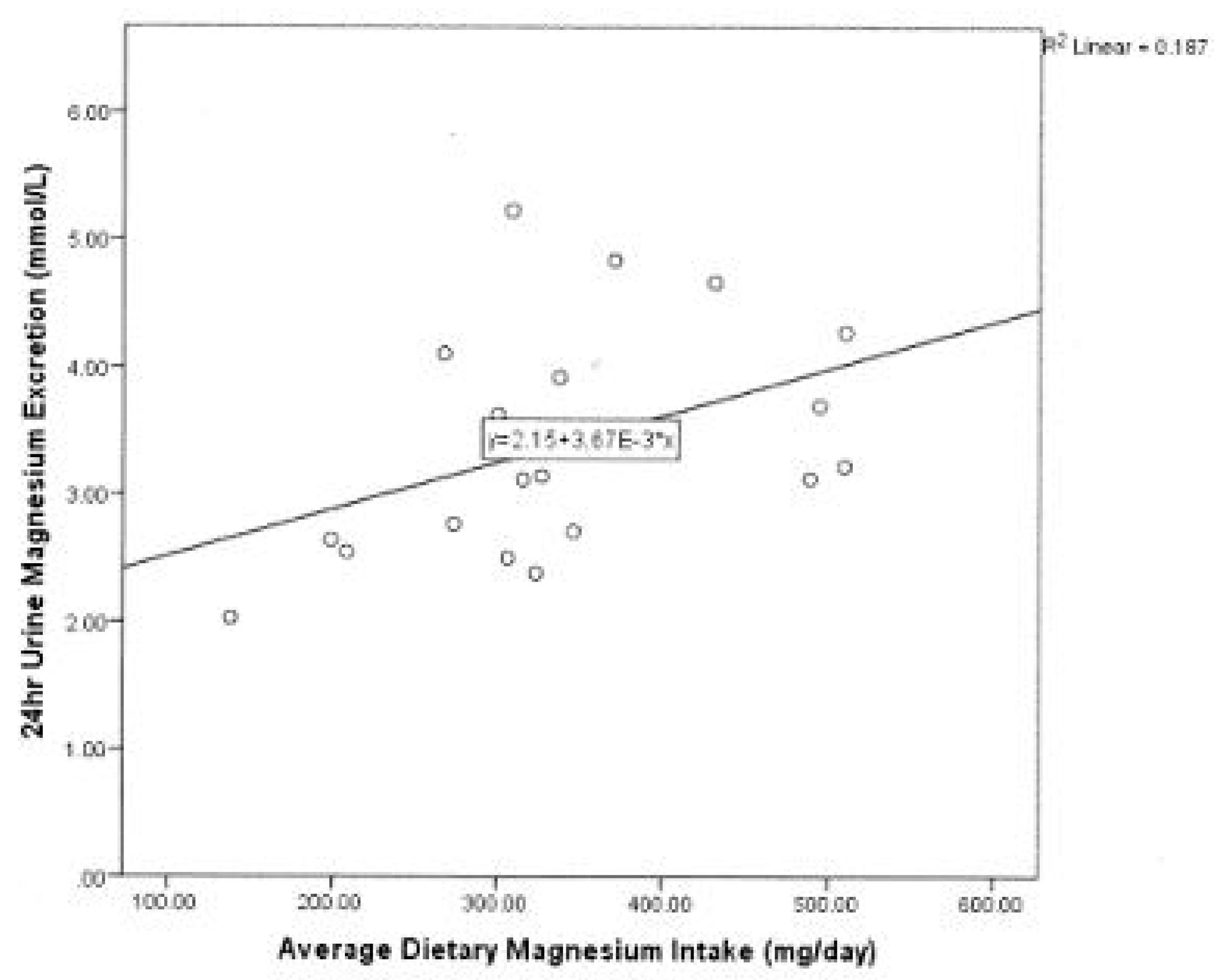
3.5. Correlations
3.6. Linear Regression Analysis
4. Discussion
4.1. Dietary Influences on FMD
4.2. Serum Magnesium
4.3. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Buttar, H.S.; Li, T.; Ravi, N. Prevention of cardiovascular diseases: Role of exercise, dietary interventions, obesity and smoking cessation. Exp. Clin. Cardiol. 2005, 10, 229–249. [Google Scholar] [PubMed]
- Qu, X.; Jin, F.; Hao, Y.; Li, H.; Tang, T.; Wang, H.; Yan, W.; Dai, K. Magnesium and the risk of cardiovascular events: A meta-analysis of prospective cohort studies. PLoS ONE 2013, 8, e57720. [Google Scholar] [CrossRef]
- Chiuve, S.E.; Korngold, E.C.; Januzzi, J.L., Jr.; Gantzer, M.L.; Albert, C.M. Plasma and dietary magnesium and risk of sudden cardiac death in women. Am. J. Clin. Nutr. 2011, 93, 253–260. [Google Scholar] [CrossRef]
- Chiuve, S.E.; Sun, Q.; Curhan, G.C.; Taylor, E.N.; Spiegelman, D.; Willett, W.C.; Manson, J.E.; Rexrode, K.M.; Albert, C.M. Dietary and plasma magnesium and risk of coronary heart disease among women. J. Am. Heart Assoc. 2013, 2, e000114. [Google Scholar] [CrossRef] [PubMed]
- Del Gobbo, L.C.; Imamura, F.; Wu, J.H.; de Oliveira Otto, M.C.; Chiuve, S.E.; Mozaffarian, D. Circulating and dietary magnesium and risk of cardiovascular disease: A systematic review and meta-analysis of prospective studies. Am. J. Clin. Nutr. 2013, 98, 160–173. [Google Scholar] [CrossRef]
- Rooney, M.R.; Alonso, A.; Folsom, A.R.; Michos, E.D.; Rebholz, C.M.; Misialek, J.R.; Chen, L.Y.; Dudley, S.; Lutsey, P.L. Serum magnesium and the incidence of coronary artery disease over a median 27 years of follow-up in the atherosclerosis risk in communities (aric) study and a meta-analysis. Am. J. Clin. Nutr. 2020, 111, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, A.; Naghshi, S.; Sadeghi, O.; Larijani, B.; Esmaillzadeh, A. Total, dietary, and supplemental magnesium intakes and risk of all-cause, cardiovascular, and cancer mortality: A systematic review and dose-response meta-analysis of prospective cohort studies. Adv. Nutr. 2021. Epub ahead of print. [Google Scholar] [CrossRef]
- Yusuf, S.; Hawken, S.; Ounpuu, S.; Dans, T.; Avezum, A.; Lanas, F.; McQueen, M.; Budaj, A.; Pais, P.; Varigos, J.; et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the interheart study): Case-control study. Lancet 2004, 364, 937–952. [Google Scholar] [CrossRef]
- Dickinson, K.M.; Clifton, P.M.; Burrell, L.M.; Barrett, P.H.R.; Keogh, J.B. Postprandial effects of a high salt meal on serum sodium, arterial stiffness, markers of nitric oxide production and markers of endothelial function. Atherosclerosis 2014, 232, 211–216. [Google Scholar] [CrossRef]
- Dickinson, K.M.; Clifton, P.M.; Keogh, J.B. Endothelial function is impaired after a high-salt meal in healthy subjects. Am. J. Clin. Nutr. 2011, 93, 500–505. [Google Scholar] [CrossRef]
- Dickinson, K.M.; Clifton, P.M.; Keogh, J.B. A reduction of 3 g/day from a usual 9 g/day salt diet improves endothelial function and decreases endothelin-1 in a randomised cross_over study in normotensive overweight and obese subjects. Atherosclerosis 2014, 233, 32–38. [Google Scholar] [CrossRef]
- Dickinson, K.M.; Keogh, J.B.; Clifton, P.M. Effects of a low-salt diet on flow-mediated dilatation in humans. Am. J. Clin. Nutr. 2009, 89, 485–490. [Google Scholar] [CrossRef]
- Blanch, N.; Clifton, P.M.; Keogh, J.B. A systematic review of vascular and endothelial function: Effects of fruit, vegetable and potassium intake. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 253–266. [Google Scholar] [CrossRef]
- Blanch, N.; Clifton, P.M.; Petersen, K.S.; Keogh, J.B. Effect of sodium and potassium supplementation on vascular and endothelial function: A randomized controlled trial. Am. J. Clin. Nutr. 2015, 101, 939–946. [Google Scholar] [CrossRef]
- Blanch, N.; Clifton, P.M.; Petersen, K.S.; Willoughby, S.R.; Keogh, J.B. Effect of high potassium diet on endothelial function. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 983–989. [Google Scholar] [CrossRef]
- Australian National Health and Medical Research Council. Heart, Stroke & Vascular Diseases; Australian Institute of Health and Welfare: Canberra, NSW, Australia, 2019.
- Versari, D.; Daghini, E.; Virdis, A.; Ghiadoni, L.; Taddei, S. Endothelial dysfunction as a target for prevention of cardiovascular disease. Diabetes Care 2009, 32, S314–S321. [Google Scholar] [CrossRef] [PubMed]
- Ellins, E.A.; Halcox, J.P. Where are we heading with noninvasive clinical vascular physiology? Why and how should we assess endothelial function? Cardiol. Res. Pract. 2011, 2011, 870132. [Google Scholar] [CrossRef] [PubMed]
- Celermajer, D.S.; Sorensen, K.E.; Gooch, V.M.; Spiegelhalter, D.J.; Miller, O.I.; Sullivan, I.D.; Lloyd, J.K.; Deanfield, J.E. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet 1992, 340, 1111–1115. [Google Scholar] [CrossRef]
- Chacko, S.A.; Song, Y.; Nathan, L.; Tinker, L.; de Boer, I.H.; Tylavsky, F.; Wallace, R.; Liu, S. Relations of dietary magnesium intake to biomarkers of inflammation and endothelial dysfunction in an ethnically diverse cohort of postmenopausal women. Diabetes Care 2010, 33, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Li, T.Y.; van Dam, R.M.; Manson, J.E.; Hu, F.B. Magnesium intake and plasma concentrations of markers of systemic inflammation and endothelial dysfunction in women. Am. J. Clin. Nutr. 2007, 85, 1068–1074. [Google Scholar] [CrossRef]
- Joosten, M.M.; Gansevoort, R.T.; Mukamal, K.J.; van der Harst, P.; Geleijnse, J.M.; Feskens, E.J.; Navis, G.; Bakker, S.J.; Group, P.S. Urinary and plasma magnesium and risk of ischemic heart disease. Am. J. Clin. Nutr. 2013, 97, 1299–1306. [Google Scholar] [CrossRef] [PubMed]
- Joris, P.J.; Plat, J.; Bakker, S.J.L.; Mensink, R.P. Effects of long-term magnesium supplementation on endothelial function and cardiometabolic risk markers: A randomized controlled trial in overweight/obese adults. Sci. Rep. 2017, 7, 106. [Google Scholar]
- Marques, B.; Klein, M.; da Cunha, M.R.; de Souza Mattos, S.; de Paula Nogueira, L.; de Paula, T.; Correa, F.M.; Oigman, W.; Neves, M.F. Effects of oral magnesium supplementation on vascular function: A systematic review and meta-analysis of randomized controlled trials. High Blood Press Cardiovasc. Prev. 2020, 27, 19–28. [Google Scholar]
- Liu, M.; Dudley, S.C., Jr. Magnesium, oxidative stress, inflammation, and cardiovascular disease. Antioxidants 2020, 9, 907. [Google Scholar] [CrossRef]
- NHMRC. Nutrient Reference Values for Australia and New Zealand: Including Recommended Dietary Intakes; Ministry of Health, Department of Health and Ageing: Canberra, NSW, Australia, 2006.
- Rosanoff, A. Perspective: Us adult magnesium requirements need updating: Impacts of rising body weights and data-derived variance. Adv. Nutr. 2021, 12, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Sakai, T.; Sato, B.; Hara, K.; Hara, Y.; Naritomi, Y.; Koyanagi, S.; Hara, H.; Nagao, T.; Ishibashi, T. Consumption of water containing over 3.5 mg of dissolved hydrogen could improve vascular endothelial function. Vasc. Health Risk Manag. 2014, 10, 591–597. [Google Scholar]
- Akbari, C.M.; Saouaf, R.; Barnhill, D.F.; Newman, P.A.; LoGerfo, F.W.; Veves, A. Endothelium-dependent vasodilatation is impaired in both microcirculation and macrocirculation during acute hyperglycemia. J. Vascular. Surg. 1998, 28, 687–694. [Google Scholar] [CrossRef]
- Kawano, H.; Motoyama, T.; Hirashima, O.; Hirai, N.; Miyao, Y.; Sakamoto, T.; Kugiyama, K.; Ogawa, H.; Yasue, H. Hyperglycemia rapidly suppresses flow-mediated endothelium- dependent vasodilation of brachial artery. J. Am. College Cardiol. 1999, 34, 146–154. [Google Scholar] [CrossRef]
- Title, L.M.; Cummings, P.M.; Giddens, K.; Nassar, B.A. Oral glucose loading acutely attenuates endothelium-dependent vasodilation in healthy adults without diabetes: An effect prevented by vitamins c and e. J. Am. College Cardiol. 2000, 36, 2185–2191. [Google Scholar] [CrossRef]
- Beckman, J.A.; Goldfine, A.B.; Gordon, M.B.; Creager, M.A. Ascorbate restores endothelium-dependent vasodilation impaired by acute hyperglycemia in humans. Circulation 2001, 103, 1618. [Google Scholar] [CrossRef]
- Hatzistavri, L.S.; Sarafidis, P.A.; Georgianos, P.I.; Tziolas, I.M.; Aroditis, C.P.; Zebekakis, P.E.; Pikilidou, M.I.; Lasaridis, A.N. Oral magnesium supplementation reduces ambulatory blood pressure in patients with mild hypertension. Am. J. Hypertensi. 2009, 22, 1070–1075. [Google Scholar] [CrossRef] [PubMed]
- Cosaro, E.; Bonafini, S.; Montagnana, M.; Danese, E.; Trettene, M.S.; Minuz, P.; Delva, P.; Fava, C. Effects of magnesium supplements on blood pressure, endothelial function and metabolic parameters in healthy young men with a family history of metabolic syndrome. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 1213–1220. [Google Scholar] [CrossRef] [PubMed]
- Mortazavi, M.; Moeinzadeh, F.; Saadatnia, M.; Shahidi, S.; McGee, J.C.; Minagar, A. Effect of magnesium supplementation on carotid intima-media thickness and flow-mediated dilatation among hemodialysis patients: A double-blind, randomized, placebo-controlled trial. Eur. Neurol. 2013, 69, 309–316. [Google Scholar] [CrossRef] [PubMed]
| Range | Mean/Median | SD/IQR | |
|---|---|---|---|
| Age (years) | 19–75 | 39 | 16 |
| Weight (kg) | 45.8–100.9 | 59.2 (median) | 14.2 (IQR) |
| Height (cm) | 157–180 | 166.6 | 6.5 |
| BMI (kg/m2) | 17.9–31.9 | 21.1 (median) | 5.6 (IQR) |
| SBP (mmHg) | 92–134 | 113.5 | 13.8 |
| DBP (mmHg) | 65–90 | 76.2 | 7.2 |
| MAP (mmHg) | 75–101.7 | 88.5 | 8.7 |
| 24 h urine Mg excretion (mmol/L) | 2.03–5.23 | 3.39 | 0.86 |
| 24 h urine Cr excretion (mmol/L) | 3.64–20.0 | 8.82 (median) | 3.19 (IQR) |
| Timepoint 0 brachial artery diameter (mm) | 3.01–5.20 | 3.8 | 0.56 |
| Baseline serum mg (mmol) | 0.75–0.94 | 0.85 | 0.047 |
| Nutrient | Range | Mean | SD |
|---|---|---|---|
| Mg Intake (n = 19) mg/day | 138–510 | 339 | 106.7 |
| Male Mg Intake (n = 4) | 199–429 | 328 | 51.0 |
| Female Mg Intake (n = 15) | 138–510 | 343 | 97.7 |
| Saturated Fat Intake (n = 19) g/day | 6.7–57.9 | 27.7 | 13.7 |
| Male Saturated Fat Intake (n = 4) | 15.3–57.9 | 33.5 | 17.8 |
| Female Saturated Fat Intake (n = 15) | 6.7–46.4 | 26.1 | 12.7 |
| PUFA Intake (n = 19) g/day | 1.5–24.9 | 12.5 | 6.2 |
| Male PUFA Intake (n = 4) | 5.8–17.7 | 10.1 | 5.2 |
| Female PUFA Intake (n = 15) | 1.5–24.9 | 13.2 | 6.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murphy, C.; Byrne, J.; Keogh, J.B.; Headland, M.L.; Clifton, P.M. The Acute Effect of Magnesium Supplementation on Endothelial Function: A Randomized Cross-Over Pilot Study. Int. J. Environ. Res. Public Health 2021, 18, 5303. https://doi.org/10.3390/ijerph18105303
Murphy C, Byrne J, Keogh JB, Headland ML, Clifton PM. The Acute Effect of Magnesium Supplementation on Endothelial Function: A Randomized Cross-Over Pilot Study. International Journal of Environmental Research and Public Health. 2021; 18(10):5303. https://doi.org/10.3390/ijerph18105303
Chicago/Turabian StyleMurphy, Caitríona, Jennifer Byrne, Jennifer B. Keogh, Michelle L. Headland, and Peter M. Clifton. 2021. "The Acute Effect of Magnesium Supplementation on Endothelial Function: A Randomized Cross-Over Pilot Study" International Journal of Environmental Research and Public Health 18, no. 10: 5303. https://doi.org/10.3390/ijerph18105303
APA StyleMurphy, C., Byrne, J., Keogh, J. B., Headland, M. L., & Clifton, P. M. (2021). The Acute Effect of Magnesium Supplementation on Endothelial Function: A Randomized Cross-Over Pilot Study. International Journal of Environmental Research and Public Health, 18(10), 5303. https://doi.org/10.3390/ijerph18105303








