An Interaction Effect Analysis of Thermodilution-Guided Hemodynamic Optimization, Patient Condition, and Mortality after Successful Cardiopulmonary Resuscitation
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
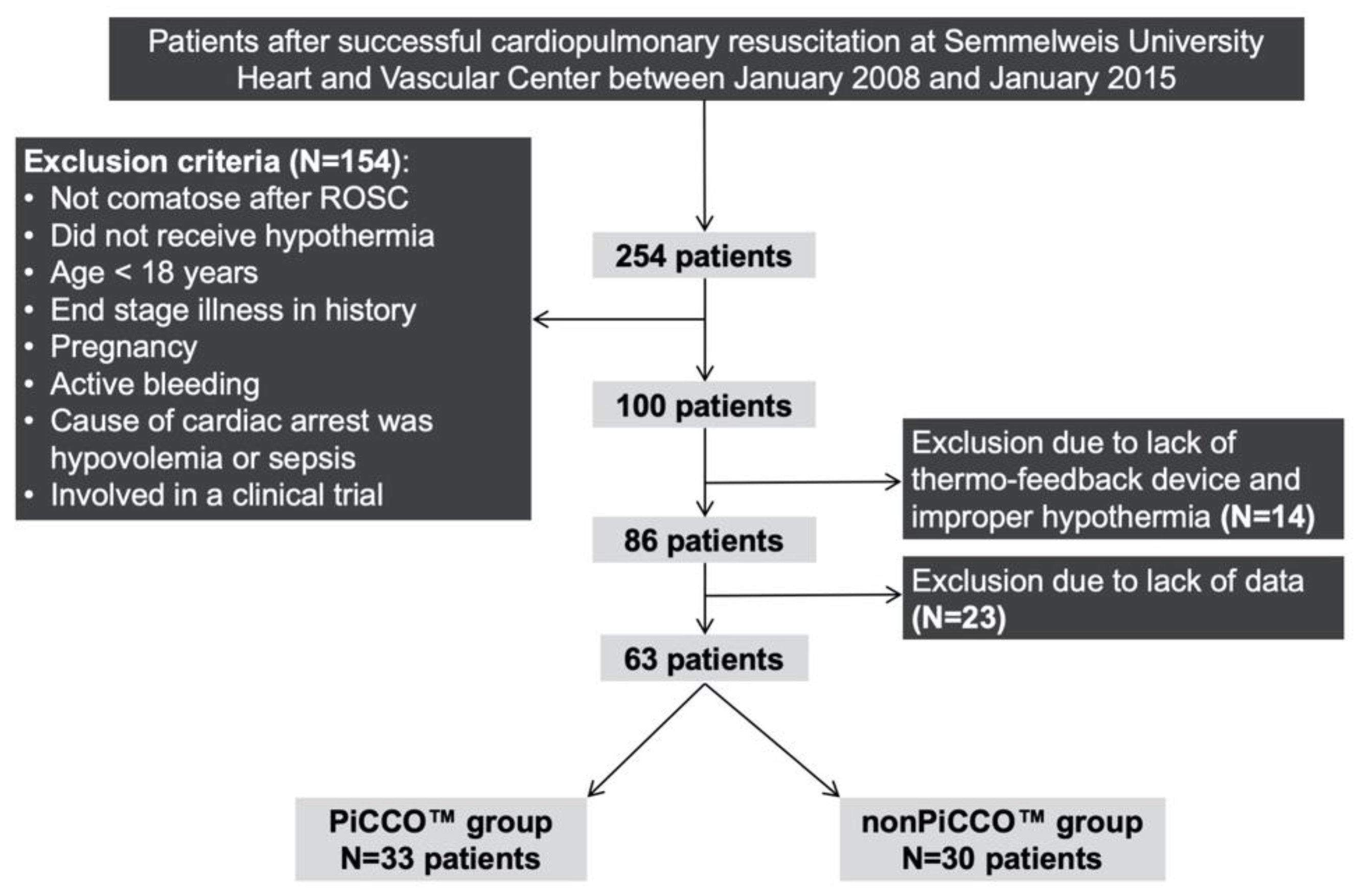
2.2. Patients
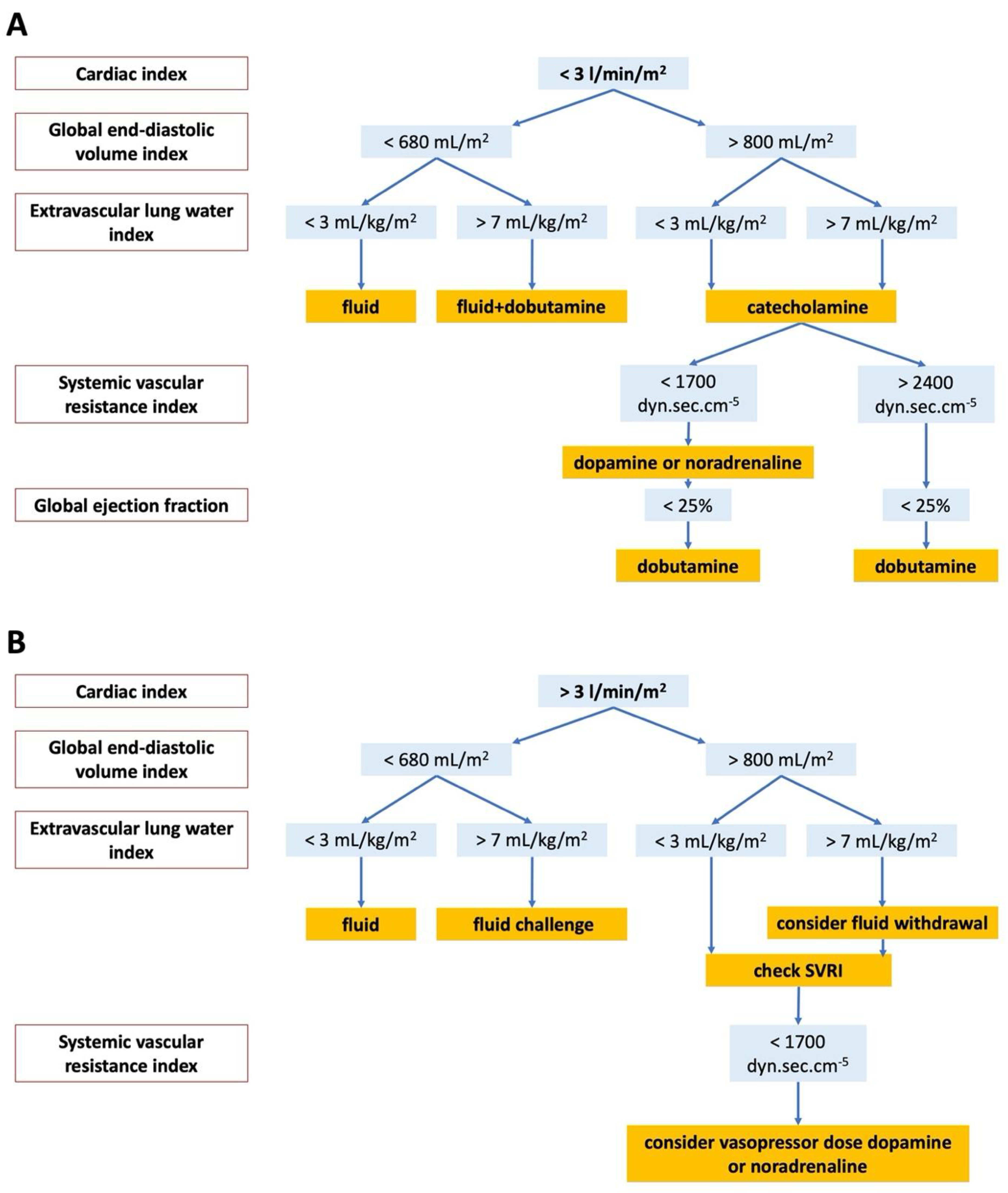
2.3. Initial Therapy
2.4. Patient Outcomes
2.5. Statistical Analysis
3. Results
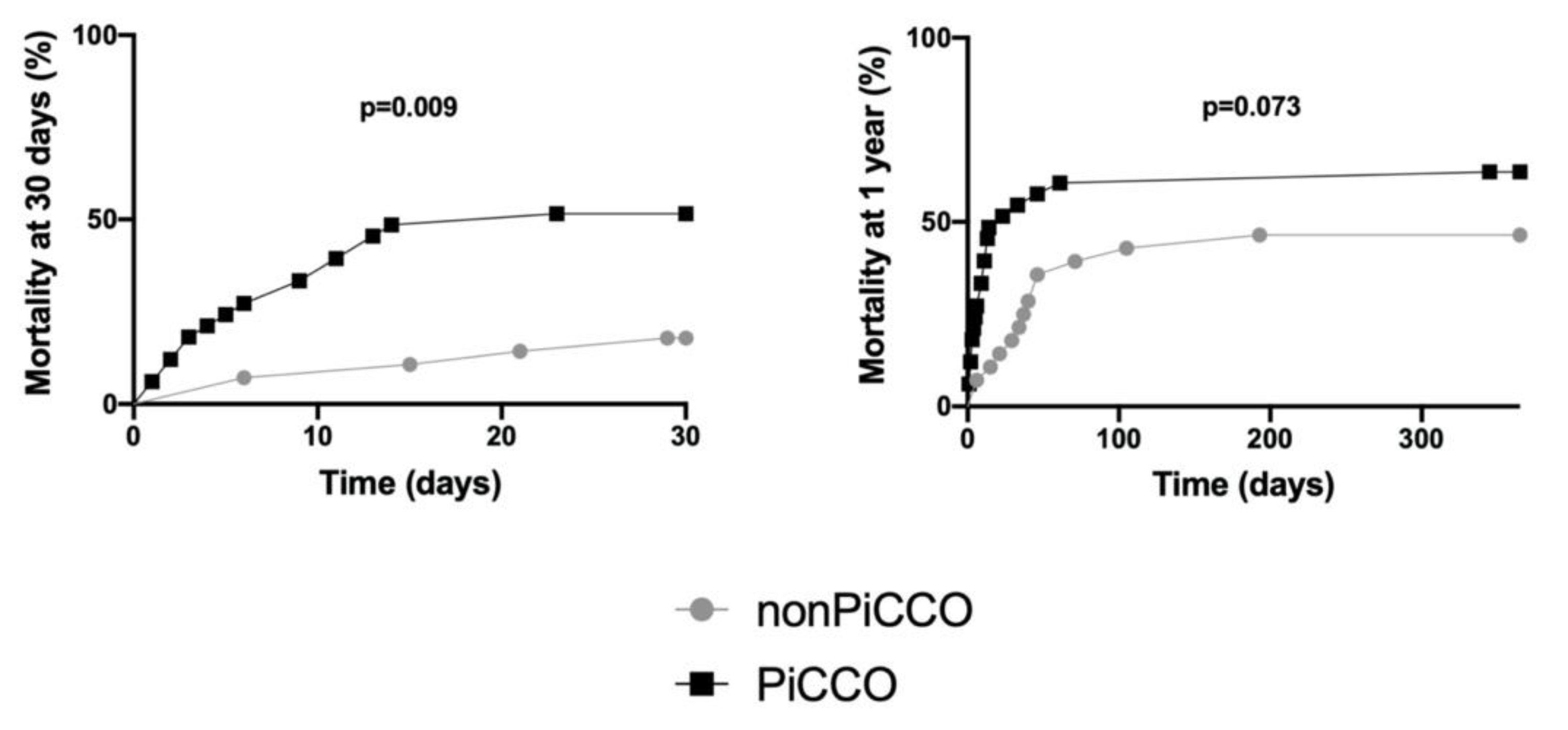
3.1. Characteristics of Patients, PiCCO™ Use, 30 Day and 1 Year Mortality
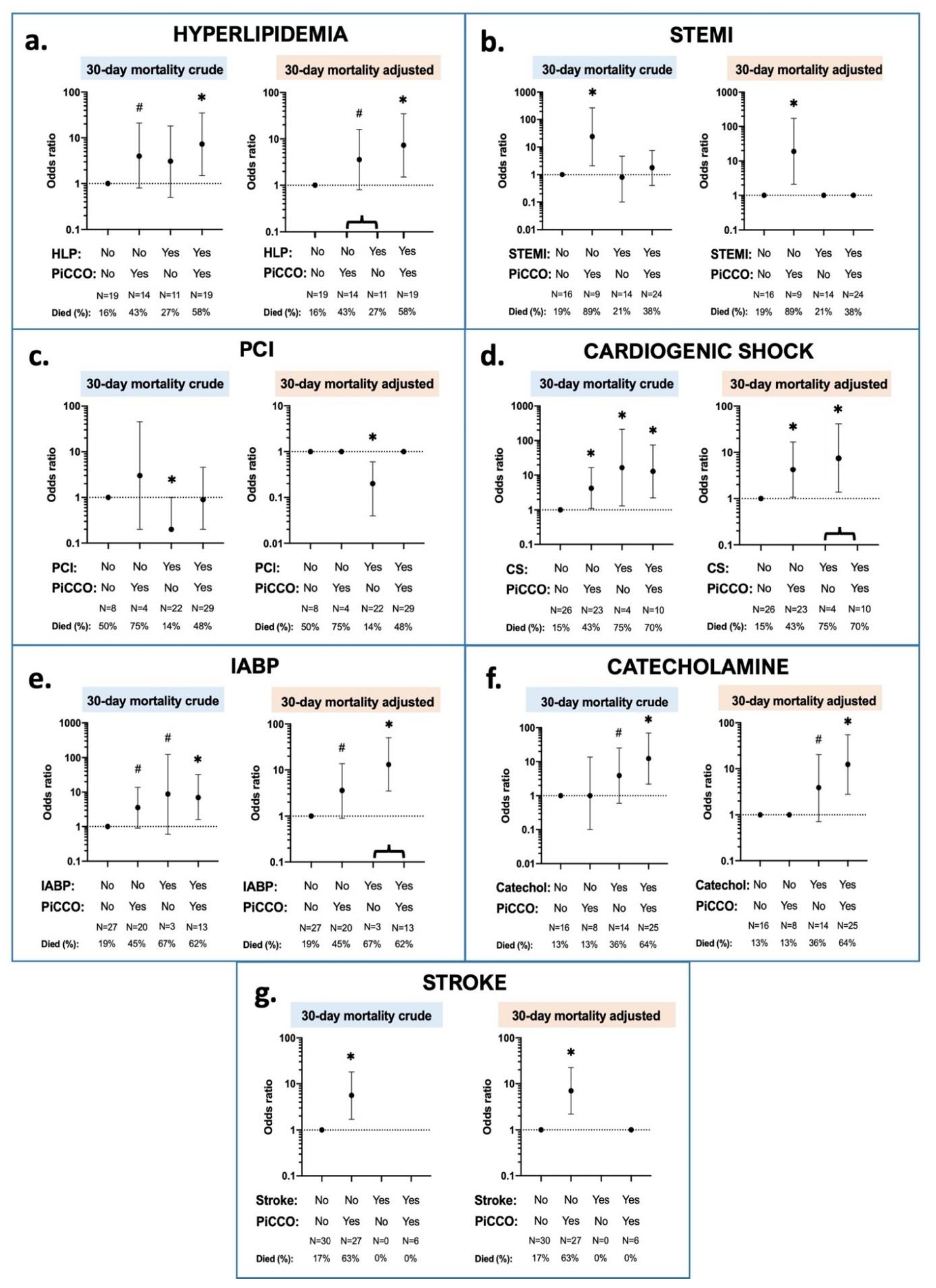
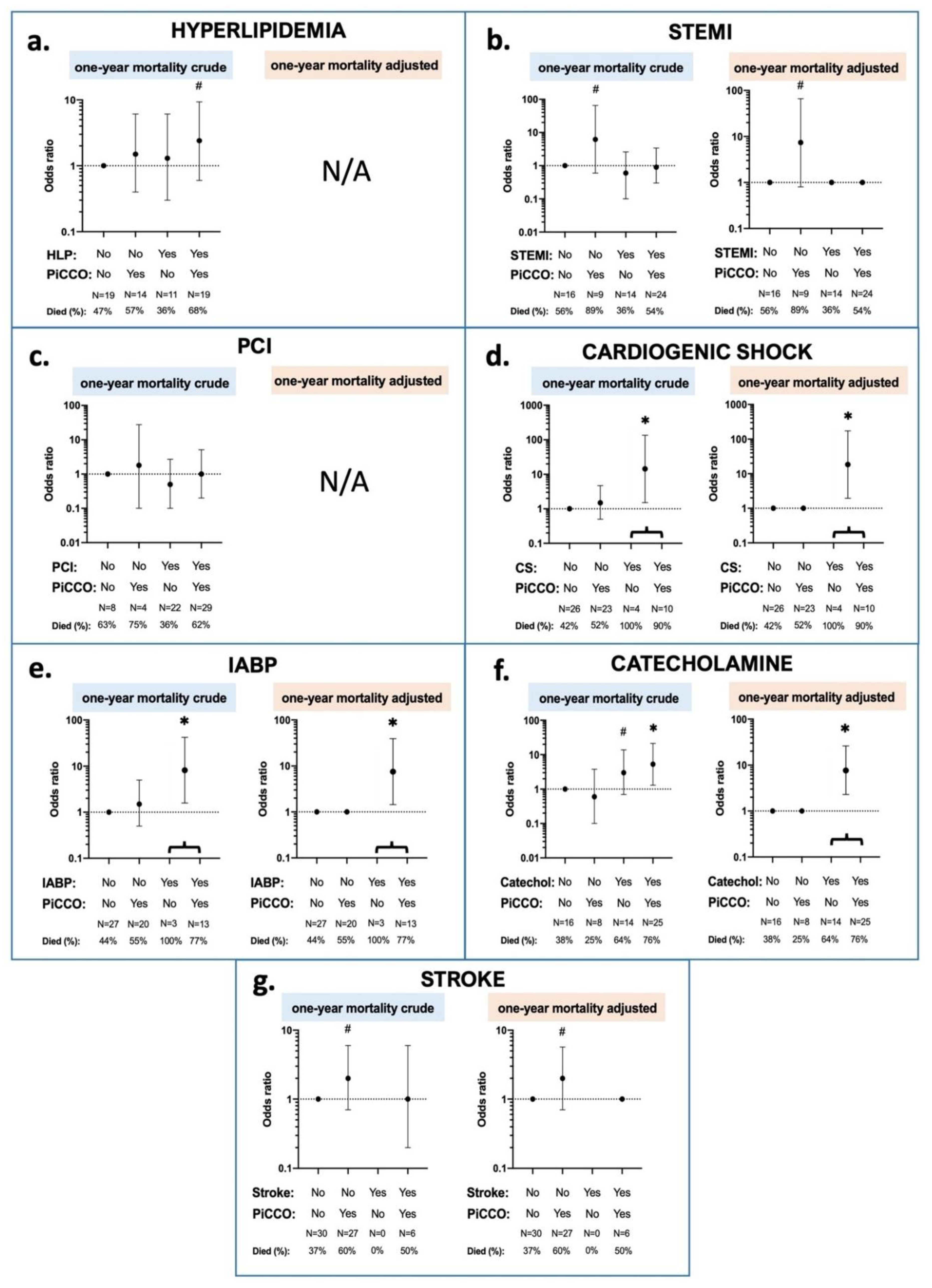
3.2. Interaction Effects between 30 Day Mortality, PiCCO™ Use and Patient Characteristics
3.3. Interaction Effects between 1 Year Mortality, PiCCO™ Use and Patient Characteristics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grasner, J.T.; Wnent, J.; Herlitz, J.; Perkins, G.D.; Lefering, R.; Tjelmeland, I.; Koster, R.W.; Masterson, S.; Rossell-Ortiz, F.; Maurer, H.; et al. Survival after out-of-hospital cardiac arrest in Europe—Results of the EuReCa TWO study. Resuscitation 2020, 148, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Kiguchi, T.; Okubo, M.; Nishiyama, C.; Maconochie, I.; Ong, M.E.H.; Kern, K.B.; Wyckoff, M.H.; McNally, B.; Christensen, E.F.; Tjelmeland, I.; et al. Out-of-hospital cardiac arrest across the World: First report from the International Liaison Committee on Resuscitation (ILCOR). Resuscitation 2020, 152, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Perkins, G.D.; Handley, A.J.; Koster, R.W.; Castren, M.; Smyth, M.A.; Olasveengen, T.; Monsieurs, K.G.; Raffay, V.; Grasner, J.T.; Wenzel, V.; et al. European Resuscitation Council Guidelines for Resuscitation 2015: Section 2. Adult basic life support and automated external defibrillation. Resuscitation 2015, 95, 81–99. [Google Scholar] [CrossRef] [PubMed]
- Nolan, J.P.; Neumar, R.W.; Adrie, C.; Aibiki, M.; Berg, R.A.; Bottiger, B.W.; Callaway, C.; Clark, R.S.; Geocadin, R.G.; Jauch, E.C.; et al. Post-cardiac arrest syndrome: Epidemiology, pathophysiology, treatment, and prognostication. A Scientific Statement from the International Liaison Committee on Resuscitation; the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; the Council on Stroke. Resuscitation 2008, 79, 350–379. [Google Scholar] [CrossRef] [PubMed]
- Nolan, J.P.; Soar, J.; Cariou, A.; Cronberg, T.; Moulaert, V.R.; Deakin, C.D.; Bottiger, B.W.; Friberg, H.; Sunde, K.; Sandroni, C. European Resuscitation Council and European Society of Intensive Care Medicine Guidelines for Post-resuscitation Care 2015: Section 5 of the European Resuscitation Council Guidelines for Resuscitation 2015. Resuscitation 2015, 95, 202–222. [Google Scholar] [CrossRef] [PubMed]
- Hypothermia after Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N. Engl. J. Med. 2002, 346, 549–556. [Google Scholar] [CrossRef]
- Bernard, S.A.; Gray, T.W.; Buist, M.D.; Jones, B.M.; Silvester, W.; Gutteridge, G.; Smith, K. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N. Engl. J. Med. 2002, 346, 557–563. [Google Scholar] [CrossRef]
- Nielsen, N.; Wetterslev, J.; Cronberg, T.; Erlinge, D.; Gasche, Y.; Hassager, C.; Horn, J.; Hovdenes, J.; Kjaergaard, J.; Kuiper, M.; et al. Targeted temperature management at 33 degrees C versus 36 degrees C after cardiac arrest. N. Engl. J. Med. 2013, 369, 2197–2206. [Google Scholar] [CrossRef]
- Kovacs, E.; Jenei, Z.; Horvath, A.; Geller, L.; Szilagyi, S.; Kiraly, A.; Molnar, L.; Sotonyi, P., Jr.; Merkely, B.; Zima, E. Physiologic effects of hypothermia. Orv. Hetil. 2011, 152, 171–181. [Google Scholar] [CrossRef]
- Delhaye, C.; Mahmoudi, M.; Waksman, R. Hypothermia therapy: Neurological and cardiac benefits. J. Am. Coll. Cardiol. 2012, 59, 197–210. [Google Scholar] [CrossRef]
- Sundgreen, C.; Larsen, F.S.; Herzog, T.M.; Knudsen, G.M.; Boesgaard, S.; Aldershvile, J. Autoregulation of cerebral blood flow in patients resuscitated from cardiac arrest. Stroke 2001, 32, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Oren-Grinberg, A. The PiCCO Monitor. Int. Anesthesiol. Clin. 2010, 48, 57–85. [Google Scholar] [CrossRef]
- Werner, M.; Wernly, B.; Lichtenauer, M.; Franz, M.; Kabisch, B.; Muessig, J.M.; Masyuk, M.; Schulze, P.C.; Hoppe, U.C.; Kelm, M.; et al. Real-world extravascular lung water index measurements in critically ill patients: Pulse index continuous cardiac output measurements: Time course analysis and association with clinical characteristics. Wien. Klin. Wochenschr. 2019, 131, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Huber, W.; Umgelter, A.; Reindl, W.; Franzen, M.; Schmidt, C.; von Delius, S.; Geisler, F.; Eckel, F.; Fritsch, R.; Siveke, J.; et al. Volume assessment in patients with necrotizing pancreatitis: A comparison of intrathoracic blood volume index, central venous pressure, and hematocrit, and their correlation to cardiac index and extravascular lung water index. Crit. Care Med. 2008, 36, 2348–2354. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Cui, N.; Su, L.; Long, Y.; Wang, X.; Zhou, X.; Chai, W.; Liu, D. Prognostic value of extravascular lung water and its potential role in guiding fluid therapy in septic shock after initial resuscitation. J. Crit. Care 2016, 33, 106–113. [Google Scholar] [CrossRef]
- Goepfert, M.S.; Reuter, D.A.; Akyol, D.; Lamm, P.; Kilger, E.; Goetz, A.E. Goal-directed fluid management reduces vasopressor and catecholamine use in cardiac surgery patients. Intensive Care Med. 2007, 33, 96–103. [Google Scholar] [CrossRef]
- Tagami, T.; Kushimoto, S.; Tosa, R.; Omura, M.; Hagiwara, J.; Hirama, H.; Yokota, H. The precision of PiCCO(R) measurements in hypothermic post-cardiac arrest patients. Anaesthesia 2012, 67, 236–243. [Google Scholar] [CrossRef]
- Cecconi, M.; De Backer, D.; Antonelli, M.; Beale, R.; Bakker, J.; Hofer, C.; Jaeschke, R.; Mebazaa, A.; Pinsky, M.R.; Teboul, J.L.; et al. Consensus on circulatory shock and hemodynamic monitoring. Task force of the European Society of Intensive Care Medicine. Intensive Care Med. 2014, 40, 1795–1815. [Google Scholar] [CrossRef]
- Nolan, J.P.; Hazinski, M.F.; Aickin, R.; Bhanji, F.; Billi, J.E.; Callaway, C.W.; Castren, M.; de Caen, A.R.; Ferrer, J.M.; Finn, J.C.; et al. Part 1: Executive summary: 2015 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science with Treatment Recommendations. Resuscitation 2015, 95, e1–e31. [Google Scholar] [CrossRef]
- Koster, R.W.; Sayre, M.R.; Botha, M.; Cave, D.M.; Cudnik, M.T.; Handley, A.J.; Hatanaka, T.; Hazinski, M.F.; Jacobs, I.; Monsieurs, K.; et al. Part 5: Adult basic life support: 2010 International consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations. Resuscitation 2010, 81 (Suppl. 1), e48–e70. [Google Scholar] [CrossRef]
- Pulsion Medical System, PiCCO2 Technical Datasheet Ref: MPI850305_R05. Available online: https://www.getinge.com/dam/hospital/documents/english/picco_technology_brochure-en-non_us.pdf (accessed on 13 May 2021).
- Laurent, I.; Monchi, M.; Chiche, J.D.; Joly, L.M.; Spaulding, C.; Bourgeois, B.; Cariou, A.; Rozenberg, A.; Carli, P.; Weber, S.; et al. Reversible myocardial dysfunction in survivors of out-of-hospital cardiac arrest. J. Am. Coll. Cardiol. 2002, 40, 2110–2116. [Google Scholar] [CrossRef]
- Pellis, T.; Sanfilippo, F.; Ristagno, G. The optimal hemodynamics management of post-cardiac arrest shock. Best Pract. Res. Clin. Anaesthesiol. 2015, 29, 485–495. [Google Scholar] [CrossRef]
- Kozar, M.; Javorka, K.; Javorka, M.; Matasova, K.; Zibolen, M. Changes of cardiovascular regulation during rewarming in newborns undergoing whole-body hypothermia. Neuro Endocrinol. Lett. 2015, 36, 434–438. [Google Scholar] [PubMed]
- Demirgan, S.; Erkalp, K.; Sevdi, M.S.; Aydogmus, M.T.; Kutbay, N.; Firincioglu, A.; Ozalp, A.; Alagol, A. Cardiac condition during cooling and rewarming periods of therapeutic hypothermia after cardiopulmonary resuscitation. BMC Anesthesiol. 2014, 14, 78. [Google Scholar] [CrossRef] [PubMed]
- Sunde, K.; Pytte, M.; Jacobsen, D.; Mangschau, A.; Jensen, L.P.; Smedsrud, C.; Draegni, T.; Steen, P.A. Implementation of a standardised treatment protocol for post resuscitation care after out-of-hospital cardiac arrest. Resuscitation 2007, 73, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Gaieski, D.F.; Band, R.A.; Abella, B.S.; Neumar, R.W.; Fuchs, B.D.; Kolansky, D.M.; Merchant, R.M.; Carr, B.G.; Becker, L.B.; Maguire, C.; et al. Early goal-directed hemodynamic optimization combined with therapeutic hypothermia in comatose survivors of out-of-hospital cardiac arrest. Resuscitation 2009, 80, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Beylin, M.E.; Perman, S.M.; Abella, B.S.; Leary, M.; Shofer, F.S.; Grossestreuer, A.V.; Gaieski, D.F. Higher mean arterial pressure with or without vasoactive agents is associated with increased survival and better neurological outcomes in comatose survivors of cardiac arrest. Intensive Care Med. 2013, 39, 1981–1988. [Google Scholar] [CrossRef]
- Kilgannon, J.H.; Roberts, B.W.; Jones, A.E.; Mittal, N.; Cohen, E.; Mitchell, J.; Chansky, M.E.; Trzeciak, S. Arterial blood pressure and neurologic outcome after resuscitation from cardiac arrest*. Crit. Care Med. 2014, 42, 2083–2091. [Google Scholar] [CrossRef]
- Walters, E.L.; Morawski, K.; Dorotta, I.; Ramsingh, D.; Lumen, K.; Bland, D.; Clem, K.; Nguyen, H.B. Implementation of a post-cardiac arrest care bundle including therapeutic hypothermia and hemodynamic optimization in comatose patients with return of spontaneous circulation after out-of-hospital cardiac arrest: A feasibility study. Shock 2011, 35, 360–366. [Google Scholar] [CrossRef]
- Litton, E.; Morgan, M. The PiCCO monitor: A review. Anaesth. Intensive Care 2012, 40, 393–409. [Google Scholar] [CrossRef]
- Swan, H.J.; Ganz, W.; Forrester, J.; Marcus, H.; Diamond, G.; Chonette, D. Catheterization of the heart in man with use of a flow-directed balloon-tipped catheter. N. Engl. J. Med. 1970, 283, 447–451. [Google Scholar] [CrossRef] [PubMed]
- Gassanov, N.; Caglayan, E.; Nia, A.; Erdmann, E.; Er, F. Hemodynamic monitoring in the intensive care unit: Pulmonary artery catheter versus PiCCO. Dtsch. Med. Wochenschr. 2011, 136, 376–380. [Google Scholar] [CrossRef]
- Gassanov, N.; Caglayan, E.; Nia, A.; Erdmann, E.; Er, F. The PiCCO catheter. Dtsch. Med. Wochenschr. 2010, 135, 2311–2314. [Google Scholar] [CrossRef]
- Monnet, X.; Teboul, J.L. Transpulmonary thermodilution: Advantages and limits. Crit. Care 2017, 21, 147. [Google Scholar] [CrossRef] [PubMed]
- Lichtwarck-Aschoff, M.; Zeravik, J.; Pfeiffer, U.J. Intrathoracic blood volume accurately reflects circulatory volume status in critically ill patients with mechanical ventilation. Intensive Care Med. 1992, 18, 142–147. [Google Scholar] [CrossRef]
- Zhang, Y.B.; Zhang, Z.Z.; Li, J.X.; Wang, Y.H.; Zhang, W.L.; Tian, X.L.; Han, Y.F.; Yang, M.; Liu, Y. Application of pulse index continuous cardiac output system in elderly patients with acute myocardial infarction complicated by cardiogenic shock: A prospective randomized study. World J. Clin. Cases 2019, 7, 1291–1301. [Google Scholar] [CrossRef] [PubMed]
- Feneck, R.O.; Sherry, K.M.; Withington, P.S.; Oduro-Dominah, A.; European Milrinone Multicenter Trial, G. Comparison of the hemodynamic effects of milrinone with dobutamine in patients after cardiac surgery. J. Cardiothorac. Vasc. Anesth. 2001, 15, 306–315. [Google Scholar] [CrossRef]
- De Backer, D.; Biston, P.; Devriendt, J.; Madl, C.; Chochrad, D.; Aldecoa, C.; Brasseur, A.; Defrance, P.; Gottignies, P.; Vincent, J.L.; et al. Comparison of dopamine and norepinephrine in the treatment of shock. N. Engl. J. Med. 2010, 362, 779–789. [Google Scholar] [CrossRef]
- Hamzaoui, O.; Jozwiak, M.; Geffriaud, T.; Sztrymf, B.; Prat, D.; Jacobs, F.; Monnet, X.; Trouiller, P.; Richard, C.; Teboul, J.L. Norepinephrine exerts an inotropic effect during the early phase of human septic shock. Br. J. Anaesth. 2018, 120, 517–524. [Google Scholar] [CrossRef]
- Friesenecker, B.E.; Tsai, A.G.; Martini, J.; Ulmer, H.; Wenzel, V.; Hasibeder, W.R.; Intaglietta, M.; Dunser, M.W. Arteriolar vasoconstrictive response: Comparing the effects of arginine vasopressin and norepinephrine. Crit. Care 2006, 10, R75. [Google Scholar] [CrossRef]
- Bai, X.; Yu, W.; Ji, W.; Lin, Z.; Tan, S.; Duan, K.; Dong, Y.; Xu, L.; Li, N. Early versus delayed administration of norepinephrine in patients with septic shock. Crit. Care 2014, 18, 532. [Google Scholar] [CrossRef] [PubMed]

| Patient Characteristics | Total |
|---|---|
| n (%) | |
| or | |
| Median (IQR) | |
| Total | 63 (100%) |
| Age | 64 (56, 69) |
| Gender (female in %) | 19 (30%) |
| IHCA | 11 (17 %) |
| Prior history: | |
| Hypertension | 45 (71%) |
| Diabetes | 18 (29%) |
| Hyperlipidemia | 30 (48%) |
| AMI | 15 (24%) |
| Stroke | 6 (10%) |
| Circumstances of CPR: | |
| Patient on monitor when collapsed | 9 (14%) |
| BLS performed by bystanders | 49 (78%) |
| Time to ROSC (minutes) | 20 (15, 30) |
| Initial rhythm: | |
| VF | 42 (67%) |
| VT | 2 (3%) |
| PEA | 10 (16%) |
| Asystole | 9 (14%) |
| Cause of cardiac arrest: | |
| STEMI | 38 (60%) |
| NSTEMI | 8 (13%) |
| Cardiac condition after ROSC: | |
| Cardiogenic shock (clinical signs) | 14 (22%) |
| EF after ROSC (%) | 36 (29, 48) |
| Therapy after ROSC: | |
| Catecholamine therapy | 39 (62%) |
| Acute PCI | 51 (81%) |
| Levosimendan | 7 (11%) |
| IABP use | 16 (25%) |
| Time to reach target temperature (hours) | 3,8 (2.0, 5.1) |
| PiCCO™ application rate | 33 (52%) |
| Died at 30 days | 24 (38%) |
| Died at 1 year | 36 (57%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kovács, E.; Gyarmathy, V.A.; Pilecky, D.; Fekete-Győr, A.; Szakál-Tóth, Z.; Gellér, L.; Hauser, B.; Gál, J.; Merkely, B.; Zima, E. An Interaction Effect Analysis of Thermodilution-Guided Hemodynamic Optimization, Patient Condition, and Mortality after Successful Cardiopulmonary Resuscitation. Int. J. Environ. Res. Public Health 2021, 18, 5223. https://doi.org/10.3390/ijerph18105223
Kovács E, Gyarmathy VA, Pilecky D, Fekete-Győr A, Szakál-Tóth Z, Gellér L, Hauser B, Gál J, Merkely B, Zima E. An Interaction Effect Analysis of Thermodilution-Guided Hemodynamic Optimization, Patient Condition, and Mortality after Successful Cardiopulmonary Resuscitation. International Journal of Environmental Research and Public Health. 2021; 18(10):5223. https://doi.org/10.3390/ijerph18105223
Chicago/Turabian StyleKovács, Enikő, Valéria Anna Gyarmathy, Dávid Pilecky, Alexandra Fekete-Győr, Zsófia Szakál-Tóth, László Gellér, Balázs Hauser, János Gál, Béla Merkely, and Endre Zima. 2021. "An Interaction Effect Analysis of Thermodilution-Guided Hemodynamic Optimization, Patient Condition, and Mortality after Successful Cardiopulmonary Resuscitation" International Journal of Environmental Research and Public Health 18, no. 10: 5223. https://doi.org/10.3390/ijerph18105223
APA StyleKovács, E., Gyarmathy, V. A., Pilecky, D., Fekete-Győr, A., Szakál-Tóth, Z., Gellér, L., Hauser, B., Gál, J., Merkely, B., & Zima, E. (2021). An Interaction Effect Analysis of Thermodilution-Guided Hemodynamic Optimization, Patient Condition, and Mortality after Successful Cardiopulmonary Resuscitation. International Journal of Environmental Research and Public Health, 18(10), 5223. https://doi.org/10.3390/ijerph18105223







