Staphylococcus aureus—An Additional Parameter of Bathing Water Quality for Crowded Urban Beaches
Abstract
1. Introduction
2. Materials and Methods
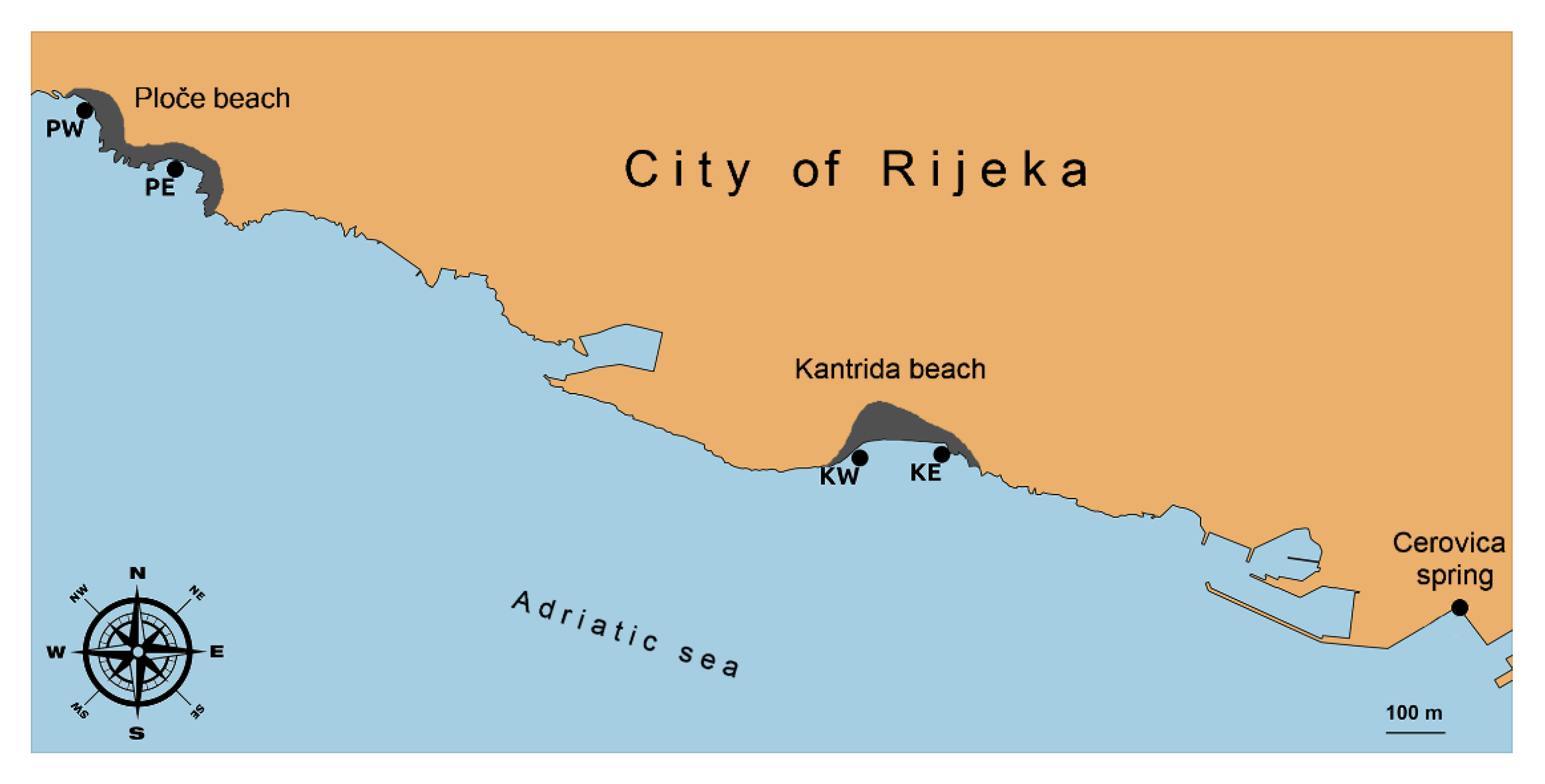
2.1. Study Area and Sampling
2.2. Sample Analysis
2.2.1. Microbiological Analysis
2.2.2. Physical/Chemical Analysis
2.2.3. Data Analysis
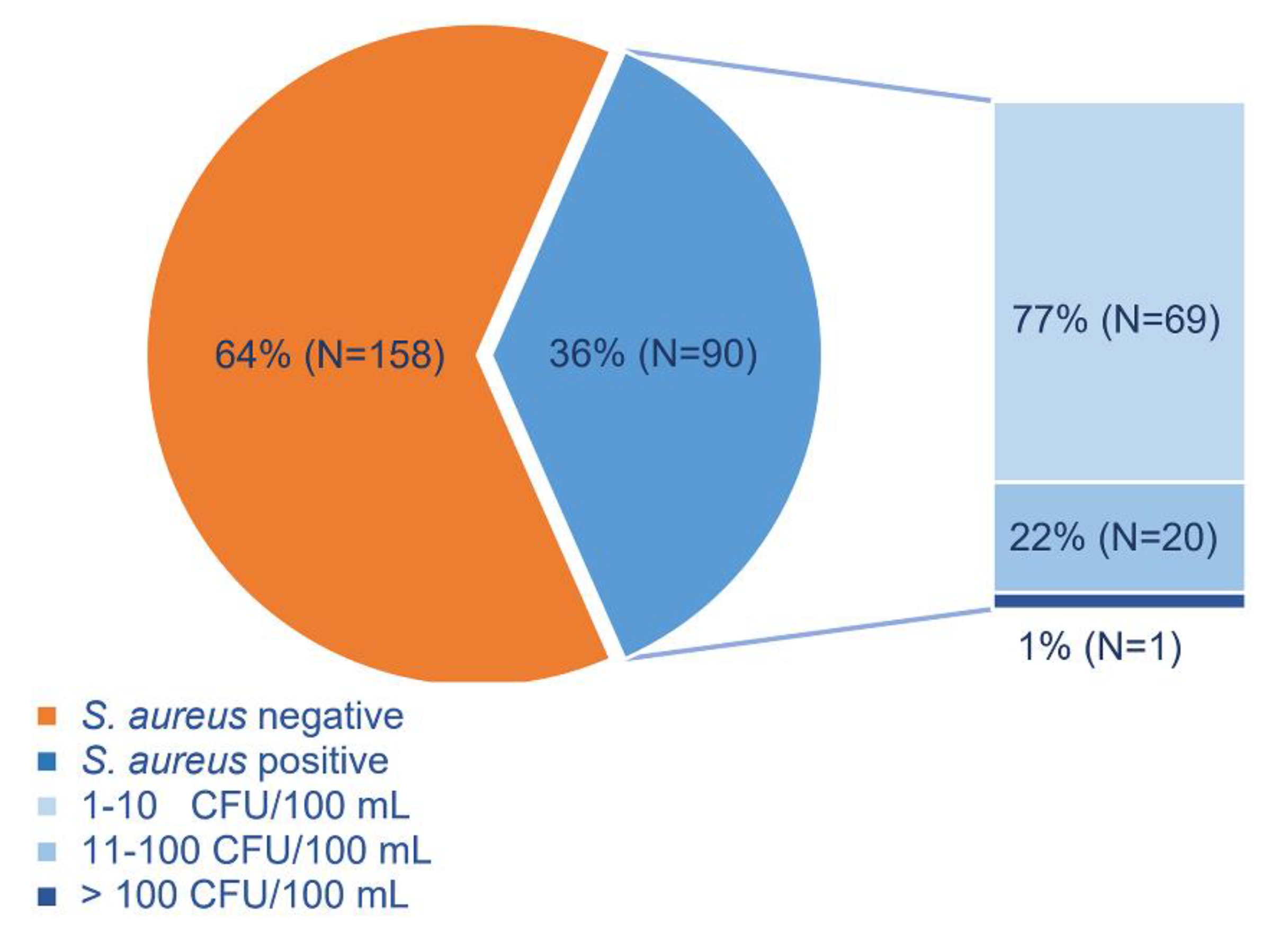
3. Results
3.1. Official vs. Supplemental Monitoring
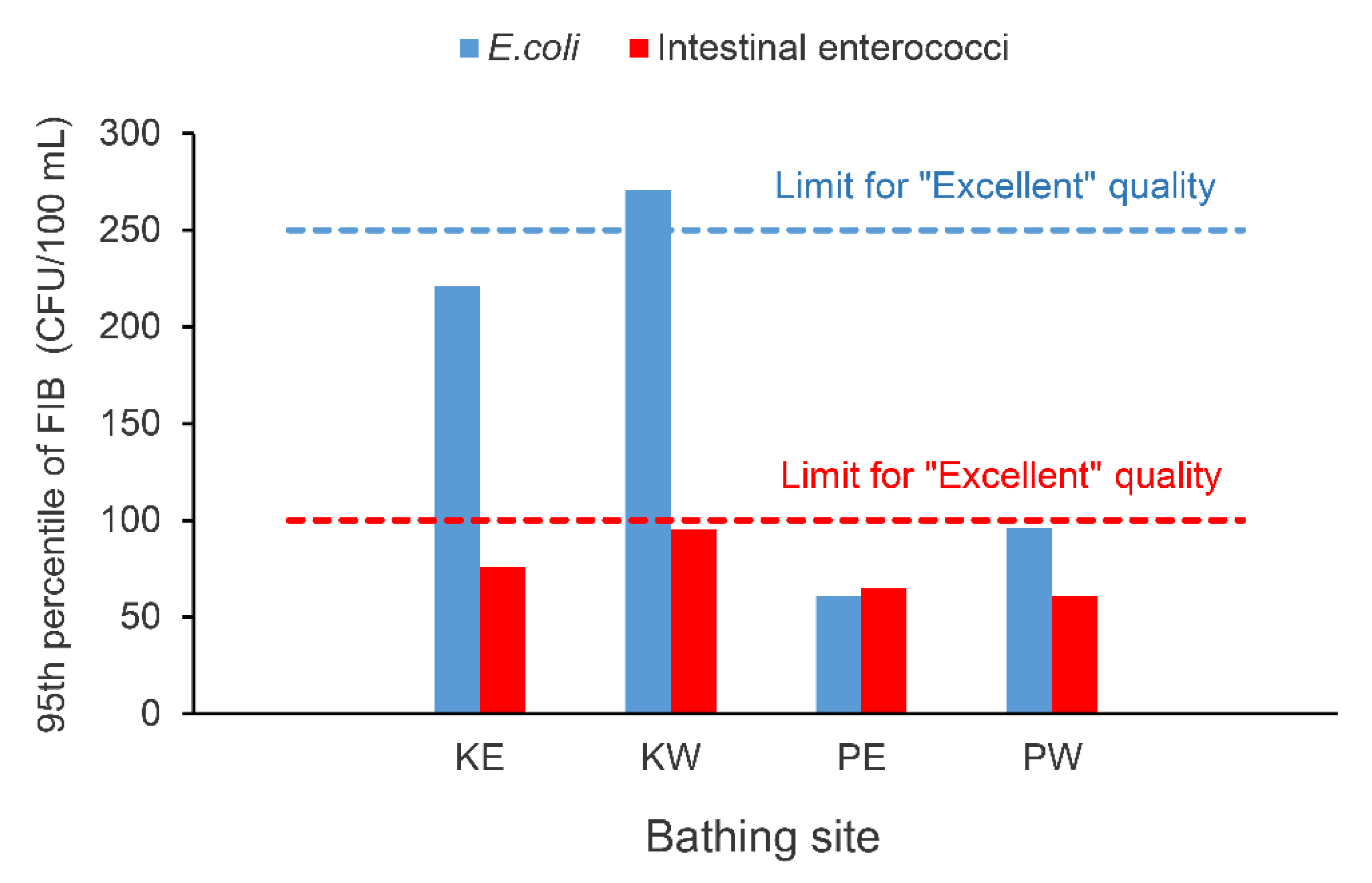
3.2. Kantrida vs. Ploče Beaches
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- The Council of the European Communities. Council Directive 76/160/EEC of 8 December 1975 Concerning the Quality of Bathing Water; European Communities: Brussels, Belgium, 1975.
- The European Parliament and the Council of the European Union. Directive 2006/7/EC of 15 February 2006 Concerning the Management of Bathing Water Quality and Repealing Directive 76/160/EEC.; European Union: Brussels, Belgium, 2006.
- World Health Organization (WHO). WHO Recommendations on Scientific, Analytical and Epidemiological Developments Relevant to the Parameters for Bathing Water Quality in the Bathing Water Directive (2006/7/EC). 2018. Available online: https://circabc.europa.eu/d/d/workspace/SpacesStore/9e89152c-7cfe-4391-9bcf-c173519e8181/WHO%20Recommendations%20on%20EC%20BWD.pdf (accessed on 20 April 2020).
- King, S.; Exley, J.; Winpenny, E.; Alves, L.; Henham, M.L.; Larkin, J. The Health Risks of Bathing in Recreational Waters: A Rapid Evidence Assessment of Water Quality and Gastrointestinal Illness. Rand Health Q. 2015, 4, 5. [Google Scholar]
- Papastergiou, P.; Mouchtouri, V.; Pinaka, O.; Katsiaflaka, A.; Rachiotis, G.; Hadjichristodoulou, C. Elevated bathing-associated disease risks despite certified water quality: A cohort study. Int. J. Environ. Res. Public Health 2012, 9, 1548–1565. [Google Scholar] [CrossRef] [PubMed]
- Korajkic, A.; McMinn, B.R.; Harwood, V.J. Relationships between Microbial Indicators and Pathogens in Recreational Water Settings. Int. J. Environ. Res. Public Health 2018, 15, 2842. [Google Scholar] [CrossRef] [PubMed]
- Enns, A.A.; Vogel, L.J.; Abdelzaher, A.M.; Solo-Gabriele, H.M.; Plano, L.R.W.; Gidley, M.L.; Phillips, M.C.; Klaus, J.S.; Piggot, A.M.; Feng, Z.; et al. Spatial and temporal variation in indicator microbe sampling is influential in beach management decisions. Water Res. 2012, 46, 2237–2246. [Google Scholar] [CrossRef] [PubMed]
- Zabed, H.; Suely, A.; Faruq, G.; Sahu, J.N. Water quality assessment of an unusual ritual well in Bangladesh and impact of mass bathing on this quality. Sci. Total Environ. 2014, 472, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Curiel-Ayala, F.; Quiñones-Ramírez, E.I.; Pless, R.C.; González-Jasso, E. Comparative studies on Enterococcus, Clostridium perfringens and Staphylococcus aureus as quality indicators in tropical seawater at a Pacific Mexican beach resort. Mar. Pollut. Bull. 2012, 64, 2193–2198. [Google Scholar] [CrossRef] [PubMed]
- Yoshpe-Purer, Y.; Golderman, S. Occurrence of Staphylococcus aureus and Pseudomonas aeruginosa in Israeli coastal water. Appl. Environ. Microbiol. 1987, 53, 1138–1141. [Google Scholar] [CrossRef] [PubMed]
- Bruins, M.J.; Juffer, P.; Wolfhagen, M.J.; Ruijs, G.J. Salt tolerance of methicillin-resistant and methicillin-susceptible Staphylococcus aureus. J. Clin. Microbiol. 2007, 45, 682–683. [Google Scholar] [CrossRef][Green Version]
- Chambers, H.F.; Deleo, F.R. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat. Rev. Microbiol. 2009, 7, 629–641. [Google Scholar] [CrossRef]
- Food Standards Australia New Zealand (FSANZ) Staphylococcus aureus—Food Standards Australia. Available online: https://www.foodstandards.gov.au/publications/Documents/Staphylococcus%20aureus.pdf (accessed on 15 November 2019).
- Taheri, N.; Ardebili, A.; Amouzandeh-Nobaveh, A.; Ghaznavi-Rad, E. Frequency of antiseptic resistance among Staphylococcus aureus and coagulase-negative Staphylococci isolated from a University hospital in central Iran. Oman Med. J. 2016, 31, 426–432. [Google Scholar] [CrossRef]
- Kloos, W.E.; Bannerman, T.L. Update on clinical significance of coagulase-negative staphylococci. Clin. Microbiol. Rev. 1994, 7, 117–140. [Google Scholar] [CrossRef] [PubMed]
- Al-Zu’bi, E.; Bdour, S.; Shehabi, A.A. Antibiotic resistance patterns of mecA-positive Staphylococcus aureus isolates from clinical specimens and nasal carriage. Microb. Drug Resist. 2004, 10, 321–324. [Google Scholar] [CrossRef]
- Kluytmans, J.A.; Wertheim, H.F. Nasal carriage of Staphylococcus aureus and prevention of nosocomial infections. Infection 2005, 33, 3–8. [Google Scholar] [CrossRef]
- Kuehnert, M.J.; Kruszon-Moran, D.; Hill, H.A.; McQuillan, G.; McAllister, S.K.; Fosheim, G.; McDougal, L.K.; Chaitram, J.; Jensen, B.; Fridkin, S.K.; et al. Prevalence of Staphylococcus aureus nasal colonization in the United States, 2001–2002. J. Infect. Dis. 2006, 193, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Boost, M.V.; O’Donoghue, M.M.; James, A. Prevalence of Staphylococcus aureus carriage among dogs and their owners. Epidemiol. Infect. 2008, 136, 953–964. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, K.D.; McNay, M.; Cao, Y.; Ebentier, D.; Madison, M.; Griffith, J.F. A multi-beach study of Staphylococcus aureus, MRSA, and enterococci in seawater and beach sand. Water Res. 2012, 46, 4195–4207. [Google Scholar] [CrossRef]
- Gilmore, S. Characterization and Isolation of Fecal Indicator Bacteria, Staphylococcus aureus, and Methicillin-Resistant Staphylococcus aureus from Pacific Northwest Marine Beach Samples. Master’s Thesis, University of Washington, Seattle, WA, USA, 2012. [Google Scholar]
- Elmir, S.M.; Wright, M.E.; Abdelzaher, A.; Solo-Gabriele, H.M.; Fleming, L.E.; Miller, G.; Rybolowik, M.; Peter Shih, M.T.; Pillai, S.P.; Cooper, J.A.; et al. Quantitative evaluation of bacteria released by bathers in a marine water. Water Res. 2007, 41, 3–10. [Google Scholar] [CrossRef]
- Plano, L.R.W.; Garza, A.C.; Shibata, T.; Elmir, S.M.; Kish, J.; Sinigalliano, C.D.; Gidley, M.L.; Miller, G.; Withum, K.; Fleming, L.E.; et al. Shedding of Staphylococcus aureus and methicillin-resistant Staphylococcus aureus from adult and pediatric bathers in marine waters. BMC Microbiol. 2011, 11. [Google Scholar] [CrossRef] [PubMed]
- Cheung, W.H.; Chang, K.C.; Hung, R.P.; Kleevens, J.W. Health effects of beach water pollution in Hong Kong. Epidemiol. Infect. 1990, 105, 139–162. [Google Scholar] [CrossRef]
- Gabutti, G.; De Donno, A.; Bagordo, F.; Montagna, M.T. Comparative Survival of Faecal and Human Contaminants and Use of Staphylococcus aureus as an Effective Indicator of Human Pollution. Mar. Pollut. Bull. 2000, 40, 697–700. [Google Scholar] [CrossRef]
- Vukić Lušić, D.; Lušić, D.; Pešut, D.; Mićović, V.; Glad, M.; Bilajac, L.; Peršić, V. Evaluation of equivalence between different methods for enumeration of fecal indicator bacteria before and after adoption of the new Bathing Water Directive and risk assessment of pollution. Mar. Pollut. Bull. 2013, 73, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Jozić, S.; Vukić Lušić, D. Report on Validation of Temperature Modified ISO 9308-1:2014 Method for the Enumeration of Escherichia coli in Bathing Water Samples in Croatia; Institute of Oceanography and Fisheries: Croatia, Balkan, 2018. [Google Scholar]
- Jozić, S.; Vukić Lušić, D.; Ordulj, M.; Frlan, E.; Cenov, A.; Diković, S.; Kauzlarić, V.; Fiorido Đurković, L.; Stilinović Totić, J.; Ivšinović, D.; et al. Performance characteristics of the temperature-modified ISO 9308-1 method for the enumeration of Escherichia coli in marine and inland bathing waters. Mar. Pollut. Bull. 2018, 135, 150–158. [Google Scholar] [CrossRef]
- Baird, R.; Bridgewater, L. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association: Washington, DC, USA, 2017. [Google Scholar]
- World Health Organization (WHO). Guidelines for Safe Recreational Water Environments Volume 1: Coastal and Fresh Waters; World Health Organization: Switzerland, Geneva, 2003. [Google Scholar]
- Cheung, W.H.; Chang, K.C.; Hung, R.P. Variations in microbial indicator densities in beach waters and health-related assessment of bathing water quality. Epidemiol. Infect. 1991, 106, 329–344. [Google Scholar] [CrossRef] [PubMed]
- Šolić, M.; Krstulović, N. Presence and survival of Staphylococcus aureus in the coastal area of Split (Adriatic Sea). Mar. Pollut. Bull. 1994, 28, 696–700. [Google Scholar] [CrossRef]
- El-Shenawy, M.A. Staphylococcus aureus and fecal indicators in Egyptian coastal waters of Agaba gulf, Suez gulf and Red sea. Egypt. J. Aquat. Res. 2004, 31, 113–124. [Google Scholar]
- Charoenca, N.; Fujioka, R.S. Assessment of Staphylococcus bacteria in Hawaii’s marine recreational waters. Water Sci. Technol. 1993, 27, 283–289. [Google Scholar] [CrossRef]
- Cragg, J.; Clayton, Y.M. Bacterial and fungal flora of seagull droppings in Jersey. J. Clin. Pathol. 1971, 24, 317–319. [Google Scholar] [CrossRef] [PubMed]
- Biberstein, E.L.; Jang, S.S.; Hirsh, D.C. Species distribution of coagulase-positive staphylococci in animals. J. Clin. Microbiol. 1984, 19, 610–615. [Google Scholar] [CrossRef]
- Efstratiou, M.A.; Mavridou, A.; Richardson, S.C.; Papadakis, J.A. Correlation of bacterial indicator organisms with Salmonella spp., Staphylococcus aureus and Candida albicans in sea water. Lett. Appl. Microbiol. 1998, 26, 342–346. [Google Scholar] [CrossRef]
- Bujas, L.; Unić Klarin, B. Sea quality on the beaches of the Šibenik-Knin County in 2015. In XXII Working Meeting of County Public Health Institutes, Administrative Departments and Institutes Responsible for Environmental Protection, Physical Planning, Construction, Communal Economy, Sustainable Development and Maritime Affairs of the Adriatic Area; Ministry of Environmental Protection and Energy: Croatia, Balkan, 2016. [Google Scholar]
- Krstulović, N.; Šolić, M. Die-off rate of Staphylococcus aureus in sea-water. In Proceedings of the Rapport du XXXIVE Congrès de la CIESM, Valletta, Malta, 23 March–1 April 1995; Commission Internationale pour L’exploration Scientifique de la mer Méditerranée (CIESM): Monaco, 1995; p. 158. [Google Scholar]
- Vukić Lušić, D.; Kranjčević, L.; Mačešić, S.; Lušić, D.; Jozić, S.; Linšak, Z.; Bilajac, L.; Grbčić, L.; Bilajac, N. Temporal variations analyses and predictive modeling of microbiological seawater quality. Water Res. 2017, 119, 160–170. [Google Scholar] [CrossRef]
- Davies-Colley, R.J.; Bell, R.G.; Donnison, A.M. Sunlight inactivation of enterococci and fecal coliforms in sewage effluent diluted in seawater. Appl. Environ. Microbiol. 1994, 60, 2049–2058. [Google Scholar] [CrossRef] [PubMed]
- Fujioka, R.S.; Hashimoto, H.H.; Siwak, E.B.; Young, R.H. Effect of sunlight on survival of indicator bacteria in seawater. Appl. Environ. Microbiol. 1981, 41, 690–696. [Google Scholar] [CrossRef] [PubMed]
- Jozić, S.; Morović, M.; Šolić, M.; Krstulović, N.; Ordulj, M. Effect of solar radiation, temperature and salinity on the survival of two different strains of Escherichia coli. Fresenius Environ. Bull. 2014, 23, 1852–1859. [Google Scholar]
- Sinton, L.W.; Davies-Colley, R.J.; Bell, R.G. Inactivation of enterococci and fecal coliforms from sewage and meatworks effluents in seawater chambers. Appl. Environ. Microbiol. 1994, 60, 2040–2048. [Google Scholar] [CrossRef]
- Institute of Oceanography and Fisheries (IOF) Sea Bathing Water Quality in Croatia. Available online: http://baltazar.izor.hr/plazepub/kakvoca_detalji10 (accessed on 7 September 2020).
- Mance, D.; Mance, D.; Vukić Lušić, D. Marine pollution differentiation with stable isotopes of groundwater. Pomorstvo 2018, 31, 80–87. [Google Scholar] [CrossRef]
- Esiobu, N.; Green, M.; Echeverry, A.; Bonilla, T.D.; Stinson, C.M.; Hartz, A.; Rogerson, A.; Mc Corquodale, D.S. High numbers of Staphylococcus aureus at three bathing beaches in South Florida. Int. J. Environ. Res. Public Health 2013, 23, 46–57. [Google Scholar] [CrossRef]
- Chareonca, N.; Fujioka, R.S. Association of staphylococcal skin infections and swimming. Water Sci. Technol. 1995, 31, 11–17. [Google Scholar] [CrossRef]
- Schets, F.M.; van der Berg, H.H.J.L.; Lynch, G.; de Rijk, S.; de Roda Husman, A.M.; Schijven, J.H. Evaluation of water quality guidelines for public swimming ponds. Environ. Int. 2020, 137. [Google Scholar] [CrossRef] [PubMed]
- Favero, M.S.; Drake, C.H.; Randall, G.B. Use of staphylococci as indicators of swimming pool pollution. Public Health Rep. 1964, 79, 61–70. [Google Scholar] [CrossRef] [PubMed]
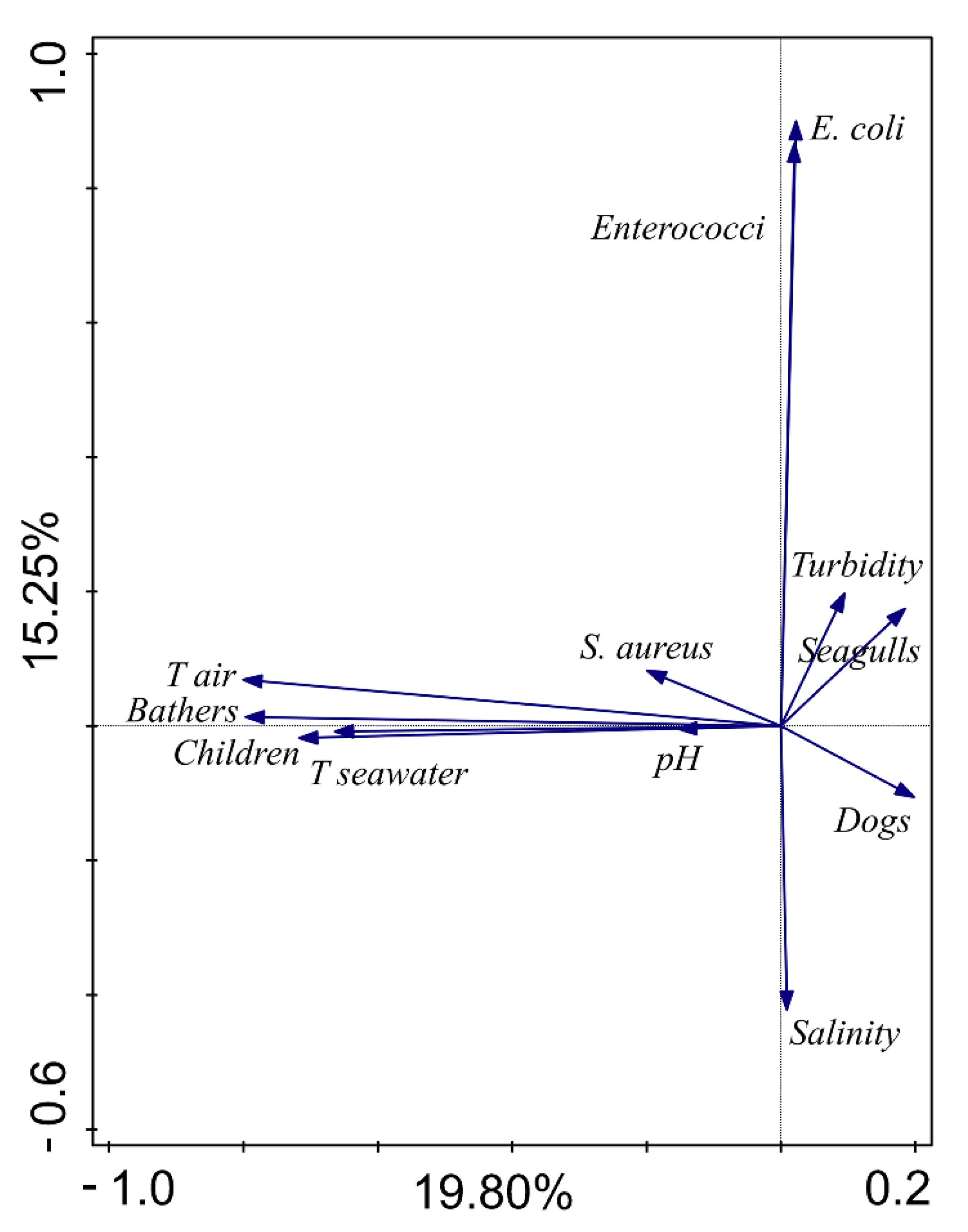
| Variables | S. Aureus (CFU/100 mL) | Salinity | ph | Turbidity (NTU) | E. coli (CFU/100 mL) | Enterococci (CFU/100 mL) | Air Temperature (°C) | Water Temperature (°C) | N Bathers | N Children in Diapers | N Dogs |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Salinity | −0.033 | ||||||||||
| pH | 0.048 | −0.020 | |||||||||
| Turbidity (NTU) | 0.046 | −0.353 | −0.025 | ||||||||
| E. coli (CFU/100 mL) | 0.084 | −0.395 | −0.094 | 0.211 | |||||||
| Enterococci (CFU/100 mL) | 0.088 | −0.379 | −0.057 | 0.208 | 0.695 | ||||||
| Air temperature (°C) | 0.147 | 0.111 | 0.052 | 0.008 | −0.273 | −0.276 | |||||
| Water temperature (°C) | 0.243 | 0.066 | 0.274 | −0.252 | −0.319 | −0.307 | 0.416 | ||||
| N bathers | 0.321 | 0.131 | 0.081 | −0.226 | −0.136 | −0.161 | 0.367 | 0.498 | |||
| N children in diapers | 0.203 | 0.117 | 0.014 | −0.130 | −0.220 | −0.176 | 0.355 | 0.395 | 0.673 | ||
| N dogs | −0.065 | −0.286 | −0.066 | 0.287 | 0.294 | 0.264 | −0.341 | −0.419 | −0.390 | −0.261 | |
| N seagulls | 0.035 | −0.208 | 0.039 | 0.221 | 0.316 | 0.216 | −0.166 | −0.221 | −0.229 | −0.382 | 0.252 |
| Parameter | Median (IQR 25–75) | |
|---|---|---|
| Ploče | Kantrida | |
| S. aureus (CFU/100 mL) | 2 (1–3) | 6 (2–14) |
| E. coli (CFU/100 mL) | 7 (3–22) | 29.5 (11.5–77) |
| Intestinal enterococci (CFU/100 mL) | 7 (3–18) | 18 (10–35) |
| Salinity | 36.1 (34.2–37.2) | 35.0 (33.0–36.7) |
| Water temperature (°C) | 25.0 (23.0–25.0) | 24.0 (23.0–25.0) |
| Air temperature (°C) | 26.0 (25.0–28.0) | 26.0 (25.0–28.0) |
| Turbidity (NTU) | 0.50 (0.38–0.7) | 0.62 (0.49–0.85) |
| pH | 8.0 (7.9–8.0) | 8.0 (7.9–8.0) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Topić, N.; Cenov, A.; Jozić, S.; Glad, M.; Mance, D.; Lušić, D.; Kapetanović, D.; Mance, D.; Vukić Lušić, D. Staphylococcus aureus—An Additional Parameter of Bathing Water Quality for Crowded Urban Beaches. Int. J. Environ. Res. Public Health 2021, 18, 5234. https://doi.org/10.3390/ijerph18105234
Topić N, Cenov A, Jozić S, Glad M, Mance D, Lušić D, Kapetanović D, Mance D, Vukić Lušić D. Staphylococcus aureus—An Additional Parameter of Bathing Water Quality for Crowded Urban Beaches. International Journal of Environmental Research and Public Health. 2021; 18(10):5234. https://doi.org/10.3390/ijerph18105234
Chicago/Turabian StyleTopić, Nancy, Arijana Cenov, Slaven Jozić, Marin Glad, Diana Mance, Dražen Lušić, Damir Kapetanović, Davor Mance, and Darija Vukić Lušić. 2021. "Staphylococcus aureus—An Additional Parameter of Bathing Water Quality for Crowded Urban Beaches" International Journal of Environmental Research and Public Health 18, no. 10: 5234. https://doi.org/10.3390/ijerph18105234
APA StyleTopić, N., Cenov, A., Jozić, S., Glad, M., Mance, D., Lušić, D., Kapetanović, D., Mance, D., & Vukić Lušić, D. (2021). Staphylococcus aureus—An Additional Parameter of Bathing Water Quality for Crowded Urban Beaches. International Journal of Environmental Research and Public Health, 18(10), 5234. https://doi.org/10.3390/ijerph18105234







