Factors Associated with SARS-CoV-2 Infection in Physician Trainees in New York City during the First COVID-19 Wave
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Setting and Design
2.2. Participant Enrollment
2.3. Institutional Process for Employee COVID-19 Testing
2.4. Assessment of SARS-CoV-2 Infection
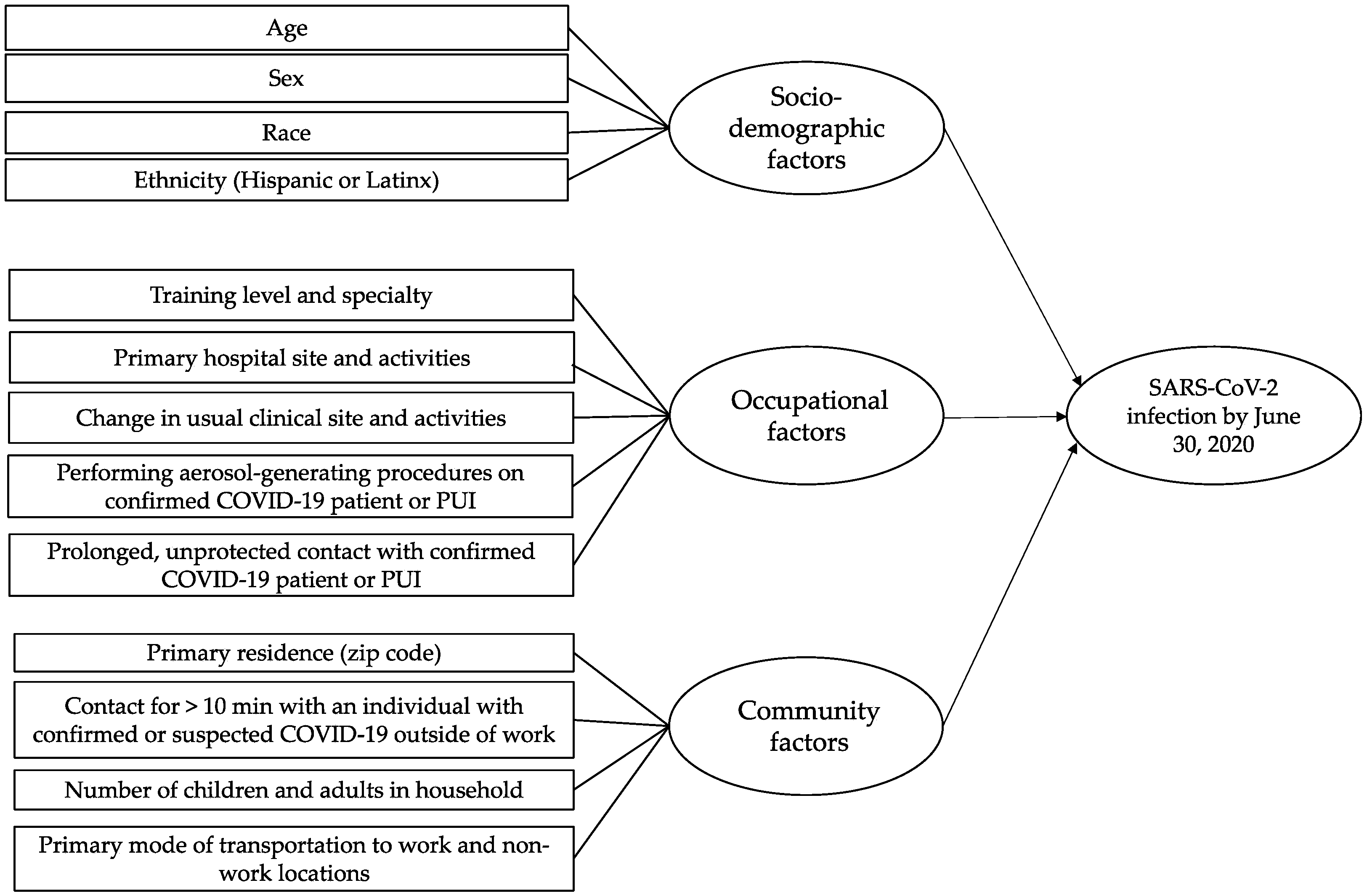
2.5. Assessment of Potential Risk Factors for SARS-CoV-2 Infection
2.6. Statistical Analysis
3. Results
3.1. Survey Response
3.2. Participant Characteristics
3.3. SARS-CoV-2 Infection
3.4. Sociodemographic Factors and SARS-CoV-2 Infection
3.5. Occupational Factors and SARS-CoV-2 Infection
3.6. Community Factors and SARS-CoV-2 Infection
3.7. Structural Equational Model
3.8. Sensitivity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Centers for Disease Control and Prevention. Compare Trends in COVID-19 Cases and Deaths in the US. 2020. Available online: https://covid.cdc.gov/covid-data-tracker/#compare-trends_totalcases (accessed on 17 October 2020).
- Centers for Disease Control and Prevention. Trends in Number of COVID-19 Cases and Deaths in the US Reported to CDC, by State/Territory. 2020. Available online: https://covid.cdc.gov/covid-data-tracker/#trends_dailytrendscases (accessed on 23 November 2020).
- Stadlbauer, D.; Tan, J.; Jiang, K.; Hernandez, M.M.; Fabre, S.; Amanat, F.; Teo, C.; Arunkumar, G.; McMahon, M.; Capuano, C.; et al. Repeated cross-sectional sero-monitoring of SARS-CoV-2 in New York City. Nature 2020, 590, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Interim Infection Prevention and Control Recommendations for Healthcare Personnel during the Coronavirus Disease 2019 (COVID-19) Pandemic. 2020. Available online: https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control-recommendations.html (accessed on 17 October 2020).
- Chou, R.; Dana, T.; Buckley, D.I.; Selph, S.; Fu, R.; Totten, A.M. Epidemiology of and Risk Factors for Coronavirus Infection in Health Care Workers: A Living Rapid Review. Ann. Intern. Med. 2020, 173, 120–136. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y. Clinical Characteristics of 138 Hospitalized Patients with 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069. [Google Scholar] [CrossRef]
- Chou, R.; Dana, T.; Buckley, D.I.; Selph, S.; Fu, R.; Totten, A.M. Update Alert 6: Epidemiology of and Risk Factors for Coronavirus Infection in Health Care Workers. Ann. Intern. Med. 2021, 174, W18–W19. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.M.; Nelson, K.N.; Overton, E.; Lopman, B.A.; Lash, T.L.; Photakis, M.; Jacob, J.T.; Roback, J.; Fridkin, S.; Steinberg, J.P. Quantification of Occupational and Community Risk Factors for SARS-CoV-2 Seropositivity among Health Care Workers in a Large U.S. Health Care System. Ann. Intern. Med. 2021. [Google Scholar] [CrossRef]
- Staiger, D.O.; Auerbach, D.I.; Buerhaus, P.I. Trends in the work hours of physicians in the United States. JAMA 2010, 303, 747–753. [Google Scholar] [CrossRef] [Green Version]
- Restivo, V.; Costantino, C.; Mammina, C.; Vitale, F. Influenza like Illness among Medical Residents Anticipates Influenza Diffusion in General Population: Data from a National Survey among Italian Medical Residents. PLoS ONE 2016, 11, e0168546. [Google Scholar] [CrossRef] [Green Version]
- Breazzano, M.P.; Shen, J.; Abdelhakim, A.H.; Glass, L.R.D.; Horowitz, J.D.; Xie, S.X.; De Moraes, C.G.; Chen-Plotkin, A.; Chen, R.W.S.; on behalf of the New York City Residency Program Directors COVID-19 Research Group. New York City COVID-19 resident physician exposure during exponential phase of pandemic. J. Clin. Investig. 2020, 130, 4726–4733. [Google Scholar] [CrossRef]
- Nobel, T.B.; Marin, M.; Divino, C.M. Lessons in flexibility from a general surgery program at the epicenter of the pandemic in New York City. Surgery 2020, 168, 11–13. [Google Scholar] [CrossRef]
- Nassar, A.H.; Zern, N.K.; McIntyre, L.K.; Lynge, D.; Smith, C.A.; Petersen, R.P.; Horvath, K.; Wood, D.E. Emergency Restructuring of a General Surgery Residency Program during the Coronavirus Disease 2019 Pandemic: The University of Washington Experience. JAMA Surg. 2020, 155, 624–627. [Google Scholar] [CrossRef] [PubMed]
- Accreditation Council for Graduate Medical Education. ACGME Common Program Requirements (Residency). 2019. Available online: https://www.acgme.org/Portals/0/PFAssets/ProgramRequirements/CPRResidency2019.pdf (accessed on 17 October 2020).
- Gonzalez-Reiche, A.S.; Hernandez, M.M.; Sullivan, M.J.; Ciferri, B.; Alshammary, H.; Obla, A.; Fabre, S.; Kleiner, G.; Polanco, J.; Khan, Z.; et al. Introductions and early spread of SARS-CoV-2 in the New York City area. Science 2020, 369, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Thompson, C.N.; Baumgartner, J.; Pichardo, C.; Toro, B.; Li, L.; Arciuolo, R.; Chan, P.; Chen, J.; Culp, G.; Davidson, A.; et al. COVID-19 Outbreak—New York City, 29 February–1 June 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 1725–1729. [Google Scholar] [CrossRef] [PubMed]
- United States Food and Drug Administration. EUA Authorized Serology Test Performance. 2020. Available online: https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices/eua-authorized-serology-test-performance (accessed on 6 December 2020).
- Weissleder, R.; Lee, H.; Ko, J.; Pittet, M.; COVID-19 Diagnostics in Context. Updated 1 November 2020. 2020. Available online: https://csb.mgh.harvard.edu/covid (accessed on 6 December 2020).
- Accorsi, E.; Qiu, X.; Rumpler, E.; Kennedy-Shaffer, L.; Kahn, R.; Joshi, K.; Goldstein, E.; Stensrud, M.J.; Niehus, R.; Cevik, M.; et al. How to detect and reduce potential sources of biases in epidemiologic studies of SARS-CoV-2. 2020. Available online: https://osf.io/46am5/ (accessed on 6 December 2020).
- Chung, Y.; Gelman, A.; Rabe-Hesketh, S.; Liu, J.; Dorie, V. Weakly Informative Prior for Point Estimation of Covariance Matrices in Hierarchical Models. J. Educ. Behav. Stat. 2015, 40, 136–157. [Google Scholar] [CrossRef] [Green Version]
- Yan, X.; Yang, Y. RMSEA, CFI, and TLI in structural equation modeling with ordered categorical data: The story they tell depends on the estimation methods. Behav. Res. 2019, 51, 409–428. [Google Scholar]
- van Buuren, S.; Groothuis-Oudshoom, K. Mice: Multivariate Imputation by Chained Equations in R. J. Stat. Softw. 2011, 45, 1–67. [Google Scholar] [CrossRef] [Green Version]
- Rosseel, Y. lavaan: An R Package for Structural Equation Modeling. J. Stat. Softw. 2012, 48, 1–36. [Google Scholar] [CrossRef] [Green Version]
- Moscola, J.; Sembajwe, G.; Jarrett, M.; Farber, B.; Chang, T.; McGinn, T.; Davidson, K.; Northwell Health COVID-19 Research Consortium. Prevalence of SARS-CoV-2 Antibodies in Health Care Personnel in the New York City Area. JAMA 2020, 324, 893–895. [Google Scholar] [CrossRef]
- Jeremias, A.; Nguyen, J.; Levine, J.; Pollack, S.; Engellenner, W.; Thakore, A.; Lucore, C. Prevalence of SARS-CoV-2 Infection among Health Care Workers in a Tertiary Community Hospital. JAMA Intern. Med. 2020, 180, 1707–1709. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, E.S.; Tesoriero, J.M.; Rosenthal, E.M.; Chung, R.; Barranco, M.A.; Styer, L.M.; Parker, M.M.; Leung, S.-Y.; Morne, J.; Greene, D.; et al. Cumulative incidence and diagnosis of SARS-CoV-2 infection in New York. Ann. Epidemiol. 2020, 48, 23–29.e24. [Google Scholar] [CrossRef]
- Amendola, A.; Tanzi, E.; Folgori, L.; Barcellini, L.; Bianchi, S.; Gori, M.; Cammi, G.; Albani, E.; Zuccotti, G.V. Low seroprevalence of SARS-CoV-2 infection among healthcare workers of the largest children hospital in Milan during the pandemic wave. Infect. Control Hosp. Epidemiol. 2020, 41, 1468–1469. [Google Scholar] [CrossRef]
- Blairon, L.; Mokrane, S.; Wilmet, A.; Dessilly, G.; Kabamba-Mukadi, B.; Beukinga, I.; Tré-Hardy, M. Large-scale, molecular and serological SARS-CoV-2 screening of healthcare workers in a 4-site public hospital in Belgium after COVID-19 outbreak. J. Infect. 2020, 82, 159–198. [Google Scholar] [CrossRef]
- Grant, J.J.; Wilmore, S.M.S.; McCann, N.S.; Donnelly, O.; Lai, R.W.; Kinsella, M.J.; Rochford, H.; Patel, T.; Kelsey, M.; Andrews, J. Seroprevalence of SARS-CoV-2 antibodies in healthcare workers at a London NHS Trust. Infect. Control Hosp. Epidemiol. 2020, 42, 212–214. [Google Scholar] [CrossRef]
- Lackermair, K.; William, F.; Grzanna, N.; Lehmann, E.; Fichtner, S.; Kucher, H.B.; Wilhelm, K.; Estner, H. Infection with SARS-CoV-2 in primary care health care workers assessed by antibody testing. Fam. Pract. 2020, 38, 76–79. [Google Scholar] [CrossRef] [PubMed]
- Olalla, J.; Correa, A.M.; Martín-Escalante, M.D.; Hortas, M.L.; Martín-Sendarrubias, M.J.; Fuentes, V.; Sena, G.; García-Alegría, J. Search for asymptomatic carriers of SARS-CoV-2 in healthcare workers during the pandemic: A Spanish experience. QJM 2020, 113, 794–798. [Google Scholar] [CrossRef] [PubMed]
- Sotgiu, G.; Barassi, A.; Miozzo, M.; Saderi, L.; Piana, A.; Orfeo, N.; Colosio, C.; Felisati, G.; Davi, M.; Gerli, A.; et al. SARS-CoV-2 specific serological pattern in healthcare workers of an Italian COVID-19 forefront hospital. BMC Pulm. Med. 2020, 20, 203. [Google Scholar] [CrossRef]
- Chou, R.; Dana, T.; Buckley, D.I.; Selph, S.; Fu, R.; Totten, A.M. Update Alert 4: Epidemiology of and Risk Factors for Coronavirus Infection in Health Care Workers. Ann. Intern. Med. 2020, 173, 143–144. [Google Scholar] [CrossRef]
- Mansour, M.; Leven, E.; Muellers, K.; Stone, K.; Mendu, D.R.; Wajnberg, A. Prevalence of SARS-CoV-2 Antibodies among Healthcare Workers at a Tertiary Academic Hospital in New York City. J. Gen. Intern. Med. 2020, 35, 2485–2486. [Google Scholar] [CrossRef]
- Iverson, K.; Bundgaard, H.; Hasselbalch, R.B.; Kristensen, J.H.; Nielsen, P.B.; Pries-Heje, M.; Knudsen, A.; Christensen, C.; Fogh, K.; Norsk, J.B.; et al. Risk of COVID-19 in health-care workers in Denmark: An observational cohort study. Lancet Infect. Dis. 2020, 20, 1401–1408. [Google Scholar] [CrossRef]
- Canelli, R.; Connor, C.W.; Gonzalez, M.; Nozari, A.; Ortega, R. Barrier Enclosure during Endotracheal Intubation. N. Engl. J. Med. 2020, 382, 1957–1958. [Google Scholar] [CrossRef] [PubMed]
- Heinzerling, A.; Stuckey, M.J.; Scheuer, T.; Xu, K.; Perkins, K.M.; Resseger, H.; Magill, S.; Verani, J.R.; Jain, S.; Acosta, M.; et al. Transmission of COVID-19 to Health Care Personnel during Exposures to a Hospitalized Patient—Solano County, California, February 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 472–476. [Google Scholar] [CrossRef] [Green Version]
- Weissman, D.N.; de Perio, M.A.; Radonovich, L.J., Jr. COVID-19 and Risks Posed to Personnel during Endotracheal Intubation. JAMA 2020, 323, 2027–2028. [Google Scholar] [CrossRef] [PubMed]
- Carrion, D.; Colicino, E.; Pedretti, N.F.; Arfer, K.B.; Rush, J.; DeFelice, N.; Just, A.C. Assessing capacity to social distance and neighborhood-level health disparities during the COVID-19 pandemic. medRxiv 2020. preprint. [Google Scholar] [CrossRef]
- Lentz, R.J.; Colt, H.; Chen, H.; Cordovilla, R.; Popevic, S.; Tahura, S.; Candoli, P.; Tomassetti, S.; Meachery, G.J.; Cohen, B.P.; et al. Assessing coronavirus disease 2019 (COVID-19) transmission to healthcare personnel: The global ACT-HCP case-control study. Infect. Control Hosp. Epidemiol. 2020, 42, 381–387. [Google Scholar] [CrossRef] [PubMed]

| Variable | Negative SARS-CoV-2 Test (n = 262) | Positive SARS-CoV-2 Test (n = 66) | p-Value |
|---|---|---|---|
| Sociodemographic factors | |||
| Age, years, median (IQR) | 31 (29–33) | 30 (28–33) | 0.36 |
| Sex, no. (%) | 0.27 | ||
| Female | 155 (82) | 34 (18) | |
| Male | 107 (77) | 32 (23) | |
| Race, no. (%) | 0.25 | ||
| White | 156 (77) | 46 (23) | |
| Asian | 71 (87) | 11 (13) | |
| Black | 19 (73) | 7 (27) | |
| Other | 10 (83) | 2 (17) | |
| Missing | 6 | 0 | |
| Hispanic/Latinx, no. (%) | 0.18 | ||
| No | 237 (81) | 56 (19) | |
| Yes | 24 (71) | 10 (29) | |
| Missing | 1 | 0 | |
| Occupational factors | |||
| Training specialty, no. (%) | 0.002 | ||
| Hospital-based, primarily non-procedural | 180 (85) | 33 (15) | |
| High-risk procedural | 32 (62) | 20 (38) | |
| Surgical | 41 (77) | 12 (23) | |
| Missing | 9 | 1 | |
| PGY level, no. (%) | 0.57 | ||
| 1 | 55 (75) | 18 (25) | |
| 2 | 51 (82) | 11 (18) | |
| ≥3 | 156 (81) | 37 (19) | |
| Resident or fellowship, no. (%) | 0.88 | ||
| Fellowship | 69 (81) | 16 (19) | |
| Residency | 193 (79) | 50 (21) | |
| Primary hospital site, no. (%) | 0.27 | ||
| Beth Israel Medical Center | 23 (82) | 5 (18) | |
| Elmhurst Hospital Center | 15 (100) | 0 (0) | |
| Institute for Family Health | 4 (67) | 2 (33) | |
| Mount Sinai Hospital | 166 (79) | 45 (21) | |
| North Central Bronx | 1 (100) | 0 (0) | |
| Queens Hospital Center | 6 (86) | 1 (14) | |
| South Nassau Communities Hospital | 2 (50) | 2 (50) | |
| St. Luke’s Roosevelt Hospital | 45 (80) | 11 (20) | |
| Occupational setting | |||
| Medical-surgical unit, no. (%) | 0.24 | ||
| No | 89 (84) | 17 (16) | |
| Yes | 173 (78) | 49 (22) | |
| Emergency department, no. (%) | 0.64 | ||
| No | 194 (80) | 47 (20) | |
| Yes | 68 (78) | 19 (22) | |
| ICU, no. (%) | >0.99 | ||
| No | 154 (80) | 39 (20) | |
| Yes | 108 (80) | 27 (20) | |
| Ambulatory clinic, no. (%) | 0.04 | ||
| No | 174 (77) | 53 (23) | |
| Yes | 88 (87) | 13 (13) | |
| Telemedicine, no. (%) | 0.047 | ||
| No | 181 (77) | 54 (23) | |
| Yes | 81 (87) | 12 (13) | |
| High-risk occupational exposures | |||
| Direct care for confirmed COVID-19 case or PUI, no. (%) | 0.29 | ||
| No | 33 (87) | 5 (13) | |
| Yes | 229 (79) | 61 (21) | |
| Performed or attended an AGP on confirmed COVID-19 case or PUI, no. (%) | 0.05 | ||
| No | 127 (85) | 23 (15) | |
| Yes | 134 (76) | 43 (24) | |
| Missing | 1 | 0 | |
| Contact > 10 mins with confirmed without N95 COVID-19 case or PUI, no. (%) | 0.07 | ||
| No | 182 (83) | 37 (17) | |
| Once | 42 (76) | 13 (24) | |
| Twice or more | 36 (69) | 16 (31) | |
| Missing | 2 | 0 | |
| Contact > 10 mins without eye protection with confirmed COVID-19 case or PUI, no. (%) | 0.09 | ||
| No | 155 (83) | 31 (17) | |
| Once | 44 (80) | 11 (20) | |
| Twice or more | 61 (72) | 24 (28) | |
| Missing | 2 | 0 | |
| Contact > 10 mins without gown with confirmed COVID-19 case or PUI, no. (%) | 0.01 | ||
| No | 174 (84) | 32 (16) | |
| Once | 37 (77) | 11 (23) | |
| Twice or more | 48 (68) | 23 (32) | |
| Missing | 3 | 0 | |
| Contact > 10 mins without gloves with confirmed COVID-19 case or PUI, no. (%) | 0.12 | ||
| None | 225 (81) | 52 (19) | |
| Once or more | 34 (71) | 14 (29) | |
| Missing | 3 | 0 | |
| Deployment factors | |||
| Change in usual hospital, no. (%) | 0.59 | ||
| No | 212 (79) | 56 (21) | |
| Yes | 50 (83) | 10 (17) | |
| Change in usual clinical activities, no. (%) | 0.87 | ||
| No | 206 (80) | 53 (20) | |
| Yes | 56 (81) | 13 (19) | |
| Change in usual patient population, no. (%) | <0.001 | ||
| No | 230 (78) | 66 (22) | |
| Yes | 32 (100) | 0 (0) | |
| Change in usual department, no. (%) | 0.34 | ||
| No | 193 (78) | 53 (22) | |
| Yes | 69 (84) | 13 (16) | |
| More time on telemedicine than usual, no. (%) | 0.05 | ||
| No | 226 (78) | 63 (22) | |
| Yes | 36 (92) | 3 (8) | |
| Community factors | |||
| Primary residence, no. (%) | 0.06 | ||
| Manhattan | 202 (77) | 60 (23) | |
| Queens | 28 (93) | 2 (7) | |
| Brooklyn | 12 (100) | 0 (0) | |
| Bronx | 5 (100) | 0 (0) | |
| Outside of NYC | 13 (76) | 4 (24) | |
| Missing | 2 | 0 | |
| Contact > 10 mins with individual confirmed or suspected COVID-19 outside of work, no. (%) | 0.008 | ||
| No | 212 (83) | 43 (17) | |
| Yes | 50 (68) | 23 (32) | |
| Number of adults in household, no. (%) | 0.64 | ||
| 1 (self) | 72 (82) | 16 (18) | |
| ≥ 2 | 189 (79) | 50 (21) | |
| Missing | 1 | 0 | |
| Number of children in household, no. (%) | 0.19 | ||
| 0 | 214 (78) | 59 (22) | |
| ≥ 1 | 46 (87) | 7 (13) | |
| Missing | 2 | 0 | |
| Primary mode of transportation to work | |||
| Public transit (subway or bus), no. (%) | 0.32 | ||
| No | 165 (82) | 37 (18) | |
| Yes | 97 (77) | 29 (23) | |
| Cab or rideshare, no. (%) | 0.37 | ||
| No | 183 (81) | 42 (19) | |
| Yes | 79 (77) | 24 (23) | |
| Private vehicle, bicycle or walking, no. (%) | 0.86 | ||
| No | 53 (82) | 12 (18) | |
| Yes | 209 (79) | 54 (21) | |
| Primary mode of transportation to non-work location | |||
| Public transit (subway or bus), no. (%) | 0.07 | ||
| No | 220 (82) | 49 (18) | |
| Yes | 42 (71) | 17 (29) | |
| Cab or rideshare, no. (%) | 0.049 | ||
| No | 220 (82) | 48 (18) | |
| Yes | 42 (70) | 18 (30) | |
| Private vehicle, bicycle or walking, no. (%) | 0.08 | ||
| No | 12 (63) | 7 (37) | |
| Yes | 250 (81) | 59 (19) | |
| Variable | Model 1: Sociodemographic Factors | Model 2: Occupational Factors | Model 3: Community Factors | Model 4: Final Adjusted Model | ||||
|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Race | ||||||||
| White (ref) | 1.00 | - | 1.00 | - | ||||
| Asian | 0.53 | 0.23, 1.24 | 0.53 | 0.24, 1.15 | ||||
| Black | 1.34 | 0.45, 3.98 | 1.42 | 0.50, 4.01 | ||||
| Other | 0.43 | 0.08, 2.47 | 0.64 | 0.14, 2.92 | ||||
| Hispanic/Latinx | ||||||||
| No (ref) | 1.00 | - | 1.00 | - | ||||
| Yes | 2.18 | 0.73, 6.47 | 1.98 | 0.72, 5.46 | ||||
| Change in usual patient population | ||||||||
| No (ref) | 1.00 | - | 1.00 | - | ||||
| Yes | 0.09 | 0.01, 0.67 | 0.16 | 0.03, 0.73 | ||||
| Medical/surgical unit | ||||||||
| No (ref) | 1.00 | - | 1.00 | - | ||||
| Yes | 2.96 | 1.27, 6.91 | 2.51 | 1.18, 5.34 | ||||
| Ambulatory clinic | ||||||||
| No (ref) | 1.00 | - | 1.00 | - | ||||
| Yes | 0.53 | 0.24, 1.17 | 0.61 | 0.29, 1.30 | ||||
| Contact >10 mins without N95 with confirmed COVID-19 case | ||||||||
| Never (ref) | 1.00 | - | 1.00 | - | ||||
| Once | 1.47 | 0.62, 3.48 | 1.24 | 0.55, 2.75 | ||||
| Twice or more | 1.72 | 0.75, 3.94 | 1.59 | 0.74, 3.43 | ||||
| Training specialty | ||||||||
| Hospital-based, primarily non-procedural (ref) | 1.00 | - | 1.00 | - | ||||
| High-risk procedural | 4.29 | 1.62, 11.33 | 2.93 | 1.24, 6.92 | ||||
| Surgical | 1.98 | 0.81, 4.89 | 1.51 | 0.65, 3.50 | ||||
| Number of children in household | ||||||||
| 0 (ref) | 1.00 | - | 1.00 | - | ||||
| ≥ 1 | 0.52 | 0.20, 1.38 | 0.59 | 0.23, 1.48 | ||||
| Contact > 10 mins with individual confirmed or suspected COVID-19 outside of work | ||||||||
| No (ref) | 1.00 | - | 1.00 | - | ||||
| Yes | 2.38 | 1.14, 4.98 | 1.58 | 0.78, 3.17 | ||||
| Primary mode of transportation to location other than work: public transit (subway or bus) | ||||||||
| No (ref) | 1.00 | - | 1.00 | - | ||||
| Yes | 2.25 | 1.01, 5.01 | 1.85 | 0.85, 3.99 | ||||
| Primary mode of transportation to location other than work: private vehicle, bicycle, walking | ||||||||
| No (ref) | 1.00 | - | 1.00 | - | ||||
| Yes | 0.44 | 0.14, 1.40 | 0.42 | 0.14, 1.27 | ||||
| Primary residence (zip code) | ||||||||
| Manhattan (ref) | 1.00 | - | 1.00 | - | ||||
| Queens | 0.24 | 0.06, 0.94 | 0.34 | 0.10, 1.20 | ||||
| Brooklyn | 0.21 | 0.03, 1.64 | 0.30 | 0.06, 1.62 | ||||
| Bronx | 0.40 | 0.04, 3.98 | 0.48 | 0.08, 3.08 | ||||
| Outside of NYC | 1.48 | 0.40, 5.49 | 1.51 | 0.44, 5.20 | ||||
| Exposure Latent Functions | SEM 1 a | SEM 2 b | SEM 3 c | SEM 4 d | ||||
|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Sociodemographic factors | 0.09 | −0.07, 0.25 | 0.13 | −0.06, 0.31 | ||||
| Occupational factors | 0.33 | 0.13, 0.53 | 0.35 | 0.15, 0.54 | ||||
| Community factors | 0.12 | −0.08, 0.32 | 0.10 | −0.12, 0.33 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pawloski, K.R.; Kolod, B.; Khan, R.F.; Midya, V.; Chen, T.; Oduwole, A.; Camins, B.; Colicino, E.; Leitman, I.M.; Nabeel, I.; et al. Factors Associated with SARS-CoV-2 Infection in Physician Trainees in New York City during the First COVID-19 Wave. Int. J. Environ. Res. Public Health 2021, 18, 5274. https://doi.org/10.3390/ijerph18105274
Pawloski KR, Kolod B, Khan RF, Midya V, Chen T, Oduwole A, Camins B, Colicino E, Leitman IM, Nabeel I, et al. Factors Associated with SARS-CoV-2 Infection in Physician Trainees in New York City during the First COVID-19 Wave. International Journal of Environmental Research and Public Health. 2021; 18(10):5274. https://doi.org/10.3390/ijerph18105274
Chicago/Turabian StylePawloski, Kate R., Betty Kolod, Rabeea F. Khan, Vishal Midya, Tania Chen, Adeyemi Oduwole, Bernard Camins, Elena Colicino, I. Michael Leitman, Ismail Nabeel, and et al. 2021. "Factors Associated with SARS-CoV-2 Infection in Physician Trainees in New York City during the First COVID-19 Wave" International Journal of Environmental Research and Public Health 18, no. 10: 5274. https://doi.org/10.3390/ijerph18105274
APA StylePawloski, K. R., Kolod, B., Khan, R. F., Midya, V., Chen, T., Oduwole, A., Camins, B., Colicino, E., Leitman, I. M., Nabeel, I., Oliver, K., & Valvi, D. (2021). Factors Associated with SARS-CoV-2 Infection in Physician Trainees in New York City during the First COVID-19 Wave. International Journal of Environmental Research and Public Health, 18(10), 5274. https://doi.org/10.3390/ijerph18105274








