Reported Neighborhood Traffic and the Odds of Asthma/Asthma-Like Symptoms: A Cross-Sectional Analysis of a Multi-Racial Cohort of Children
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Questionnaires
2.3. Exposure Variables
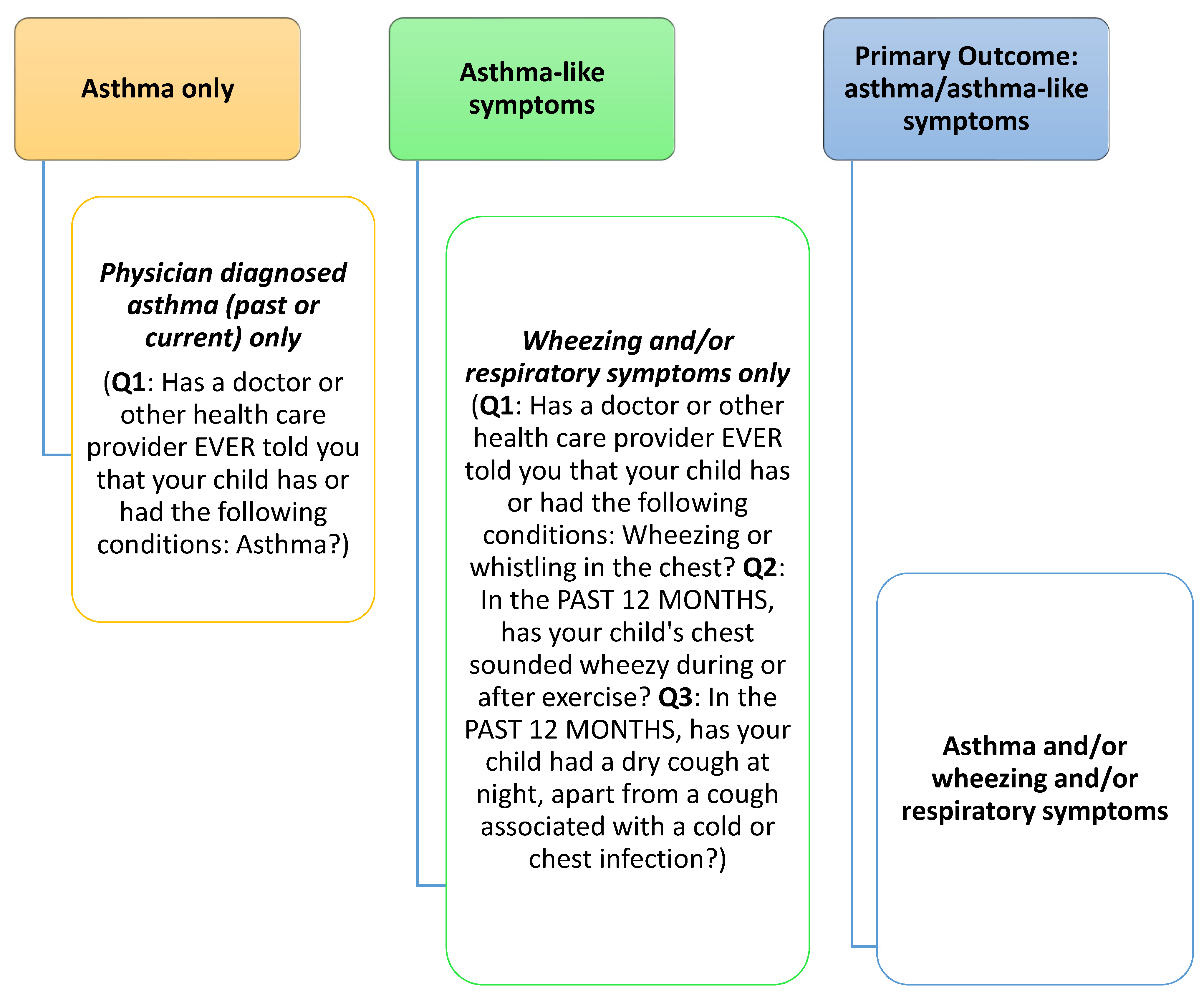
2.4. Outcome Variable
2.5. Covariates
2.6. Statistical Analysis
3. Results
Overview
- Differences by race-ethnic group
- Bivariate analysis—neighborhood traffic
- Bivariate analysis—asthma/asthma-like symptoms
- Multiple logistic regression models
- Sensitivity analysis
- Other variables independently associated with asthma/asthma-like symptoms
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Variable | Participants in Current Study (n = 855) | Participants Eligible for ECHO Study (n = 2373) | p-Value for χ2 or t Test |
|---|---|---|---|
| Mother’s age, mean (SD) in years | 28.6 (5.6) | 28.3 (5.5) | 0.2 |
| Mother’s education | <0.0001 | ||
| Less than or up to high school | 21.6% | 29.6% | |
| More than high school | 78.4% | 70.4% | |
| Mother’s race | <0.0001 | ||
| Non-Hispanic White (NHW) | 34.0% | 26.3% | |
| Non-Hispanic Black (NHB) | 29.6% | 27.8% | |
| Hispanic | 22.8% | 28.7% | |
| Asian | 13.6% | 17.2% | |
| Male child | 51.2% | 50.8% | 0.8 |
| Child’s median year of birth (range) | 2011 (2010 to 2013) | 2011 (2009 to 2013) | 0.5 |
| Child’s gestational age at delivery, mean (SD) in weeks | 39.2 (1.5) | 39.1 (1.9) | 0.6 |
| Child’s birthweight, mean (SD) in kg | 3.2 (0.5) | 3.3 (0.5) | 0.06 |
References
- Wenzel, S.E. Asthma phenotypes: The evolution from clinical to molecular approaches. Nat. Med. 2012, 18, 716–725. [Google Scholar] [CrossRef] [PubMed]
- Hekking, P.-P.W.; Bel, E.H. Developing and emerging clinical asthma phenotypes. J. Allergy Clin. Immunol. Pr. 2014, 2, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Martinez, F.D. Genes, environments, development and asthma: A reappraisal. Eur. Respir. J. 2007, 29, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Pierangeli, I.; Nieuwenhuijsen, M.J.; Cirach, M.; Rojas-Rueda, D. Health equity and burden of childhood asthma—Related to air pollution in Barcelona. Environ. Res. 2020, 186, 109067. [Google Scholar] [CrossRef]
- Gong, T.; Lundholm, C.; Rejnö, G.; Mood, C.; Långström, N.; Almqvist, C. Parental socioeconomic status, childhood asthma and medication use—A population-based study. PLoS ONE 2014, 9, e106579. [Google Scholar] [CrossRef]
- Ehteshami-Afshar, S.; FitzGerald, J.; Doyle-Waters, M.; Sadatsafavi, M. The global economic burden of asthma and chronic obstructive pulmonary disease. Int. J. Tuberc. Lung Dis. 2016, 20, 11–23. [Google Scholar] [CrossRef]
- Nurmagambetov, T.; Khavjou, O.; Murphy, L.; Orenstein, D. State-level medical and absenteeism cost of asthma in the United States. J. Asthma 2017, 54, 357–370. [Google Scholar] [CrossRef]
- Sullivan, P.W.; Slejko, J.F.; Ghushchyan, V.H.; Sucher, B.; Globe, D.R.; Lin, S.-L.; Globe, G. The relationship between asthma, asthma control and economic outcomes in the United States. J. Asthma 2014, 51, 769–778. [Google Scholar] [CrossRef]
- American Lung Association. Epidemiology & Statistics Unit, Research and Program Services. Trends in Asthma Morbidity and Mortality. September 2012. 2012. Available online: http://www.lung.org/assets/documents/research/asthma-trend-report.pdf (accessed on 26 June 2017).
- Nurmagambetov, T.; Kuwahara, R.; Garbe, P. The Economic Burden of Asthma in the United States, 2008–2013. Ann. Am. Thorac. Soc. 2018, 15, 348–356. [Google Scholar] [CrossRef]
- Gilmour, M.I.; Jaakkola, M.S.; London, S.J.; Nel, A.E.; Rogers, C.A. How exposure to environmental tobacco smoke, outdoor air pollutants, and increased pollen burdens influences the incidence of asthma. Environ. Health Perspect. 2006, 114, 627–633. [Google Scholar] [CrossRef]
- Braback, L.; Forsberg, B. Does traffic exhaust contribute to the development of asthma and allergic sensitization in children: Findings from recent cohort studies. Environ. Health 2009, 8, 17. [Google Scholar] [CrossRef] [PubMed]
- Gaviola, C.; Miele, C.H.; Wise, R.A.; Gilman, R.H.; Jaganath, D.; Miranda, J.J.; Bernabe-Ortiz, A.; Hansel, N.N.; Checkley, W. Urbanisation but not biomass fuel smoke exposure is associated with asthma prevalence in four resource-limited settings. Thorax 2016, 71, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Venn, A.J.; Lewis, S.A.; Cooper, M.; Hubbard, R.; Britton, J. Living near a main road and the risk of wheezing illness in children. Am. J. Respir. Crit. Care Med. 2001, 164, 2177–2180. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Salam, M.T.; Eckel, S.P.; Breton, C.V.; Gilliland, F.D. Chronic effects of air pollution on respiratory health in Southern California children: Findings from the Southern California Children’s Health Study. J. Thorac. Dis. 2015, 7, 46–58. [Google Scholar]
- Escamilla-Nunez, M.C.; Barraza-Villarreal, A.; Hernandez-Cadena, L.; Moreno-Macias, H.; Ramirez-Aguilar, M.; Sienra-Monge, J.J.; Cortez-Lugo, M.; Texcalac, J.L.; del Rio-Navarro, B.; Romieu, I. Traffic-related air pollution and respiratory symptoms among asthmatic children, resident in Mexico City: The EVA cohort study. Respir. Res. 2008, 9, 74. [Google Scholar] [CrossRef]
- Rice, M.B.; Rifas-Shiman, S.L.; Litonjua, A.A.; Gillman, M.W.; Liebman, N.; Kloog, I.; Luttmann-Gibson, H.; Coull, B.A.; Schwartz, J.; Koutrakis, P.; et al. Lifetime air pollution exposure and asthma in a pediatric birth cohort. J. Allergy Clin. Immunol. 2018, 141, 1932–1934. [Google Scholar] [CrossRef]
- Brunst, K.J.; Ryan, P.H.; Brokamp, C.; Bernstein, D.; Reponen, T.; Lockey, J.; Khurana Hershey, G.K.; Levin, L.; Grinshpun, S.A.; LeMasters, G. Timing and Duration of Traffic-related Air Pollution Exposure and the Risk for Childhood Wheeze and Asthma. Am. J. Respir. Crit. Care Med. 2015, 192, 421–427. [Google Scholar] [CrossRef]
- Gehring, U.; Wijga, A.H.; Hoek, G.; Bellander, T.; Berdel, D.; Brüske, I.; Fuertes, E.; Gruzieva, O.; Heinrich, J.; Hoffmann, B.; et al. Exposure to air pollution and development of asthma and rhinoconjunctivitis throughout childhood and adolescence: A population-based birth cohort study. Lancet Respir. Med. 2015, 3, 933–942. [Google Scholar] [CrossRef]
- McCreanor, J.; Cullinan, P.; Nieuwenhuijsen, M.J.; Stewart-Evans, J.; Malliarou, E.; Jarup, L.; Harrington, R.; Svartengren, M.; Han, I.-K.; Ohman-Strickland, P.; et al. Respiratory effects of exposure to diesel traffic in persons with asthma. N. Engl. J. Med. 2007, 357, 2348–2358. [Google Scholar] [CrossRef]
- Zhang, J.J.; McCreanor, J.E.; Cullinan, P.; Chung, K.F.; Ohman-Strickland, P.; Han, I.-K.; Järup, L.; Nieuwenhuijsen, M.J. Health effects of real-world exposure to diesel exhaust in persons with asthma. Res. Rep. Health Eff. Inst. 2009, 2009, 5–109, discussion 111–123. [Google Scholar]
- Delfino, R.J.; Staimer, N.; Tjoa, T.; Gillen, D.L.; Schauer, J.J.; Shafer, M.M. Airway inflammation and oxidative potential of air pollutant particles in a pediatric asthma panel. J. Expo. Sci. Environ. Epidemiol. 2013, 23, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Alexis, N.E.; Carlsten, C. Interplay of air pollution and asthma immunopathogenesis: A focused review of diesel exhaust and ozone. Int. Immunopharmacol. 2014, 23, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Biagini Myers, J.M.; Brandt, E.B.; Brokamp, C.; Ryan, P.H.; Khurana Hershey, G.K. Air pollution, epigenetics, and asthma. Allergy Asthma Clin. Immunol. Off. J. Can. Soc. Allergy Clin. Immunol. 2016, 12, 51. [Google Scholar] [CrossRef]
- Brown, M.S.; Sarnat, S.E.; DeMuth, K.A.; Brown, L.A.; Whitlock, D.R.; Brown, S.W.; Tolbert, P.E.; Fitzpatrick, A.M. Residential proximity to a major roadway is associated with features of asthma control in children. PLoS ONE 2012, 7, e37044. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.A.; Siscovick, D.S.; Sheppard, L.; Shepherd, K.; Sullivan, J.H.; Anderson, G.L.; Kaufman, J.D. Long-term exposure to air pollution and incidence of cardiovascular events in women. N. Engl. J. Med. 2007, 2007, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Gauderman, W.J.; Avol, E.; Lurmann, F.; Kuenzli, N.; Gilliland, F.; Peters, J.; McConnell, R. Childhood asthma and exposure to traffic and nitrogen dioxide. Epidemiology 2005, 16, 737–743. [Google Scholar] [CrossRef] [PubMed]
- Oftedal, B.; Nystad, W.; Brunekreef, B.; Nafstad, P. Long-Term Traffic-Related Exposures and Asthma Onset in Schoolchildren in Oslo, Norway. Environ. Health Perspect. 2009, 117, 839–844. [Google Scholar] [CrossRef]
- Calderón-Garcidueñas, L.; Villarreal-Ríos, R. Living close to heavy traffic roads, air pollution, and dementia. Lancet 2017, 389, 675–677. [Google Scholar] [CrossRef]
- Chen, H.; Kwong, J.C.; Copes, R.; Tu, K.; Villeneuve, P.J.; van Donkelaar, A.; Hystad, P.; Martin, R.V.; Murray, B.J.; Jessiman, B.; et al. Living near major roads and the incidence of dementia, Parkinson’s disease, and multiple sclerosis: A population-based cohort study. Lancet 2017, 389, 718–726. [Google Scholar] [CrossRef]
- Wheeler, A.J.; Smith-Doiron, M.; Xu, X.; Gilbert, N.L.; Brook, J.R. Intra-urban variability of air pollution in Windsor, Ontario—Measurement and modeling for human exposure assessment. Environ. Res. 2008, 106, 7–16. [Google Scholar] [CrossRef]
- Brunekreef, B.; Brunekreef, B.; Stewart, A.W.; Anderson, H.R.; Lai, C.K.; Strachan, D.P.; Pearce, N.; ISAAC Phase 3 Study Group. Self-reported truck traffic on the street of residence and symptoms of asthma and allergic disease: A global relationship in ISAAC phase 3. Environ. Health Perspect. 2009, 117, 1791–1798. [Google Scholar] [CrossRef] [PubMed]
- Dockery, D.W.; Dockery, D.W.; Pope, C.A., 3rd; Xu, X.; Spengler, J.D.; Ware, J.H.; Fay, M.E.; Ferris, B.G., Jr.; Speizer, F.E. An Association between Air Pollution and Mortality in Six U. S. Cities. N. Engl. J. Med. 1993, 329, 1753–1759. [Google Scholar] [CrossRef] [PubMed]
- Zou, B.; Wilson, J.G.; Zhan, F.B.; Zeng, Y. Air pollution exposure assessment methods utilized in epidemiological studies. J. Environ. Monit. 2009, 11, 475–490. [Google Scholar] [CrossRef]
- Hoek, G.; Krishnan, R.M.; Beelen, R.; Peters, A.; Ostro, B.; Brunekreef, B.; Kaufman, J.D. Long-term air pollution exposure and cardio-respiratory mortality: A review. Environ. Health 2013, 12, 1–15. [Google Scholar] [CrossRef]
- Jerrett, M.; Arain, A.; Kanaroglou, P.; Beckerman, B.; Potoglou, D.; Sahsuvaroglu, T.; Morrison, J.; Giovis, C. A review and evaluation of intraurban air pollution exposure models. J. Expo. Sci. Environ. Epidemiol. 2005, 15, 185–204. [Google Scholar] [CrossRef] [PubMed]
- Nethery, E.; Leckie, S.E.; Teschke, K.; Brauer, M. From measures to models: An evaluation of air pollution exposure assessment for epidemiological studies of pregnant women. Occup. Environ. Med. 2008, 65, 579–586. [Google Scholar] [CrossRef]
- Brauer, M.; Hoek, G.; van Vliet, P.; Meliefste, K.; Fischer, P.; Gehring, U.; Heinrich, J.; Cyrys, J.; Bellander, T.; Lewne, M.; et al. Estimating long-term average particulate air pollution concentrations: Application of traffic indicators and geographic information systems. Epidemiology 2003, 14, 228–239. [Google Scholar] [CrossRef]
- Gan, W.Q.; Allen, R.W.; Brauer, M.; Davies, H.W.; Mancini, G.B.; Lear, S.A. Long-term exposure to traffic-related air pollution and progression of carotid artery atherosclerosis: A prospective cohort study. BMJ Open 2014, 4, e004743. [Google Scholar] [CrossRef]
- Lipfert, F.W.; Wyzga, R.E.; Baty, J.D.; Miller, J.P. Traffic density as a surrogate measure of environmental exposures in studies of air pollution health effects: Long-term mortality in a cohort of US veterans. Atmos. Environ. 2006, 40, 154–169. [Google Scholar] [CrossRef]
- Shi, L.; Zanobetti, A.; Kloog, I.; Coull, B.A.; Koutrakis, P.; Melly, S.J.; Schwartz, J.D. Low-concentration PM2.5 and mortality: Estimating acute and chronic effects in a population-based study. Environ. Health Perspect. 2016, 124, 46–52. [Google Scholar] [CrossRef]
- Boehmer, T.K.; Foster, S.L.; Henry, J.R.; Woghiren-Akinnifesi, E.L.; Yip, F.Y. Residential Proximity to Major Highways—United States, 2010. MMWR Morb. Mortal. Wkly. Rep. 2013, 62, 46–50. Available online: https://www.cdc.gov/mmwr/preview/mmwrhtml/su6203a8.htm (accessed on 3 December 2020).
- Pratt, G.C.; Vadali, M.L.; Kvale, D.L.; Ellickson, K.M. Traffic, air pollution, minority and socio-economic status: Addressing inequities in exposure and risk. Int. J. Environ. Res. Public Health 2015, 12, 5355–5372. [Google Scholar] [CrossRef] [PubMed]
- Cushing, L.; Faust, J.; August, L.M.; Cendak, R.; Wieland, W.; Alexeeff, G. Racial/Ethnic Disparities in Cumulative Environmental Health Impacts in California: Evidence from a Statewide Environmental Justice Screening Tool (CalEnviroScreen 1.1). Am. J. Public Health 2015, 105, 2341–2348. [Google Scholar] [CrossRef] [PubMed]
- Bateson, T.F.; Schwartz, J. Children’s response to air pollutants. J. Toxicol. Environ. Health A 2008, 71, 238–243. [Google Scholar] [CrossRef]
- Wright, R.J.; Brunst, K.J. Programming of respiratory health in childhood: Influence of outdoor air pollution. Curr. Opin. Pediatr. 2013, 25, 232–239. [Google Scholar] [CrossRef]
- Anto, J.M.; Bousquet, J.; Akdis, M.; Auffray, C.; Keil, T.; Momas, I.; Postma, D.S.; Valenta, R.; Wickman, M.; Cambon-Thomsen, A.; et al. Mechanisms of the Development of Allergy (MeDALL): Introducing novel concepts in allergy phenotypes. J. Allergy Clin. Immunol. 2017, 139, 388–399. [Google Scholar] [CrossRef]
- Grewal, J.; Grantz, K.L.; Zhang, C.; Sciscione, A.; Wing, D.A.; Grobman, W.A.; Newman, R.B.; Wapner, R.; D’Alton, M.E.; Skupski, D.; et al. Cohort Profile: NICHD Fetal Growth Studies-Singletons and Twins. Int. J. Epidemiology 2018, 47, 25–25l. [Google Scholar] [CrossRef]
- Dwyer, G.M.; Hardy, L.L.; Peat, J.K.; Baur, L.A. The validity and reliability of a home environment preschool-age physical activity questionnaire (Pre-PAQ). Int. J. Behav. Nutr. Phys. Act. 2011, 8, 86. [Google Scholar] [CrossRef]
- Asher, M.I.; Keil, U.; Anderson, H.R.; Beasley, R.; Crane, J.; Martinez, F.; Mitchell, E.A.; Pearce, N.; Sibbald, B.; Stewart, A.W.; et al. International Study of Asthma and Allergies in Childhood (ISAAC): Rationale and methods. Eur. Respir. J. 1995, 8, 483–491. [Google Scholar] [CrossRef]
- Anderson, H.R.; Gupta, R.; Kapetanakis, V.; Asher, M.I.; Clayton, T.; Robertson, C.F.; Strachan, D.P.; ISAAC Steering Committee. International correlations between indicators of prevalence, hospital admissions and mortality for asthma in children. Int. J. Epidemiol. 2008, 37, 573–582. [Google Scholar] [CrossRef]
- Kuczmarski, R.J.; Ogden, C.L.; Guo, S.S.; Grummer-Strawn, L.M.; Flegal, K.M.; Mei, Z.; Wei, R.; Curtin, L.R.; Roche, A.F.; Johnson, C.L. 2000 CDC Growth Charts for the United States: Methods and development. Vital Health Stat. 2002, 2002, 1–190. [Google Scholar]
- Division of Nutrition, Physical Activity, and Obesity, National Center for Chronic Disease Prevention and Health Promotion. A SAS Program for the 2000 CDC Growth Charts (Ages 0 to <20 Years; 2019. Available online: https://www.cdc.gov/nccdphp/dnpao/growthcharts/resources/sas.htm (accessed on 6 April 2020).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016; Available online: https://www.R-project.org/ (accessed on 6 April 2020).
- Gehring, U.; Wijga, A.H.; Brauer, M.; Fischer, P.; de Jongste, J.C.; Kerkhof, M.; Oldenwening, M.; Smit, H.A.; Brunekreef, B. Traffic-related air pollution and the development of asthma and allergies during the first 8 years of life. Am. J. Respir. Crit. Care Med. 2010, 181, 596–603. [Google Scholar] [CrossRef] [PubMed]
- Lau, N.; Norman, A.; Smith, M.J.; Sarkar, A.; Gao, Z. Association between Traffic Related Air Pollution and the Development of Asthma Phenotypes in Children: A Systematic Review. Int. J. Chronic Dis. 2018, 2018, 4047386. [Google Scholar] [CrossRef] [PubMed]
- Gruzieva, O.; Bergström, A.; Hulchiy, O.; Kull, I.; Lind, T.; Melén, E.; Moskalenko, V.; Pershagen, G.; Bellander, T. Exposure to air pollution from traffic and childhood asthma until 12 years of age. Epidemiology 2013, 24, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, D.I. Diesel exhaust exposure, wheezing and sneezing. Allergy Asthma Immunol. Res. 2012, 4, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Esposito, S.; Galeone, C.; Lelii, M.; Longhi, B.; Ascolese, B.; Senatore, L.; Prada, E.; Montinaro, V.; Malerba, S.; Patria, M.F.; et al. Impact of air pollution on respiratory diseases in children with recurrent wheezing or asthma. BMC Pulm. Med. 2014, 14, 130. [Google Scholar] [CrossRef]
- HEI Panel on the Health Effects of Traffic-Related Air Pollution, Traffic-Related Air Pollution: A Critical Review of the Literature on Emissions, Exposure, and Health Effects; Health Effects Institute: Boston, MA, USA, 2010.
- Khreis, H.; Kelly, C.; Tate, J.; Parslow, R.; Lucas, K.; Nieuwenhuijsen, M. Exposure to traffic-related air pollution and risk of development of childhood asthma: A systematic review and meta-analysis. Environ. Int. 2017, 100, 1–31. [Google Scholar] [CrossRef]
- Brauer, M.; Hoek, G.; Smit, H.A.; de Jongste, J.C.; Gerritsen, J.; Postma, D.S.; Kerkhof, M.; Brunekreef, B. Air pollution and development of asthma, allergy and infections in a birth cohort. Eur. Respir. J. 2007, 29, 879–888. [Google Scholar] [CrossRef]
- Jerrett, M.; McConnell, R.; Wolch, J.; Chang, R.; Lam, C.; Dunton, G.; Gilliland, F.; Lurmann, F.; Islam, T.; Berhane, K. Traffic-related air pollution and obesity formation in children: A longitudinal, multilevel analysis. Environ. Health 2014, 13, 49. [Google Scholar] [CrossRef]
- McConnell, R.; Islam, T.; Shankardass, K.; Jerrett, M.; Lurmann, F.; Gilliland, F.; Gauderman, J.; Avol, E.; Künzli, N.; Yao, L.; et al. Childhood incident asthma and traffic-related air pollution at home and school. Environ. Health Perspect. 2010, 118, 1021–1026. [Google Scholar] [CrossRef]
- Jerrett, M.; Shankardass, K.; Berhane, K.; Gauderman, W.J.; Künzli, N.; Avol, E.; Gilliland, F.; Lurmann, F.; Molitor, J.N.; Molitor, J.T.; et al. Traffic-Related Air Pollution and Asthma Onset in Children: A Prospective Cohort Study with Individual Exposure Measurement. Environ. Health Perspect. 2008, 116, 1433–1438. [Google Scholar] [CrossRef] [PubMed]
- Pennington, A.F.; Strickland, M.J.; Klein, M.; Zhai, X.; Bates, J.T.; Drews-Botsch, C.; Hansen, C.; Russell, A.G.; Tolbert, P.E.; Darrow, L.A. Exposure to Mobile Source Air Pollution in Early-life and Childhood Asthma Incidence: The Kaiser Air Pollution and Pediatric Asthma Study. Epidemiology 2018, 29, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Garcia, E.; Berhane, K.T.; Islam, T.; McConnell, R.; Urman, R.; Chen, Z.; Gilliland, F.D. Association of Changes in Air Quality With Incident Asthma in Children in California, 1993–2014. JAMA 2019, 321, 1906–1915. [Google Scholar] [CrossRef] [PubMed]
- Guarnieri, M.; Balmes, J.R. Outdoor air pollution and asthma. Lancet 2014, 383, 1581–1592. [Google Scholar] [CrossRef]
- Heinrich, J.; Wichmann, H.-E. Traffic related pollutants in Europe and their effect on allergic disease. Curr. Opin. Allergy Clin. Immunol. 2004, 4, 341–348. [Google Scholar] [CrossRef]
- Diaz-Sanchez, D.; Proietti, L.; Polosa, R. Diesel fumes and the rising prevalence of atopy: An urban legend? Curr. Allergy Asthma Rep. 2003, 3, 146–152. [Google Scholar] [CrossRef]
- Galobardes, B.; Shaw, M.; Lawlor, D.A.; Lynch, J.W.; Davey Smith, G. Indicators of socioeconomic position (part 1). J. Epidemiol. Community Health 2006, 60, 7–12. [Google Scholar] [CrossRef]
- Zahran, H.S.; Bailey, C.M.; Damon, S.A.; Garbe, P.L.; Breysse, P.N. Vital Signs: Asthma in Children—United States, 2001–2016. MMWR Morb. Mortal. Wkly. Rep. 2018, 67, 149–155. [Google Scholar] [CrossRef]
- Nardone, A.; Neophytou, A.M.; Balmes, J.; Thakur, N. Ambient Air Pollution and Asthma-Related Outcomes in Children of Color of the USA: A Scoping Review of Literature Published Between 2013 and 2017. Curr. Allergy Asthma Rep. 2018, 18, 29. [Google Scholar] [CrossRef]
- Carlsten, C.; Dybuncio, A.; Becker, A.; Chan-Yeung, M.; Brauer, M. Traffic-related air pollution and incident asthma in a high-risk birth cohort. Occup. Environ. Med. 2011, 68, 291–295. [Google Scholar] [CrossRef]
- Ho, W.-C.; Hartley, W.R.; Myers, L.; Lin, M.H.; Lin, Y.S.; Lien, C.H.; Lin, R.S. Air pollution, weather, and associated risk factors related to asthma prevalence and attack rate. Environ. Res. 2007, 104, 402–409. [Google Scholar] [CrossRef]
- Yang, M.; Chu, C.; Bloom, M.S.; Li, S.; Chen, G.; Heinrich, J.; Markevych, I.; Knibbs, L.D.; Bowatte, G.; Dharmage, S.C.; et al. Is smaller worse? New insights about associations of PM1 and respiratory health in children and adolescents. Environ. Int. 2018, 120, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Su, J.G.; Jerrett, M.; McConnell, R.; Berhane, K.; Dunton, G.; Shankardass, K.; Reynolds, K.; Chang, R.; Wolch, J. Factors influencing whether children walk to school. Health Place 2013, 22, 153–161. [Google Scholar] [CrossRef]
- Chillón, P.; Hales, D.; Vaughn, A.; Gizlice, Z.; Ni, A.; Ward, D.S. A cross-sectional study of demographic, environmental and parental barriers to active school travel among children in the United States. Int. J. Behav. Nutr. Phys. Act. 2014, 11, 61. [Google Scholar] [CrossRef]
- McConnell, R.; Shen, E.; Gilliland, F.D.; Jerrett, M.; Wolch, J.; Chang, C.C.; Lurmann, F.; Berhane, K. A longitudinal cohort study of body mass index and childhood exposure to secondhand tobacco smoke and air pollution: The Southern California Children’s Health Study. Environ. Health Perspect. 2015, 123, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Yue, P.; Deiuliis, J.A.; Lumeng, C.N.; Kampfrath, T.; Mikolaj, M.B.; Cai, Y.; Ostrowski, M.C.; Lu, B.; Parthasarathy, S.; et al. Ambient air pollution exaggerates adipose inflammation and insulin resistance in a mouse model of diet-induced obesity. Circulation 2009, 119, 538–546. [Google Scholar] [CrossRef]
- Xu, X.; Yavar, Z.; Verdin, M.; Ying, Z.; Mihai, G.; Kampfrath, T.; Wang, A.; Zhong, M.; Lippmann, M.; Chen, L.C.; et al. Effect of early particulate air pollution exposure on obesity in mice: Role of p47phox. Arter. Thromb. Vasc. Biol. 2010, 30, 2518–2527. [Google Scholar] [CrossRef]
- Moss, K.M.; Dobson, A.J.; Edwards, K.L.; Hesketh, K.D.; Chang, Y.T.; Mishra, G.D. Not All Play Equipment Is Created Equal: Associations between Equipment at Home and Children’s Physical Activity. J. Phys. Act. Health 2019, 1, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Salam, M.T.; Islam, T.; Gilliland, F.D. Recent evidence for adverse effects of residential proximity to traffic sources on asthma. Curr. Opin. Pulm. Med. 2008, 14, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Nowak, D.J.; Crane, D.E.; Stevens, J.C. Air pollution removal by urban trees and shrubs in the United States. Urban For. Urban Green. 2006, 4, 115–123. [Google Scholar] [CrossRef]
- Janhäll, S. Review on urban vegetation and particle air pollution—Deposition and dispersion. Atmos. Environ. 2015, 105, 130–137. [Google Scholar] [CrossRef]
- Fairclough, S.J.; Stratton, G. A review of physical activity levels during elementary school physical education. J. Teach. Phys. Educ. 2006, 25, 240–258. [Google Scholar] [CrossRef]
- Ignatius, T.; Wong, T.W.; Liu, H.J. Impact of air pollution on cardiopulmonary fitness in schoolchildren. J. Occup. Environ. Med. 2004, 46, 946–952. [Google Scholar] [CrossRef]
- McConnell, R.; Berhane, K.; Gilliland, F.; London, S.J.; Islam, T.; Gauderman, W.J.; Avol, E.; Margolis, H.G.; Peters, J.M. Asthma in exercising children exposed to ozone: A cohort study. Lancet 2002, 359, 386–391. [Google Scholar] [CrossRef]
- The Trust for Public Land. The Heat Is On—A Trust for Public Land Special Report. 2020. Available online: https://www.tpl.org/the-heat-is-on (accessed on 8 December 2020).
- Su, J.G.; Jerrett, M.; de Nazelle, A.; Wolch, J. Does exposure to air pollution in urban parks have socioeconomic, racial or ethnic gradients? Environ. Res. 2011, 111, 319–328. [Google Scholar] [CrossRef]
- Trasande, L.; Thurston, G.D. The role of air pollution in asthma and other pediatric morbidities. J. Allergy Clin. Immunol. 2005, 115, 689–699. [Google Scholar] [CrossRef]
- Korhonen, P.; Haataja, P.; Ojala, R.; Hirvonen, M.; Korppi, M.; Paassilta, M.; Uotila, J.; Gissler, M.; Luukkaala, T.; Tammela, O. Asthma and atopic dermatitis after early-, late-, and post-term birth. Pediatr. Pulmonol. 2018, 53, 269–277. [Google Scholar] [CrossRef]
- Nutten, S. Atopic dermatitis: Global epidemiology and risk factors. Ann. Nutr. Metab. 2015, 66 (Suppl. 1), 8–16. [Google Scholar] [CrossRef]
- Radlović, N.; Leković, Z.; Radlović, V.; Simić, D.; Ristić, D.; Vuletić, B. Food allergy in children. Srp. Arh. Celok. Lek. 2016, 144, 99–103. [Google Scholar] [CrossRef]
- Bowatte, G.; Lodge, C.; Lowe, A.J.; Erbas, B.; Perret, J.; Abramson, M.J.; Matheson, M.; Dharmage, S.C. The influence of childhood traffic-related air pollution exposure on asthma, allergy and sensitization: A systematic review and a meta-analysis of birth cohort studies. Allergy 2015, 70, 245–256. [Google Scholar] [CrossRef]
- Fuertes, E.; Heinrich, J. The influence of childhood traffic-related air pollution exposure on asthma, allergy and sensitization. Allergy 2015, 70, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Cori, L.; Donzelli, G.; Gorini, F.; Bianchi, F.; Curzio, O. Risk Perception of Air Pollution: A Systematic Review Focused on Particulate Matter Exposure. Int. J. Environ. Res. Public Health 2020, 17, 6424. [Google Scholar] [CrossRef] [PubMed]


| Variable | All Children in Current Study (n = 855)% | Exposed to Neighborhood Traffic (n = 129)% | Unexposed to Neighborhood Traffic (n = 726)% | p-Value for χ2 or t Test |
|---|---|---|---|---|
| Child age in years, mean (SD) | 6.9 (1.0) | 6.7 (0.9) | 6.9 (1.0) | 0.09 |
| Physician diagnosed asthma only (Asthma) [current or past] | 13.9% | 20.2% | 12.8% | 0.03 |
| Wheezing or respiratory symptoms only (current or past) | 19.1% | 25.6% | 17.9% | 0.04 |
| Primary Outcome: Asthma/asthma-like symptoms (current or past) | 23.0% | 33.3% | 21.2% | 0.003 |
| Male child | 51.2% | 55.0% | 50.6% | 0.3 |
| Race of child | ||||
| Non-Hispanic White (NHW) | 31.1% | 12.4% | 34.4% | <0.0001 |
| Non-Hispanic Black (NHB) | 29.7% | 39.5% | 28.0% | |
| Hispanic | 25.9% | 34.9% | 24.2% | |
| Asian | 13.1% | 13.2% | 13.1% | |
| Mother Education | ||||
| Less than or up to high school | 21.6% | 34.9% | 19.3% | <0.0001 |
| More than high school | 78.4% | 65.1% | 80.7% | |
| Family history of asthma | 33.5% | 34.9% | 33.2% | 0.9 |
| Child’s BMI for age percentile | ||||
| Obese (BMI for age percentile ≥ 95) | 11.2% | 15.5% | 10.5% | 0.2 |
| Child exposed to any secondhand smoke | 11.0% | 15.5% | 10.2% | 0.08 |
| Household pets | ||||
| Cat at home during child’s first year and/or in the past 12 months | 18.6% | 16.3% | 19.0% | 0.5 |
| Dog at home during child’s first year and/or in the past 12 months | 44.7% | 40.3% | 45.5% | 0.3 |
| Pets (dog and/or cat) at home during child’s first year and/or in the past 12 months | 52.8% | 43.4% | 54.4% | 0.02 |
| Inhalers and other asthma drug prescriptions (e.g., Pulmicort, Flovent, Singulair, Zyflo, Orapred, Advair, Symbicort, or Xolair) | 17.2% | 21.4% | 16.5% | 0.2 |
| Hay fever or respiratory allergy | 14.3% | 12.4% | 14.6% | 0.5 |
| Current residence in urban area | 90.9% | 92.1% | 90.7% | 0.6 |
| Preschool-aged Children’s Physical Activity Questionnaire (Pre-PAQ) Q22 Variables [strongly agree or agree] | ||||
| Safety: It is safe for my child to play outdoors in my neighborhood (if supervised). | 93.7% | 79.1% | 96.3% | <0.0001 |
| Footpaths: There are usable footpaths on most of the streets in my local area. | 78.0% | 51.9% | 82.6% | <0.0001 |
| Walk: There are major barriers or dangers to walking with my child in my neighborhood that make it hard to get from place to place (e.g., major roads, railway lines, canals, storm water drains or rivers). | 11.9% | 45.7% | 5.9% | <0.0001 |
| Sufficient light: There are sufficient traffic lights or pedestrian crossings to make it safe to walk with my child around my neighborhood. | 77.2% | 48.8% | 82.2% | <0.0001 |
| Crime: The level of crime in my neighborhood makes it unsafe to go on walks with my child during the day. | 9.5% | 31.8% | 5.5% | <0.0001 |
| Shops: The local shop(s) are within easy walking distance of my home. | 54.9% | 54.3% | 55.0% | 1.0 |
| Danger: There are dangers (e.g., dogs or undesirable people) in the local park(s) so I avoid taking my child there. | 9.4% | 32.6% | 5.2% | <0.0001 |
| Preschool-aged Children’s Physical Activity Questionnaire (Pre-PAQ) Q18 (please tick as many responses as apply) [Yes] | ||||
| Do you have access to any of the following facilities within your backyard or home environment? | ||||
| Play equipment (e.g., swing set, slide, climbing gym) | 45.7% | 31.8% | 48.2% | 0.0006 |
| Pool or spa | 19.8% | 19.4% | 19.8% | 0.9 |
| Area suitable to ride a tricycle, bike or scooter, etc. | 78.0% | 60.5% | 81.1% | <0.0001 |
| Preschool-aged Children’s Physical Activity Questionnaire (Pre-PAQ) Q21 Variables [Yes] | ||||
| Does your local neighborhood have the following places or facilities where your child can be play and be physically active? (please tick as many responses as apply) | ||||
| Open areas such as beaches, rivers, natural reserves | 41.4% | 29.5% | 43.5% | 0.009 |
| Public park or oval | 87.0% | 76.7% | 88.8% | 0.0002 |
| Playground | 88.3% | 79.1% | 89.9% | 0.0003 |
| Public swimming pool | 52.4% | 44.2% | 53.9% | 0.1 |
| Gym that offers programs for young children, e.g., kindergym, playgym, etc. | 47.6% | 35.7% | 49.7% | 0.008 |
| Club that offers activities/sports for young children, e.g., soccer, dance, etc. | 59.1% | 49.6% | 60.7% | 0.04 |
| Variable | Asthma/Asthma-Like Symptoms (n = 197)% | No Asthma/Asthma-Like Symptoms (n = 658)% | p-Value for χ2 or t Test |
|---|---|---|---|
| Child age in years, mean (SD) | 6.9 (1.0) | 6.8 (1.0) | 0.7 |
| Male child | 56.9% | 49.5% | 0.07 |
| Race-ethnicity of child | |||
| Non-Hispanic White (NHW) | 19.3% | 34.7% | <0.0001 |
| Non-Hispanic Black (NHB) | 40.1% | 26.6% | |
| Hispanic | 31.5% | 24.2% | |
| Asian | 8.6% | 14.4% | |
| Mother Education | |||
| Less than or up to high school | 25.9% | 20.4% | 0.1 |
| More than high school | 74.1% | 79.6% | |
| Family history of asthma | 55.8% | 26.8% | <0.0001 |
| Child’s BMI for age percentile | |||
| Obese (BMI for age percentile ≥ 95) | 21.3% | 8.2% | <0.0001 |
| Child exposed to any second hand smoke | 15.7% | 9.6% | 0.02 |
| Household pets | |||
| Cat at home during child’s first year and/or in the past 12 months | 17.3% | 19.0% | 0.6 |
| Dog at home during child’s first year and/or in the past 12 months | 45.2% | 44.5% | 0.9 |
| Pets (dog and/or cat) at home during child’s first year and/or in the past 12 months | 51.8% | 53.0% | 0.8 |
| Preschool-aged Children’s Physical Activity Questionnaire (Pre-PAQ) Q22 Variables [strongly agree or agree] | |||
| Safety: It is safe for my child to play outdoors in my neighborhood (if supervised). | 91.9% | 94.2% | 0.3 |
| Footpaths: There are usable footpaths on most of the streets in my local area. | 72.6% | 79.6% | 0.04 |
| Walk: There are major barriers or dangers to walking with my child in my neighborhood that make it hard to get from place to place (e.g., major roads, railway lines, canals, storm water drains or rivers). | 12.2% | 11.9% | 0.8 |
| Neighborhood traffic: There is so much traffic along the streets that it makes it difficult or dangerous to walk with my child in my neighborhood. | 21.8% | 13.1% | 0.003 |
| Sufficient light: There are sufficient traffic lights or pedestrian crossings to make it safe to walk with my child around my neighborhood. | 71.6% | 78.9% | 0.06 |
| Crime: The level of crime in my neighborhood makes it unsafe to go on walks with my child during the day. | 11.7% | 8.8% | 0.2 |
| Shops: The local shop(s) are within easy walking distance of my home. | 56.9% | 54.3% | 0.6 |
| Danger: There are dangers (e.g., dogs or undesirable people) in the local park(s) so I avoid taking my child there. | 13.2% | 8.2% | 0.04 |
| Preschool-aged Children’s Physical Activity Questionnaire (Pre-PAQ) Q18 (please tick as many responses as apply) [ Yes] | |||
| Do you have access to any of the following facilities within your backyard or home environment? | |||
| Play equipment (e.g., swing set, slide, climbing gym) | 48.7% | 44.8% | 0.3 |
| Pool or spa | 21.8% | 19.2% | 0.5 |
| Area suitable to ride a tricycle, bike or scooter, etc. | 77.2% | 78.3% | 0.9 |
| Preschool-aged Children’s Physical Activity Questionnaire (Pre-PAQ) Q21 Variables [Yes] | |||
| Does your local neighborhood have the following places or facilities where your child can be play and be physically active? (please tick as many responses as apply) | |||
| Open areas such as beaches, rivers, natural reserves | 40.1% | 41.8% | 0.7 |
| Public park or oval | 90.4% | 86.0% | 0.03 |
| Playground | 86.3% | 88.9% | 0.6 |
| Public swimming pool | 53.8% | 52.0% | 0.5 |
| Gym that offers programs for young children, e.g., kindergym, playgym, etc. | 48.7% | 47.3% | 0.4 |
| Club that offers activities/sports for young children, e.g., soccer, dance, etc. | 58.9% | 59.1% | 0.4 |
| Model 1 (n = 835) | Model 2 (n = 825) | Model 3 (n = 817) | Model 4 (n = 738) | Model 5 (n = 733) | |
|---|---|---|---|---|---|
| Male child | 1.52 (1.08, 2.15) | 1.57 (1.11, 2.23) | 1.54 (1.08, 2.19) | 1.53 (1.06, 2.22) | 1.30 (0.83, 2.04) |
| Age of child | 1.20 (0.98, 1.46) | 1.20 (0.99, 1.47) | 1.21 (0.99, 1.47) | 1.18 (0.95, 1.45) | 1.21 (0.93, 1.56) |
| Race-Ethnic of child | |||||
| Non-Hispanic White (NHW) | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Non-Hispanic Black (NHB) | 2.55 (1.52, 4.29) | 2.74 (1.61, 4.67) | 2.68 (1.56, 4.59) | 2.53 (1.43, 4.50) | 2.21 (1.10, 4.47) |
| Hispanic | 2.39 (1.44, 3.98) | 2.62 (1.55, 4.42) | 2.56 (1.52, 4.34) | 2.25 (1.28, 3.94) | 2.20 (1.11, 4.32) |
| Asian | 1.41 (0.71, 2.80) | 1.58 (0.79, 3.18) | 1.50 (0.74, 3.01) | 1.40 (0.66, 2.99) | 1.37 (0.55, 3.37) |
| Mother Education | 0.93 (0.61, 1.41) | 0.91 (0.59, 1.39) | 0.97 (0.63, 1.50) | 1.08 (0.69, 1.70) | 1.17 (0.67, 2.03) |
| Family History of asthma | 3.32 (2.34, 4.70) | 3.38 (2.38, 4.81) | 3.24 (2.27, 4.63) | 3.19 (2.20, 4.64) | 1.99 (1.26, 3.15) |
| Neighborhood Traffic | 1.59 (1.02, 2.48) | 1.77 (1.13, 2.77) | 1.93 (1.22, 3.06) | 1.78 (1.10, 2.88) | 2.01 (1.12, 3.62) |
| Play Equipment | ---- | 1.53 (1.07, 2.19) | 1.44 (1.00, 2.07) | 1.60 (1.09, 2.35) | 1.91 (1.19, 3.09) |
| Public Park | ---- | ---- | 2.02 (1.07, 3.79) | 1.86 (0.98, 3.54) | 2.65 (1.14, 6.15) |
| Obese | ---- | ---- | ---- | 2.54 (1.55, 4.17) | 2.66 (1.44, 4.92) |
| Asthma medication | ---- | ---- | ---- | ---- | 25.07 (14.81, 42.45) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Commodore, S.; Ferguson, P.L.; Neelon, B.; Newman, R.; Grobman, W.; Tita, A.; Pearce, J.; Bloom, M.S.; Svendsen, E.; Roberts, J.; et al. Reported Neighborhood Traffic and the Odds of Asthma/Asthma-Like Symptoms: A Cross-Sectional Analysis of a Multi-Racial Cohort of Children. Int. J. Environ. Res. Public Health 2021, 18, 243. https://doi.org/10.3390/ijerph18010243
Commodore S, Ferguson PL, Neelon B, Newman R, Grobman W, Tita A, Pearce J, Bloom MS, Svendsen E, Roberts J, et al. Reported Neighborhood Traffic and the Odds of Asthma/Asthma-Like Symptoms: A Cross-Sectional Analysis of a Multi-Racial Cohort of Children. International Journal of Environmental Research and Public Health. 2021; 18(1):243. https://doi.org/10.3390/ijerph18010243
Chicago/Turabian StyleCommodore, Sarah, Pamela L. Ferguson, Brian Neelon, Roger Newman, William Grobman, Alan Tita, John Pearce, Michael S. Bloom, Erik Svendsen, James Roberts, and et al. 2021. "Reported Neighborhood Traffic and the Odds of Asthma/Asthma-Like Symptoms: A Cross-Sectional Analysis of a Multi-Racial Cohort of Children" International Journal of Environmental Research and Public Health 18, no. 1: 243. https://doi.org/10.3390/ijerph18010243
APA StyleCommodore, S., Ferguson, P. L., Neelon, B., Newman, R., Grobman, W., Tita, A., Pearce, J., Bloom, M. S., Svendsen, E., Roberts, J., Skupski, D., Sciscione, A., Palomares, K., Miller, R., Wapner, R., Vena, J. E., & Hunt, K. J. (2021). Reported Neighborhood Traffic and the Odds of Asthma/Asthma-Like Symptoms: A Cross-Sectional Analysis of a Multi-Racial Cohort of Children. International Journal of Environmental Research and Public Health, 18(1), 243. https://doi.org/10.3390/ijerph18010243





