The Impact of Illegal Waste Sites on the Transmission of Zoonotic Cutaneous Leishmaniasis in Central Tunisia
Abstract
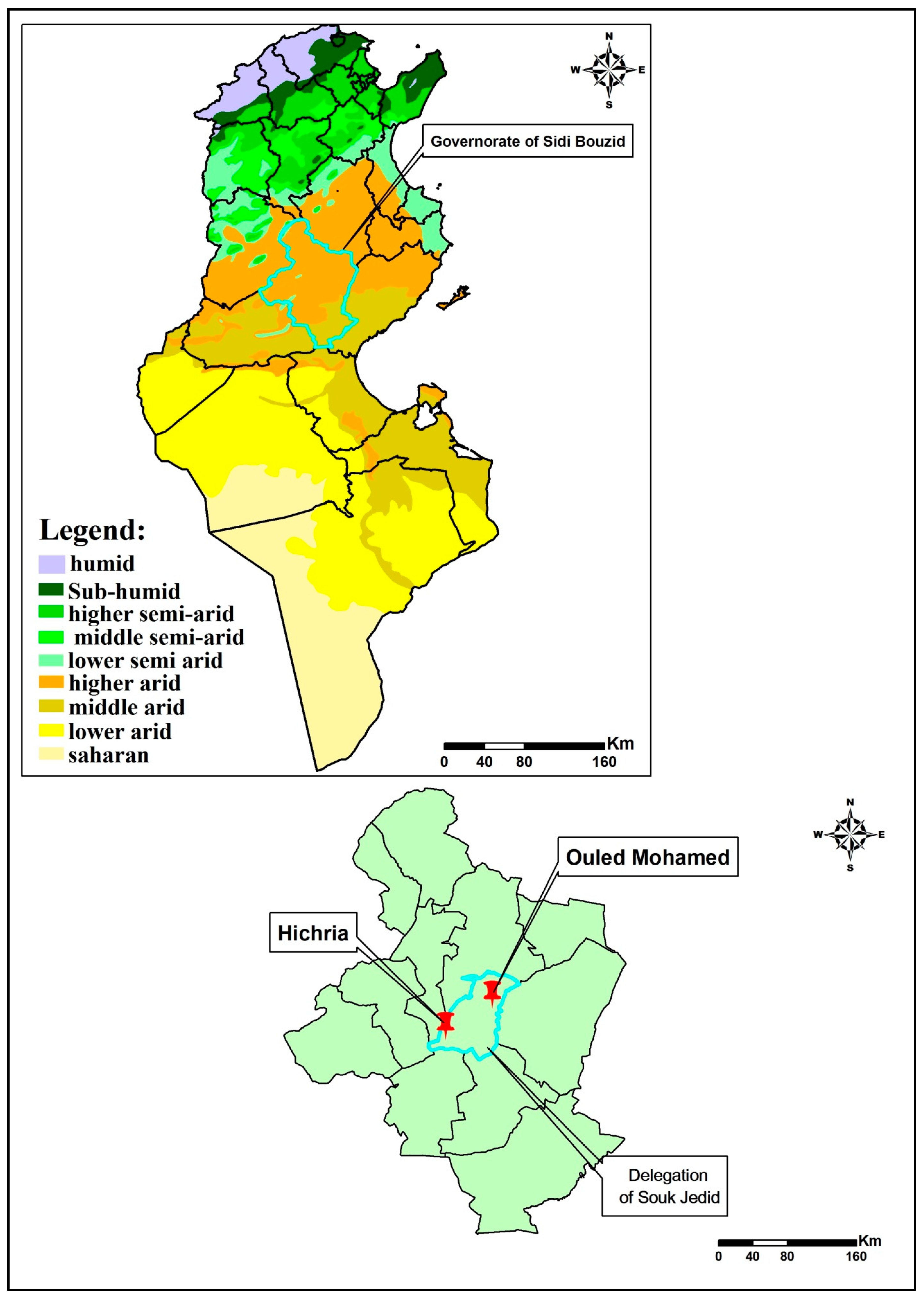
1. Introduction
2. Materials and Methods
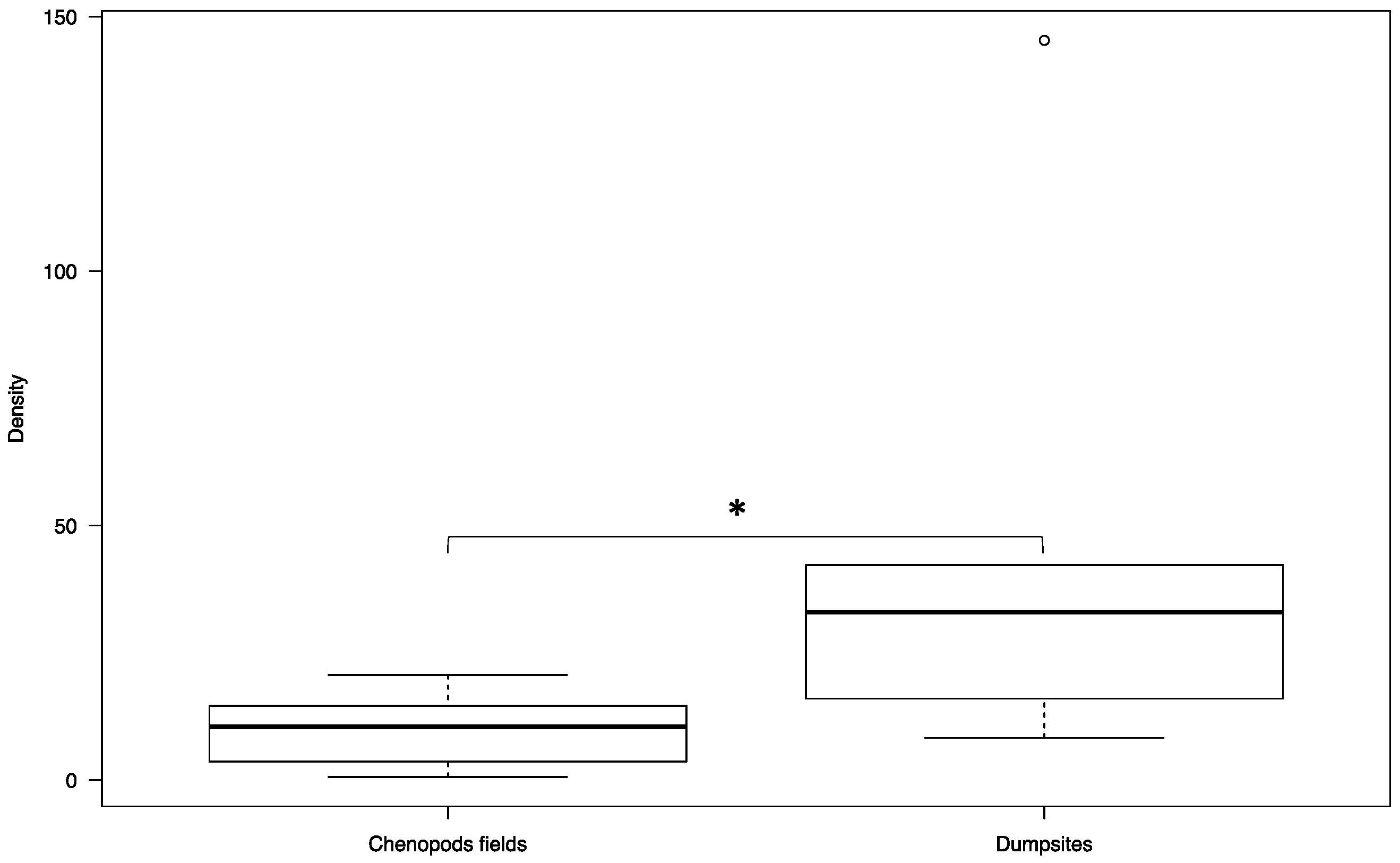
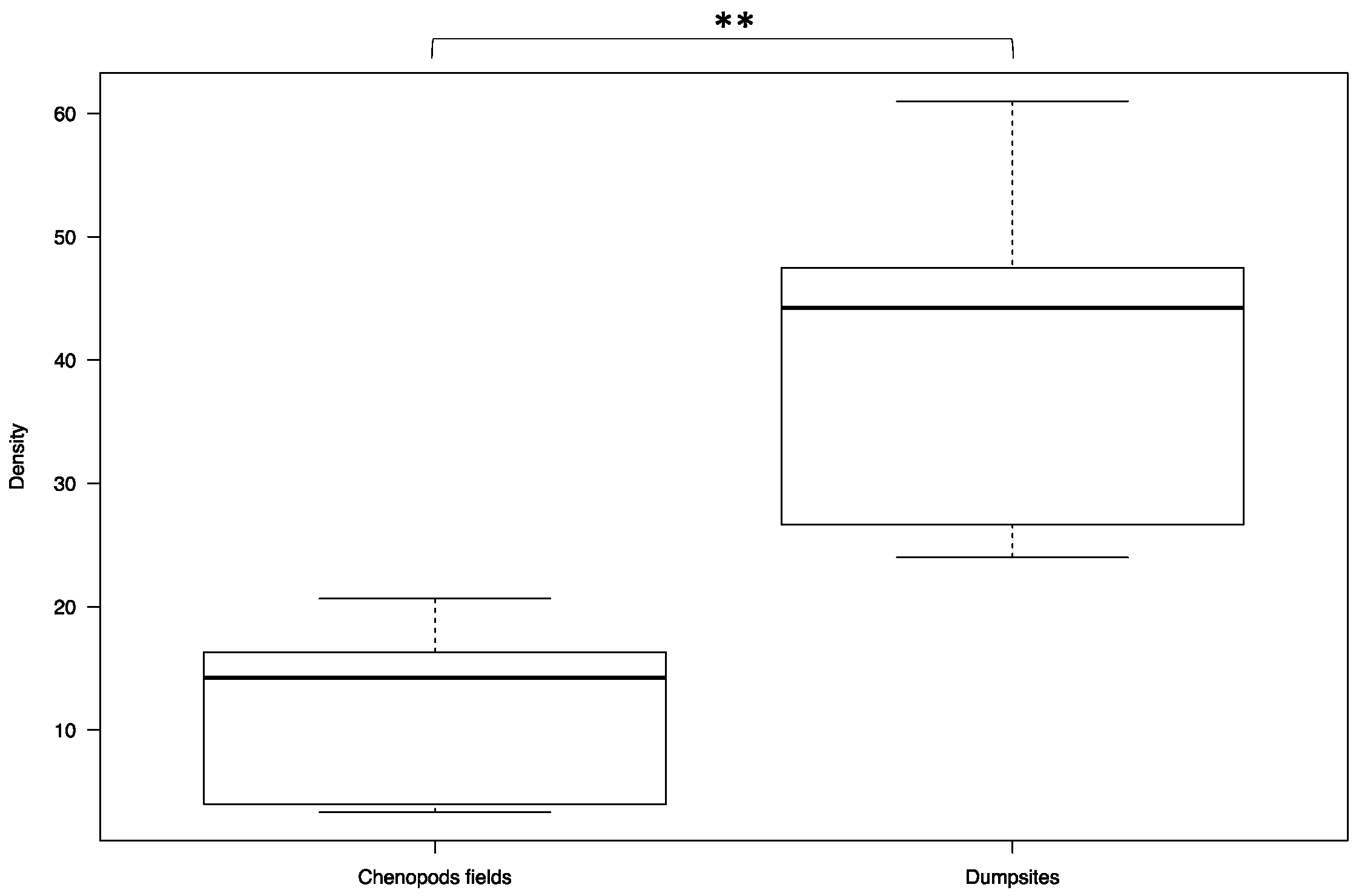
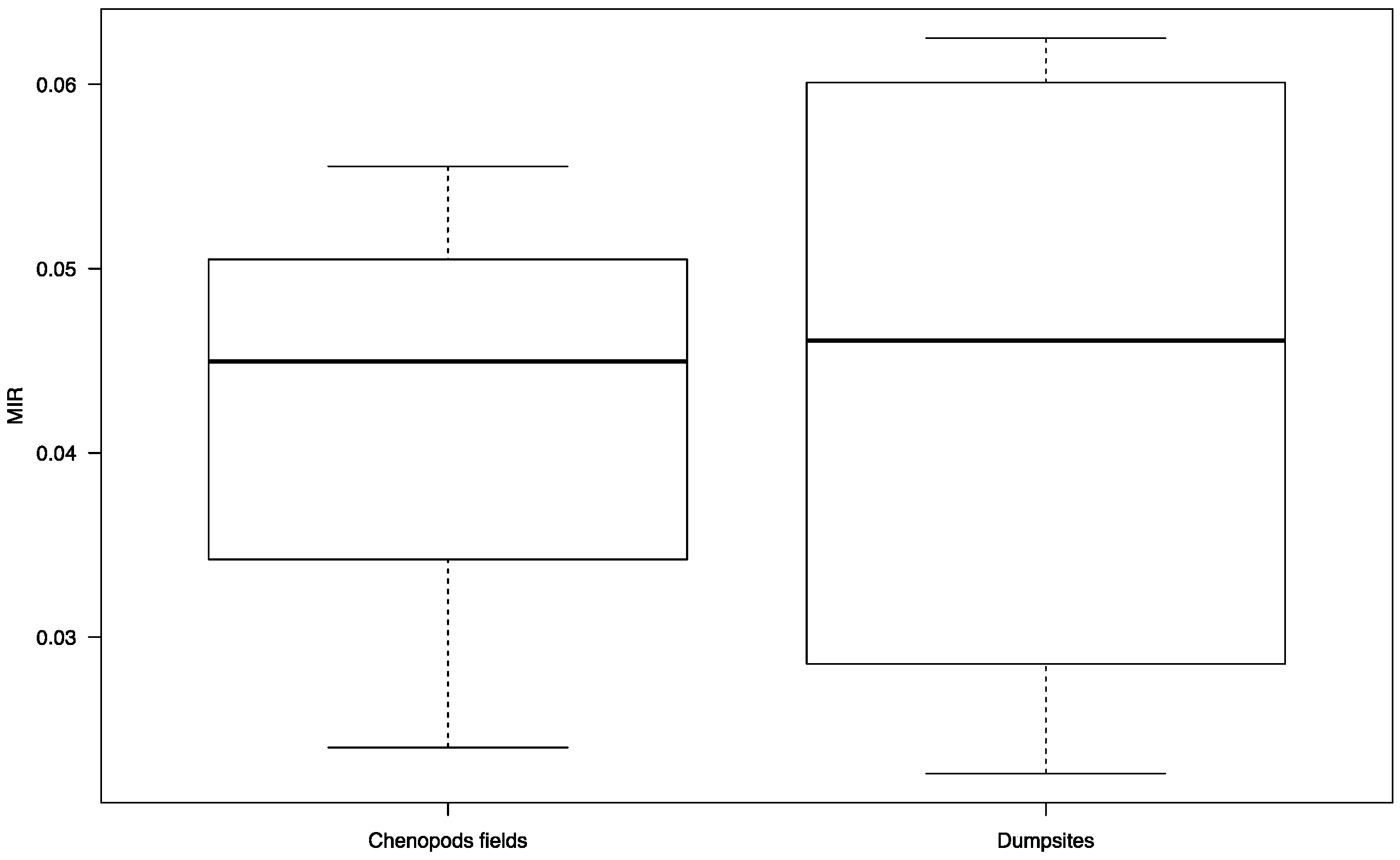
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chelbi, I.; Derbali, M.; AL-Ahmadi, Z.; Zaafouri, B.; El Fahem, A.; Zhioua, E. Phenology of Phlebotomus papatasi (Diptera: Psychodidae) relative to the seasonal prevalence of zoonotic cutaneous leishmaniasis in Central Tunisia. J. Med. Entomol. 2007, 44, 385–388. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chelbi, I.; Kaabi, B.; Bejaoui, M.; Derbali, M.; Zhioua, E. Spatial Correlation between Phlebotomus papatasi Scopoli (Diptera: Psychodidae) and Incidence of Zoonotic Cutaneous Leishmaniasis in Tunisia. J. Med Ѐntomol. 2009, 46, 400–402. [Google Scholar] [CrossRef] [PubMed]
- Ismail, R.B.; Gramiccia, M.; Gradoni, L.; Helal, H.; Rachid, M.B. Isolation of Leishmania major from Phlebotomus papatasi in Tunisia. Trans. R. Soc. Trop. Med. Hyg. 1987, 81, 749. [Google Scholar] [CrossRef]
- Derbali, M.; Chelbi, I.; Ahmed, S.B.H.; Zhioua, E. Leishmania major Yakimoff et Schokhor, 1914 (Kinetoplastida: Trypanosomatidae) chez Meriones shawi Duvernoy, 1842 (Rodentia: Gerbillidae): Persistance de l’infection du mérion et de son infectivité pour le phlébotome vecteur Phlebotomus (Phlebotomus) papatasi Scopoli, 1786 (Diptera: Psychodidae). Bull. Société Pathol. Exot. 2012, 105, 399–402. [Google Scholar] [CrossRef]
- Rioux, J.A.; Petter, F.; Zahaf, A.; Lanotte, G.; Houin, R.; Jarry, D.; Perieres, J.; Martini, A.; Sarhani, S. Isolement de Leishmania major Yakimoff et Schokhor, 1914 [Kinetoplastida-Trypanosomatidae] chez Meriones shawi (Duvernoy, 1842) [Rodentia-Gerbillidae] en Tunisie. Ann. Parasitol. Hum. Comparée 1986, 61, 139–145. [Google Scholar] [CrossRef]
- Ismail, R.B.; Rachid, M.S.B.; Gardoni, L.; Gramiccia, M.; Bach-Hamba, D. La leishmaniose cutanée zoonotique en Tunisie; étude du réservoir dans le foyer de Douara. Ann. Soc. Belge Méd. Trop. 1987, 67, 335–343. [Google Scholar]
- Rioux, J.A.; Ashford, R.W.; Khiami, A. Ecoepidemiology of leishmaniases in Syria. 3. Leishmania major infection in Psammomys obesus provides clues to life history of the rodent and possible control measures. Ann. Parasitol. Hum. Comparée 1992, 67, 163–165. [Google Scholar] [CrossRef]
- Fichet-Calvet, E.; Jomâa, I.; Ismail, R.B.; Ashford, R.W. Leishmania major infection in the fat sand rat Psammomys obesus in Tunisia: Interaction of host and parasite populations. Ann. Trop. Med. Parasitol. 2003, 97, 593–603. [Google Scholar] [CrossRef]
- Ghawar, W.; Toumi, A.; Snoussi, M.-A.; Chlif, S.; Zaatour, A.; Boukthir, A.; Hamida, N.B.H.; Chemkhi, J.; Diouani, M.F.; Ben-Salah, A. Leishmania major Infection Among Psammomys Obesus and Meriones Shawi: Reservoirs of Zoonotic Cutaneous Leishmaniasis in Sidi Bouzid (Central Tunisia). Vector Borne Zoonotic Dis. 2011, 11, 1561–1568. [Google Scholar] [CrossRef]
- Derbali, M.; Polyakova, L.; Boujaâma, A.; Burruss, D.; Cherni, S.; Barhoumi, W.; Chelbi, I.; Poché, R.; Zhioua, E. Laboratory and field evaluation of rodent bait treated with fipronil for feed through and systemic control of Phlebotomus papatasi. Acta Trop. 2014, 135, 27–32. [Google Scholar] [CrossRef]
- Chahed, M.K.; Bellali, H.; Ben Jemaa, S.; Bellaj, T. Psychological and Psychosocial Consequences of Zoonotic Cutaneous Leishmaniasis among Women in Tunisia: Preliminary Findings from an Exploratory Study. PLoS Negl. Trop. Dis. 2016, 10, e0005090. [Google Scholar] [CrossRef] [PubMed]
- Ammar, R.B.; Ismail, R.B.; Helal, H.; Bach-Hamba, D.; Chaouch, A.; Bouden, L.; Hanachi, A.; Zemzari, A.; Rachid, M.S.B. Un nouveau foyer de leishmaniose cutanée de type rural dans la region de Sidi Saad. Bull. Soc. Fr. Parasitol. 1984, 2, 9–12. [Google Scholar]
- Salah, A.B.; Kamarianakis, Y.; Chlif, S.; Alaya, N.B.; Prastacos, P.; Salah, A.B.; Alaya, N.B. Zoonotic cutaneous leishmaniasis in central Tunisia: Spatio temporal dynamics. Int. J. Epidemiol. 2007, 36, 991–1000. [Google Scholar] [CrossRef] [PubMed]
- Fichet-Calvet, E.; Jomâa, I.; Zaafouri, B.; Asford, R.W.; Ismail, R.B.; Delattre, P. The spatio-temporal distribution of a rodent reservoir host of cutaneous leishmaniais. J. Appl. Ecol. 2000, 37, 603–615. [Google Scholar] [CrossRef]
- Cherni, S. The Impact of Household Wastes on the Pullulating of Rodent Reservoir Host of Leishmania major in Central Tunisia. Master’s Thesis, University of Tunis El Manar, Tunis, Tunisia, 2017. [Google Scholar]
- Müller, G.C.; Kravchenko, V.D.; Rybalov, L.; Schlein, Y. Characteristics of resting and breeding habitats of adult sand flies in the Judean Desert. J. Vector Ecol. 2011, 36 (Suppl. 1), S195–S205. [Google Scholar] [CrossRef]
- Croset, H.; Rioux, J.-A.; Maistre, M.; Bayar, N. Les Phlébotomes de Tunisie (Diptera, Phlebotomidae). Mise au point systématique, chorologique et éthologique. Ann. Parasitol. Hum. Comparée 1978, 53, 711–749. [Google Scholar] [CrossRef]
- Noyes, H.A.; Reyburn, H.; Bailey, J.W.; Smith, D. A Nested-PCR-Based Schizodeme Method for Identifying Leishmania Kinetoplast Minicircle Classes Directly from Clinical Samples and Its Application to the Study of the Epidemiology of Leishmania tropica in Pakistan. J. Clin. Microbiol. 1998, 36, 2877–2881. [Google Scholar] [CrossRef]
- Barhoumi, W.; Fares, W.; Cherni, S.; Derbali, M.; Dachraoui, K.; Chelbi, I.; Ramalho-Ortigão, M.; Beier, J.C.; Zhioua, E. Changes of Sand Fly Populations and Leishmania infantum Infection Rates in an Irrigated Village Located in Arid Central Tunisia. Int. J. Environ. Res. Public Health 2016, 13, 329. [Google Scholar] [CrossRef]
- Fichet-Calvet, E.; Jomâa, I.; Giraudoux, P.; Asford, R.W. Estimation of sand rat abundance by using surface indices. Acta. Theriol. 1999, 44, 353–362. [Google Scholar] [CrossRef]
- Krystosik, A.; Njoroge, G.; Odhiambo, L.; Forsyth, J.E.; Mutuku, F.; LaBeaud, A.D. Solid Wastes Provide Breeding Sites, Burrows, and Food for Biological Disease Vectors, and Urban Zoonotic Reservoirs: A Call to Action for Solutions-Based Research. Front. Public Health 2020, 7, 405. [Google Scholar] [CrossRef]
- Bellali, H.; Hchaichi, A.; Harizi, C.; Mrabet, A.; Chahed, M.K. Comparison between active surveillance and passive detection of zoonotic cutaneous leishmaniasis in endemic rural areas in Central Tunisia, 2009 to 2014. Asian Pac. J. Trop. Dis. 2015, 5, 515–519. [Google Scholar] [CrossRef]
- Orshan, L.; Elbaz, S.; Ben-Ari, Y.; Akad, F.; Afik, O.; Ben-Avi, I.; Dias, D.; Ish-Shalom, D.; Studentsky, L.; Zonstein, I. Distribution and Dispersal of Phlebotomus papatasi (Diptera: Psychodidae) in a Zoonotic Cutaneous Leishmaniasis Focus, the Northern Negev, Israel. PLoS Negl. Trop. Dis. 2016, 10, e0004819. [Google Scholar] [CrossRef] [PubMed]
- Mbarki, L.; Salah, A.B.; Chlif, S.; Chahed, M.K.; Balma, A.; Chemem, N.; Garraoui, A.; Ismail, R.B. Monitoring zoonotic cutaneous leishmaniasis with GIS. In GIS for Health and Environment; Savigny, D., Wijeyaratne, P., Eds.; IDCR-Ottawa Publications: Ottawa, ON, Canada, 1995; pp. 115–125. [Google Scholar]
- Banerjee, S.; Aditya, G.; Saha, G.K. Household Wastes as Larval Habitats of Dengue Vectors: Comparison between Urban and Rural Areas of Kolkata, India. PLoS ONE 2015, 10, e0138082. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Zanzi, C.; Mason, M.; Encina, C.; Gonzalez, M.; Berg, S. Household Characteristics Associated with Rodent Presence and Leptospira Infection in Rural and Urban Communities from Southern Chile. Am. J. Trop. Med. Hyg. 2014, 90, 497–506. [Google Scholar] [CrossRef]
- Ivovic, V.; Potusek, S.; Buzan, E. Prevalence and genotype identification of Toxoplasma gondii in suburban rodents collected at waste disposal sites. Parasite 2019, 26, 27. [Google Scholar] [CrossRef]
- Duh, D.; Hasic, S.; Buzan, E. The impact of illegal waste sites on a transmission of zoonotic viruses. Virol. J. 2017, 14, 134. [Google Scholar] [CrossRef]
- Costa, C.H.N.; Werneck, G.L.; Rodrigues, L.; Santos, M.V.; Araújo, I.B.; Moura, L.S.; Moreira, S.; Gomes, R.B.B.; Lima, S.S. Household structure and urban services: Neglected targets in the control of visceral leishmaniasis. Ann. Trop. Med. Parasitol. 2005, 99, 229–236. [Google Scholar] [CrossRef]
- Singh, S.P.; Mishra, R.N.; Reddy, D.C.S.; Sundar, S. Knowledge, attitude, and practices related to kala-azar in a rural area of bihar state, india. Am. J. Trop. Med. Hyg. 2006, 75, 505–508. [Google Scholar] [CrossRef]
- Freeman, M.C.; Ogden, S.; Jacobson, J.; Abbott, D.; Addiss, D.G.; Amnie, A.G.; Beckwith, C.; Cairncross, S.; Callejas, R.; Jr, J.M.C.; et al. Integration of Water, Sanitation, and Hygiene for the Prevention and Control of Neglected Tropical Diseases: A Rationale for Inter-Sectoral Collaboration. PLoS Negl. Trop. Dis. 2013, 7, e2439. [Google Scholar] [CrossRef]



| Years | 2017 | 2018 | ||||
|---|---|---|---|---|---|---|
| Sites | Hichria | Ouled Mohamed | Hichria | Ouled Mohamed | ||
| Total numbers of sandflies | 666 | 528 | 361 | 291 | ||
| Genus | Phlebotomus | Phlebotomus | Phlebotomus | Segentomyia | Phlebotomus | |
| Species | papatasi | perniciosus | papatasi | papatasi | minuta | papatasi |
| Sex (F/M) | 205/453 | 3/5 | 148/380 | 72/285 | 0/4 | 108/183 |
| Abundance (%) | 98.8 | 1.2 | 100 | 98.2 | 1.2 | 100 |
| Villages | Sites | N. Pools/Total Number of Females | N. Positive Pools/Total Number of Females | Minimum Infection Rate | |||
|---|---|---|---|---|---|---|---|
| 2017 | 2018 | 2017 | 2018 | 2017 | 2018 | ||
| Hichria | Dumpsites | 7/177 | 3/52 | 4/177 | 3/52 | 2.25 | 5.76 |
| Chenopod fields | 1/29 | 1/16 | 1/29 | 1/16 | 3.44 | 6.25 | |
| Ouled Mohamed | Dumpsites | 5/125 | 5/90 | 3/125 | 4/90 | 2.44 | 4.44 |
| Chenopod fields | 1/22 | 1/18 | 1/22 | 1/18 | 4.54 | 5.55 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chelbi, I.; Mathlouthi, O.; Zhioua, S.; Fares, W.; Boujaama, A.; Cherni, S.; Barhoumi, W.; Dachraoui, K.; Derbali, M.; Abbass, M.; et al. The Impact of Illegal Waste Sites on the Transmission of Zoonotic Cutaneous Leishmaniasis in Central Tunisia. Int. J. Environ. Res. Public Health 2021, 18, 66. https://doi.org/10.3390/ijerph18010066
Chelbi I, Mathlouthi O, Zhioua S, Fares W, Boujaama A, Cherni S, Barhoumi W, Dachraoui K, Derbali M, Abbass M, et al. The Impact of Illegal Waste Sites on the Transmission of Zoonotic Cutaneous Leishmaniasis in Central Tunisia. International Journal of Environmental Research and Public Health. 2021; 18(1):66. https://doi.org/10.3390/ijerph18010066
Chicago/Turabian StyleChelbi, Ifhem, Olfa Mathlouthi, Sami Zhioua, Wasfi Fares, Anis Boujaama, Saifedine Cherni, Walid Barhoumi, Khalil Dachraoui, Mohamed Derbali, Mohamed Abbass, and et al. 2021. "The Impact of Illegal Waste Sites on the Transmission of Zoonotic Cutaneous Leishmaniasis in Central Tunisia" International Journal of Environmental Research and Public Health 18, no. 1: 66. https://doi.org/10.3390/ijerph18010066
APA StyleChelbi, I., Mathlouthi, O., Zhioua, S., Fares, W., Boujaama, A., Cherni, S., Barhoumi, W., Dachraoui, K., Derbali, M., Abbass, M., & Zhioua, E. (2021). The Impact of Illegal Waste Sites on the Transmission of Zoonotic Cutaneous Leishmaniasis in Central Tunisia. International Journal of Environmental Research and Public Health, 18(1), 66. https://doi.org/10.3390/ijerph18010066






