Histological and Radiological Features of a Four-Phase Injectable Synthetic Bone Graft in Guided Bone Regeneration: A Case Report
Abstract
1. Introduction
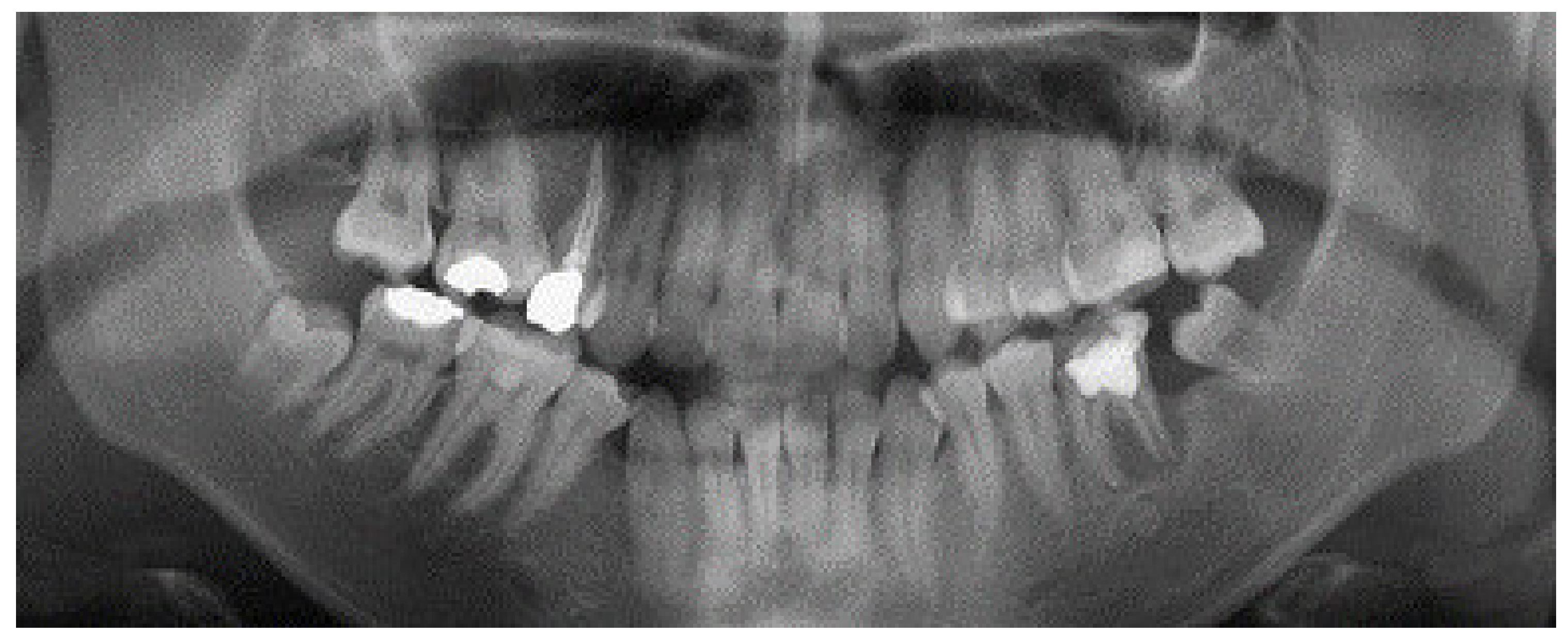
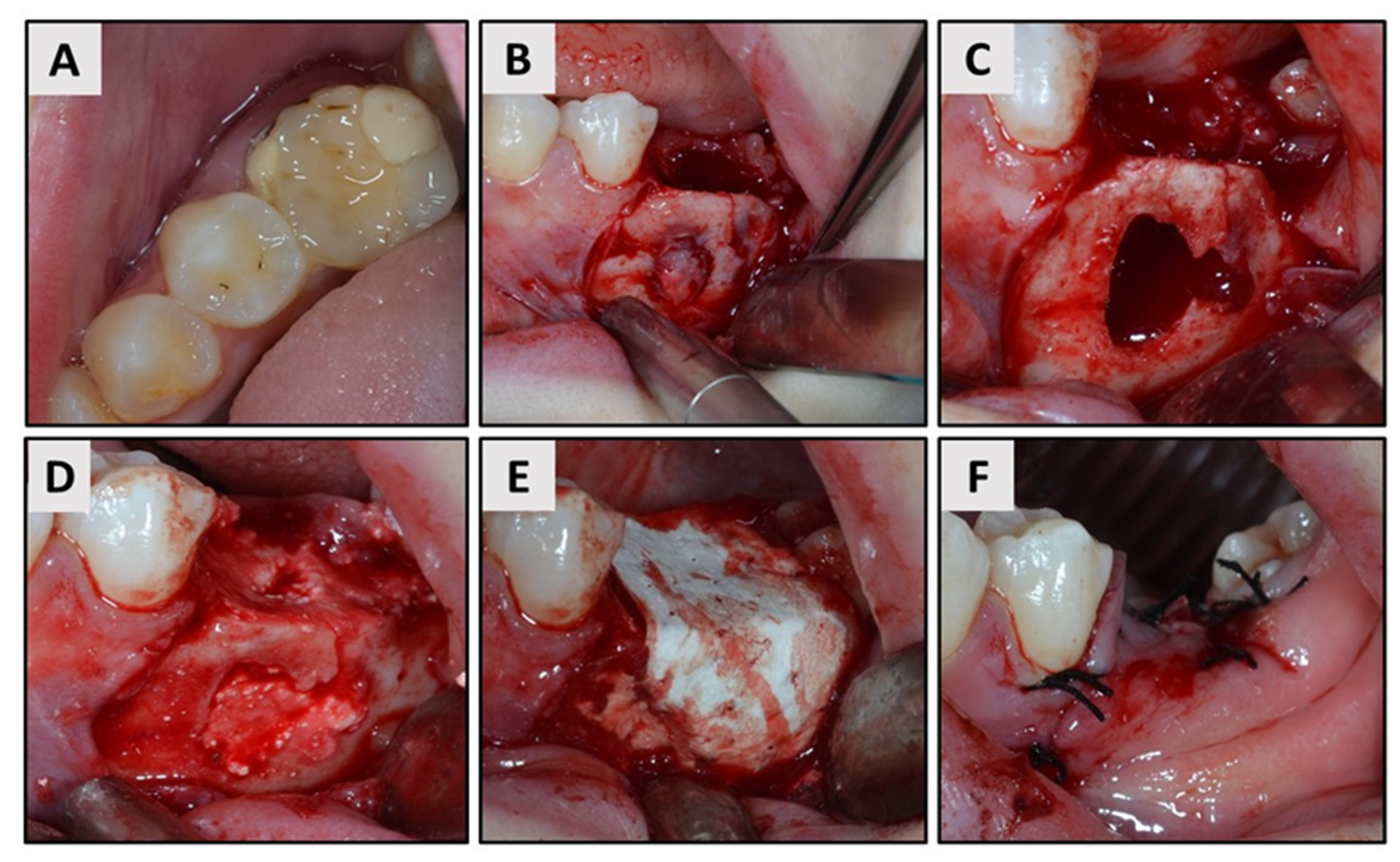
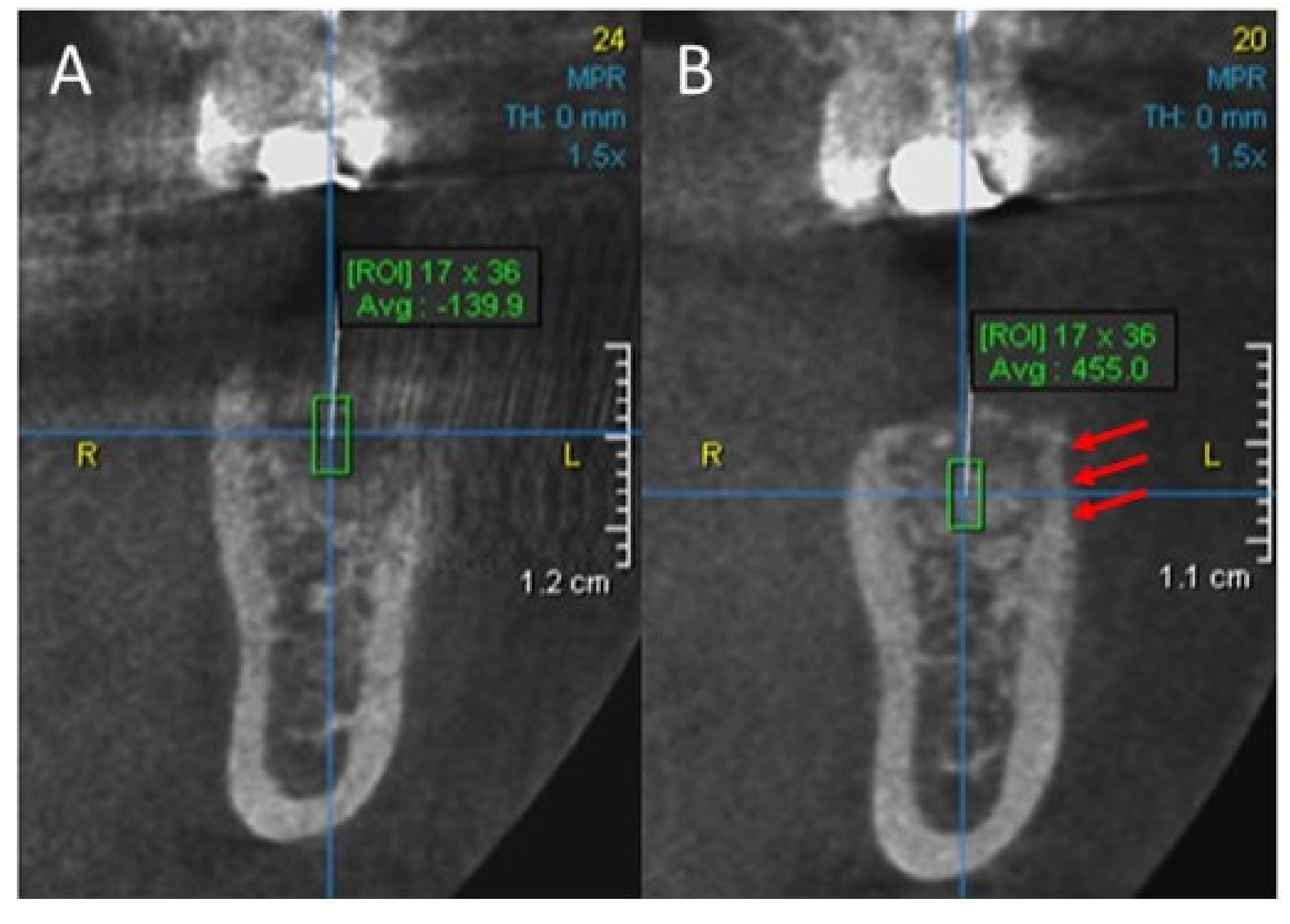
2. Case Report
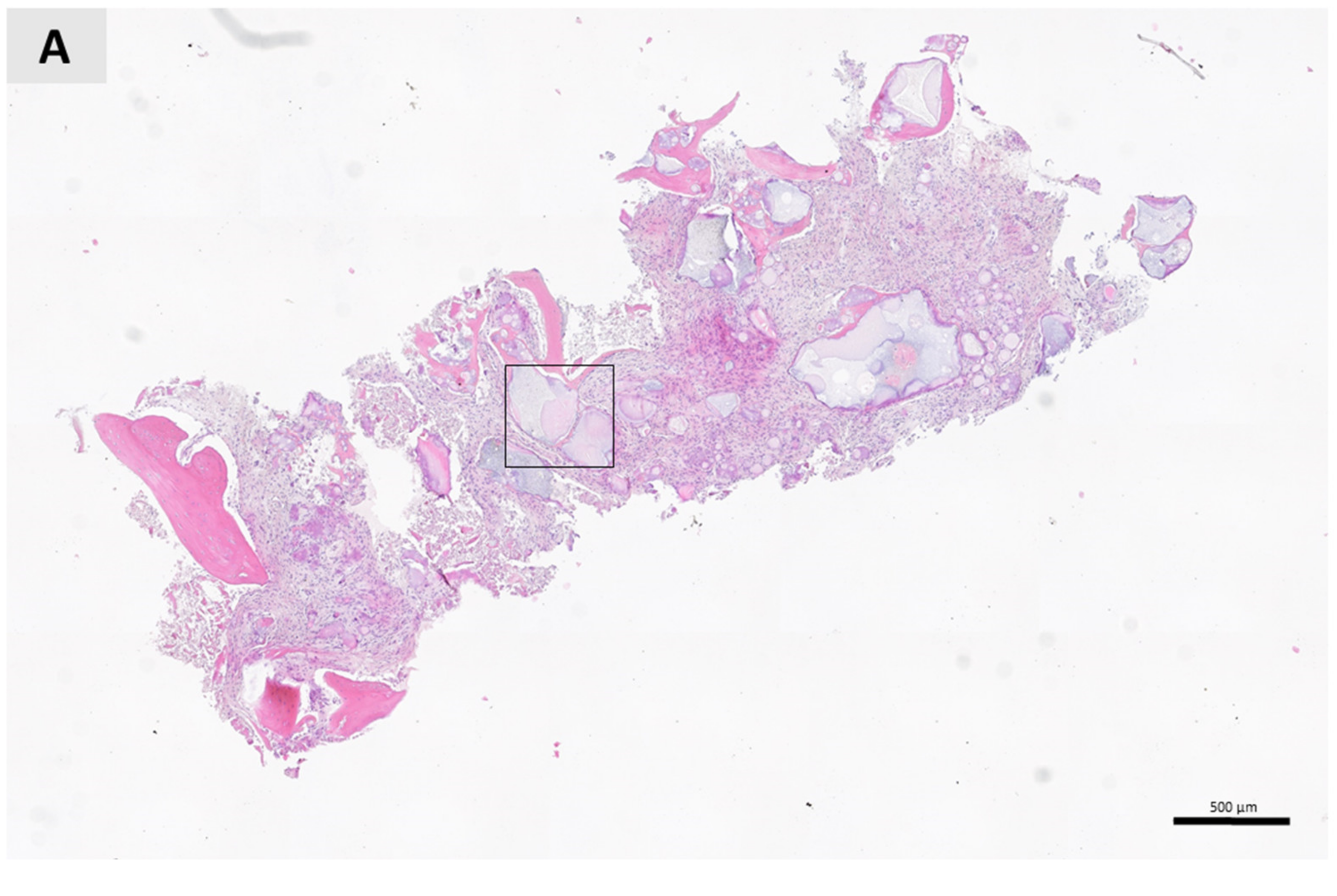
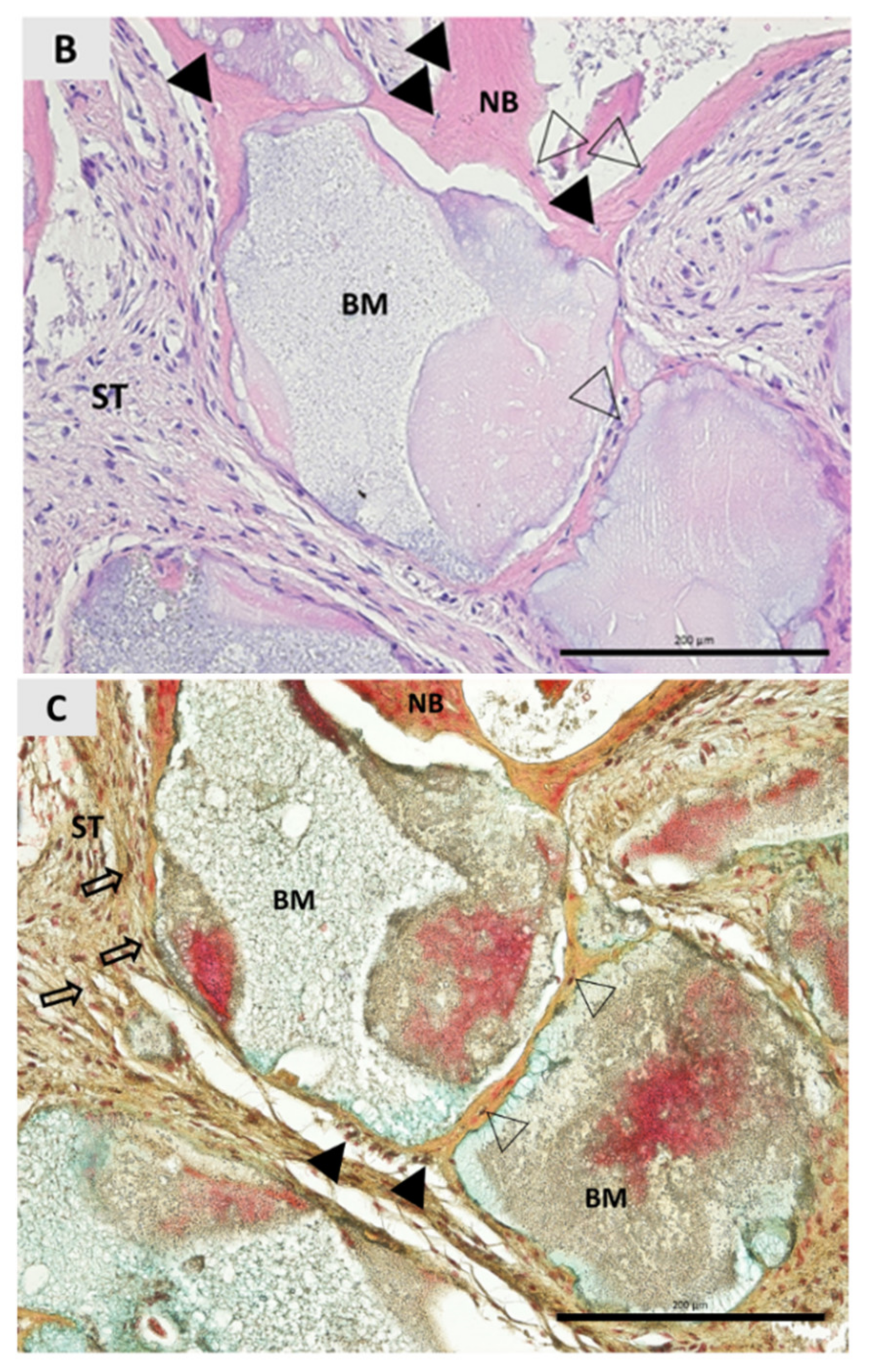
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Del Fabbro, M.; Testori, T.; Kekovic, V.; Goker, F.; Tumedei, M.; Wang, H.-L. A Systematic Review of Survival Rates of Osseointegrated Implants in Fully and Partially Edentulous Patients Following Immediate Loading. J. Clin. Med. 2019, 8, 2142. [Google Scholar] [CrossRef] [PubMed]
- Topçu, A.O.; Yamalik, N.; Güncü, G.N.; Tözüm, T.F.; El, H.; Uysal, S.; Hersek, N. Implant-Site Related and Patient-Based Factors with the Potential to Impact Patients’ Satisfaction, Quality of Life Measures and Perceptions Toward Dental Implant Treatment. Implant Dent. 2017, 26, 581–591. [Google Scholar] [CrossRef] [PubMed]
- Laudenbach, J.M.; Simon, Z. Common Dental and Periodontal Diseases: Evaluation and Management. Med. Clin. N. Am. 2014, 98, 1239–1260. [Google Scholar] [CrossRef] [PubMed]
- Koh, R.U.; Rudek, I.; Wang, H.L. Immediate implant placement: Positives and negatives. Implant Dent. 2010, 19, 98–108. [Google Scholar] [CrossRef]
- Araújo, M.G.; Lindhe, J. Dimensional ridge alterations following tooth extraction. An experimental study in the dog. J. Clin. Periodontol. 2005, 32, 212–218. [Google Scholar] [CrossRef]
- Araújo, M.G.; Silva, C.O.; Misawa, M.; Sukekava, F. Alveolar socket healing: What can we learn? Periodontol. 2000 2015, 68, 122–134. [Google Scholar] [CrossRef]
- Schropp, L.; Wenzel, A.; Kostopoulos, L.; Karring, T. Bone healing and soft tissue contour changes following single-tooth extraction: A clinical and radiographic 12-month prospective study. Int. J. Periodontics Restor. Dent. 2003, 23, 313–323. [Google Scholar]
- Park, S.; Song, Y.W.; Sanz-Martín, I.; Cha, J.; Lee, J.; Jung, U. Clinical benefits of ridge preservation for implant placement compared to natural healing in maxillary teeth: A retrospective study. J. Clin. Periodontol. 2020, 47, 382–391. [Google Scholar] [CrossRef]
- Meloni, S.M.; Lumbau, A.; Spano, G.; Baldoni, E.; Pisano, M.; Tullio, A.; Tallarico, M. Sinus augmentation grafting with anorganic bovine bone versus 50% autologous bone mixed with 50% anorganic bovine bone: 5 years after loading results from a randomised controlled trial. Int. J. Oral Implantol. (New Malden Lond. UK) 2019, 12, 483–492. [Google Scholar]
- Khaled, H. Maxillary sinus floor elevation using hydroxyapatite nano particles vs tenting technique with simultaneous implant placement: A randomized clinical trial. Clin. Implant Dent. Relat. Res. 2019, 21, 1241–1252. [Google Scholar] [CrossRef]
- Wang, R.E.; Lang, N.P. Ridge preservation after tooth extraction. Clin. Oral Implants Res. 2012, 23, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Chiapasco, M.; Casentini, P.; Zaniboni, M. Bone augmentation procedures in implant dentistry. Int. J. Oral Maxillofac. Implants 2009, 24, 237–259. [Google Scholar] [PubMed]
- Elgali, I.; Omar, O.; Dahlin, C.; Thomsen, P. Guided bone regeneration: Materials and biological mechanisms revisited. Eur. J. Oral Sci. 2017, 125, 315–337. [Google Scholar] [CrossRef] [PubMed]
- Retzepi, M.; Donos, N. Guided Bone Regeneration: Biological principle and therapeutic applications. Clin. Oral Implants Res. 2010, 21, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Wessing, B.; Lettner, S.; Zechner, W. Guided Bone Regeneration with Collagen Membranes and Particulate Graft Materials: A Systematic Review and Meta-Analysis. Int. J. Oral Maxillofac. Implants 2018, 33, 87–100. [Google Scholar] [CrossRef]
- Tanaskovic, N.; Trajkovski, B.; Kačarević, Ž.P.; Rider, P.M.; Houshmand, A.; Xiong, X.; Jung, O.; Barbeck, M. Periorbital reconstruction by “periorbital patch” technique using a pericardium-based collagen membrane and titanium mesh. Materials 2019, 12, 2343. [Google Scholar] [CrossRef]
- Trajkovski, B.; Jaunich, M.; Müller, W.D.; Beuer, F.; Zafiropoulos, G.G.; Houshmand, A. Hydrophilicity, viscoelastic, and physicochemical properties variations in dental bone grafting substitutes. Materials 2018, 11, 215. [Google Scholar] [CrossRef]
- Zafiropoulos, G.; Kačarević, Z.P.; Qasim, S.S.B.; Trajkovski, B. Open-healing socket preservation with a novel dense polytetrafluoroethylene (dPTFE) membrane: A retrospective clinical study. Medicina 2020, 56, 216. [Google Scholar] [CrossRef]
- Jelusic, D.; Zirk, M.L.; Fienitz, T.; Plancak, D.; Puhar, I.; Rothamel, D. Monophasic ß-TCP vs. biphasic HA/ß-TCP in two-stage sinus floor augmentation procedures—A prospective randomized clinical trial. Clin. Oral Implants Res. 2017, 28, e175–e183. [Google Scholar] [CrossRef]
- Gauthier, O.; Bouler, J.M.; Aguado, E.; Legeros, R.Z.; Pilet, P.; Daculsi, G. Elaboration conditions influence physicochemical properties and in vivo bioactivity of macroporous biphasic calcium phosphate ceramics. J. Mater. Sci. Mater. Med. 1999, 10, 199–204. [Google Scholar] [CrossRef]
- Schwartz, C.; Liss, P.; Jacquemaire, B.; Lecestre, P.; Frayssinet, P. Biphasic synthetic bone substitute use in orthopaedic and trauma surgery: Clinical, radiological and histological results. J. Mater. Sci. Mater. Med. 1999, 10, 821–825. [Google Scholar] [CrossRef] [PubMed]
- Daculsi, G. Biphasic calcium phosphate concept applied to artificial bone, implant coating and injectable bone substitute. Biomaterials 1998, 19, 1473–1478. [Google Scholar] [CrossRef]
- Ducheyne, P.; Radin, S.; King, L. The effect of calcium phosphate ceramic composition and structure on in vitro behavior. I. Dissolution. J. Biomed. Mater. Res. 1993, 27, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Peev, S.; Gusiyska, A.; Sabeva, E. Guided Bone Regeneration and Simultaneous Implant Placement. IJSR. 2016, 5, 1529–1530. [Google Scholar]
- Georgiev, T.; Peev, S.; Arnautska, H.; Gencheva, A.; Gerdzhikov, I. An Evaluation of Three-Dimensional Scans of the Time-Dependent Volume Changes in Bone Grafting Materials. Int. J. Sci. Res. ISSN 2015, 6, 2319–7064. [Google Scholar] [CrossRef]
- Barbeck, M.; Jung, O.; Smeets, R.; Gosau, M.; Schnettler, R.; Rider, P.; Houshmand, A.; Korzinskas, T. Implantation of an injectable bone substitute material enables integration following the principles of guided bone regeneration. In Vivo (Brooklyn) 2020, 34, 557–568. [Google Scholar] [CrossRef]
- Friedman, S. Considerations and concepts of case selection in the management of post-treatment endodontic disease (treatment failure). Endod. Top. 2002, 1, 54–78. [Google Scholar] [CrossRef]
- Touré, B.; Faye, B.; Kane, A.W.; Lo, C.M.; Niang, B.; Boucher, Y. Analysis of reasons for extraction of endodontically treated teeth: A prospective study. J. Endod. 2011, 37, 1512–1515. [Google Scholar] [CrossRef]
- Olcay, K.; Ataoglu, H.; Belli, S. Evaluation of Related Factors in the Failure of Endodontically Treated Teeth: A Cross-sectional Study. J. Endod. 2018, 44, 38–45. [Google Scholar] [CrossRef]
- Norton, M.R.; Gamble, C. Bone classification: An objective scale of bone density using the computerized tomography scan. Clin. Oral Implants Res. 2001, 12, 79–84. [Google Scholar] [CrossRef]
- Mah, P.; Reeves, T.E.; McDavid, W.D. Deriving Hounsfield units using grey levels in cone beam computed tomography. Dentomaxillofac. Radiol. 2010, 39, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Fujioka-Kobayashi, M.; Schaller, B.; Zhang, Y.; Pippenger, B.E.; Miron, R.J. In vitro evaluation of an injectable biphasic calcium phosphate (BCP) carrier system combined with recombinant human bone morphogenetic protein (rhBMP)-9. Biomed. Mater. Eng. 2017, 28, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Papanchev, G.; Georgiev, T.; Peev, S.; Arnautska, H.; Zgurova, N.; Borisova-Papancheva, T.; Dzhongova, E. Comparison of the rates of bone regeneration in Sinus lift grafting with a Calcium-Phosphate paste between the 6th and the 9th month—A clinical case. Scr. Sci. Med. Dent. 2015, 1, 41. [Google Scholar] [CrossRef][Green Version]
- Soldatos, N.K.; Stylianou, P.; Angelov, N.; Koidou, P.; Yukna, R.; Romanos, G.E. Limitations and options using resorbable versus nonresorbable membranes for successful guided bone regeneration. Quintessence Int. 2017, 48, 131–147. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Čandrlić, M.; Perić Kačarević, Ž.; Ivanišević, Z.; Tomas, M.; Včev, A.; Faj, D.; Matijević, M. Histological and Radiological Features of a Four-Phase Injectable Synthetic Bone Graft in Guided Bone Regeneration: A Case Report. Int. J. Environ. Res. Public Health 2021, 18, 206. https://doi.org/10.3390/ijerph18010206
Čandrlić M, Perić Kačarević Ž, Ivanišević Z, Tomas M, Včev A, Faj D, Matijević M. Histological and Radiological Features of a Four-Phase Injectable Synthetic Bone Graft in Guided Bone Regeneration: A Case Report. International Journal of Environmental Research and Public Health. 2021; 18(1):206. https://doi.org/10.3390/ijerph18010206
Chicago/Turabian StyleČandrlić, Marija, Željka Perić Kačarević, Zrinka Ivanišević, Matej Tomas, Aleksandar Včev, Dario Faj, and Marko Matijević. 2021. "Histological and Radiological Features of a Four-Phase Injectable Synthetic Bone Graft in Guided Bone Regeneration: A Case Report" International Journal of Environmental Research and Public Health 18, no. 1: 206. https://doi.org/10.3390/ijerph18010206
APA StyleČandrlić, M., Perić Kačarević, Ž., Ivanišević, Z., Tomas, M., Včev, A., Faj, D., & Matijević, M. (2021). Histological and Radiological Features of a Four-Phase Injectable Synthetic Bone Graft in Guided Bone Regeneration: A Case Report. International Journal of Environmental Research and Public Health, 18(1), 206. https://doi.org/10.3390/ijerph18010206







