Particulate Matter Decreases Intestinal Barrier-Associated Proteins Levels in 3D Human Intestinal Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Tissue Culture
2.2. Transepithelial Resistance (TEER) Measurements
2.3. Tissue Collection
2.4. Tissue Immunofluorescence
3. Results
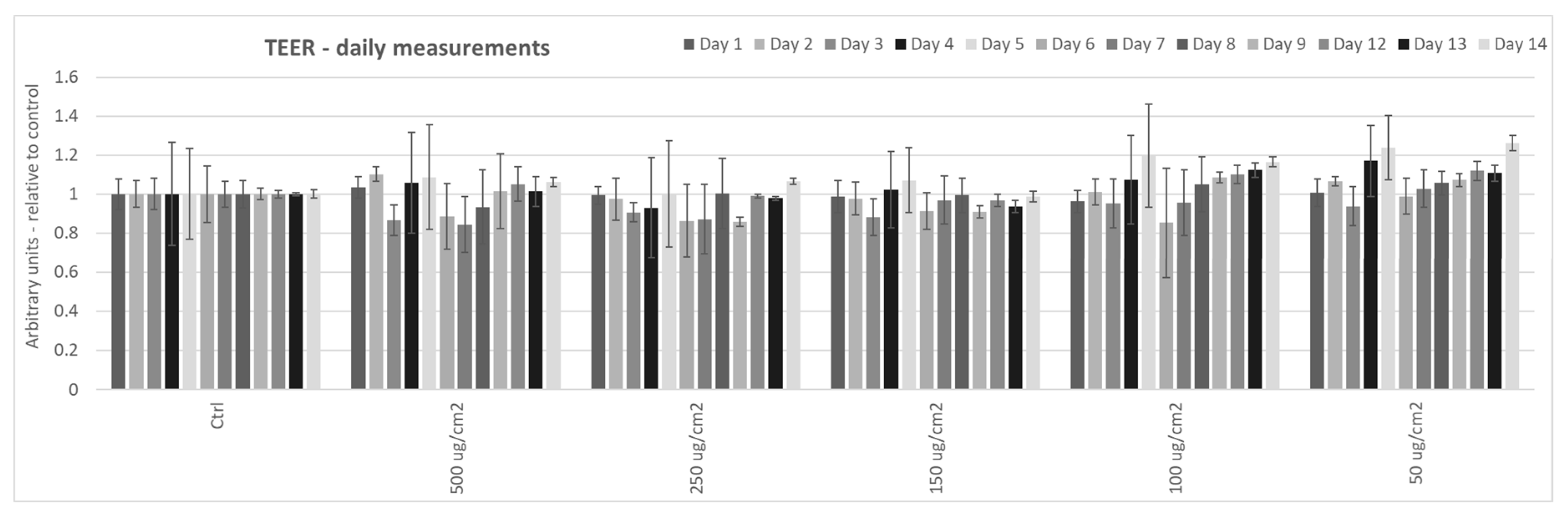
3.1. Effect of Particulate Matter On Transepithelial Electrical Resistance (TEER) in 3D Tissues
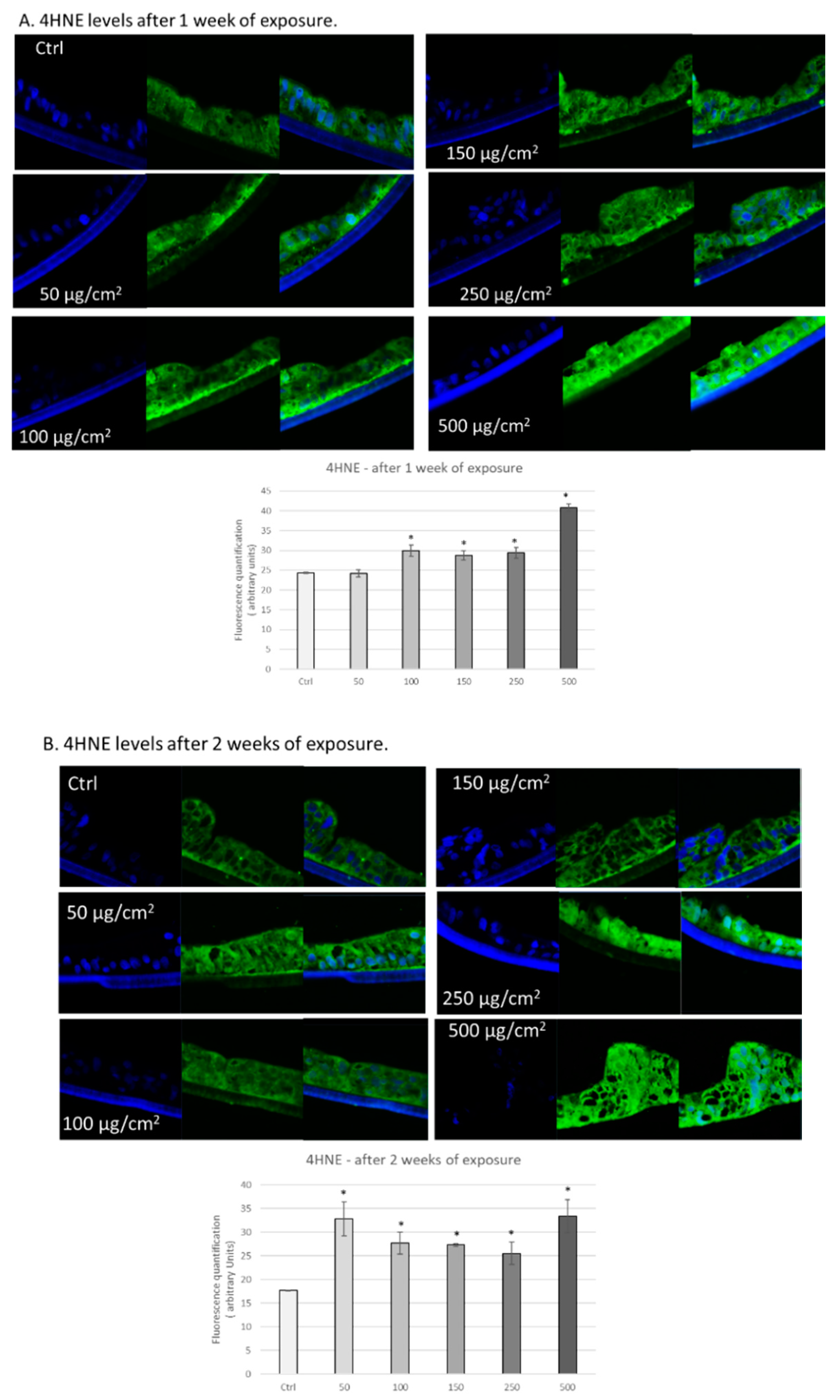
3.2. PM Exposure Increased 4-Hydroxynonenal Levels in 3D Human Intestinal Tissues
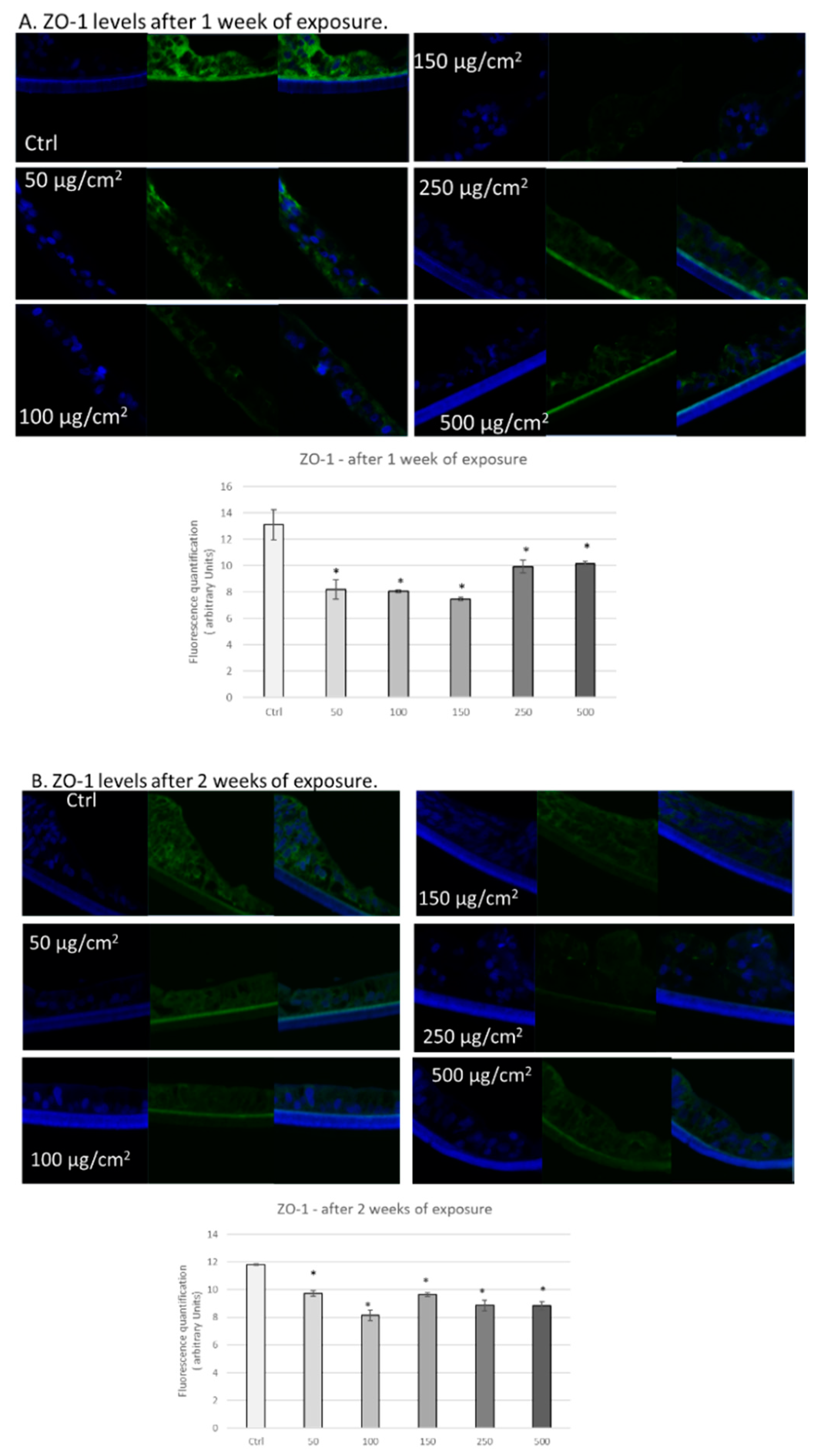
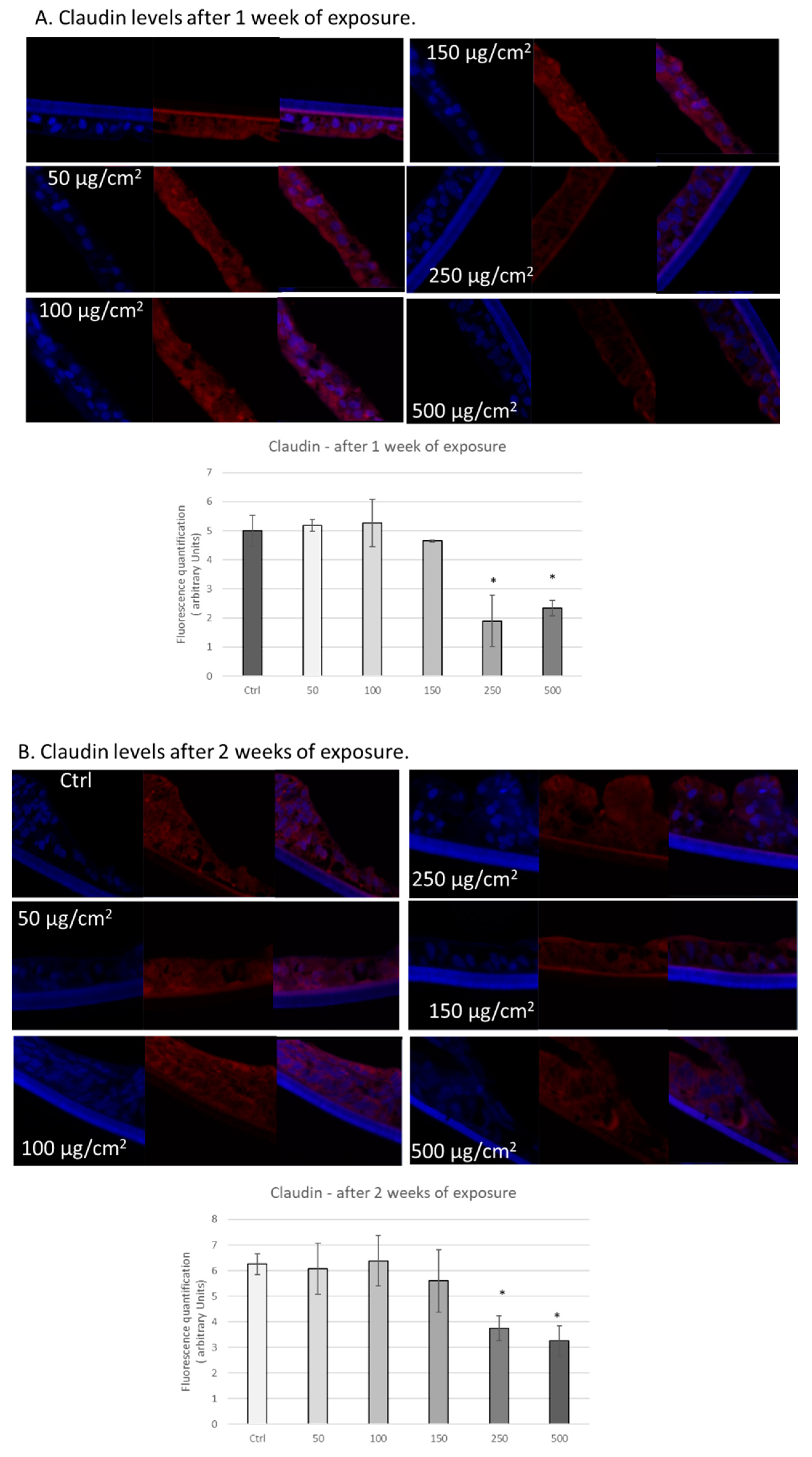
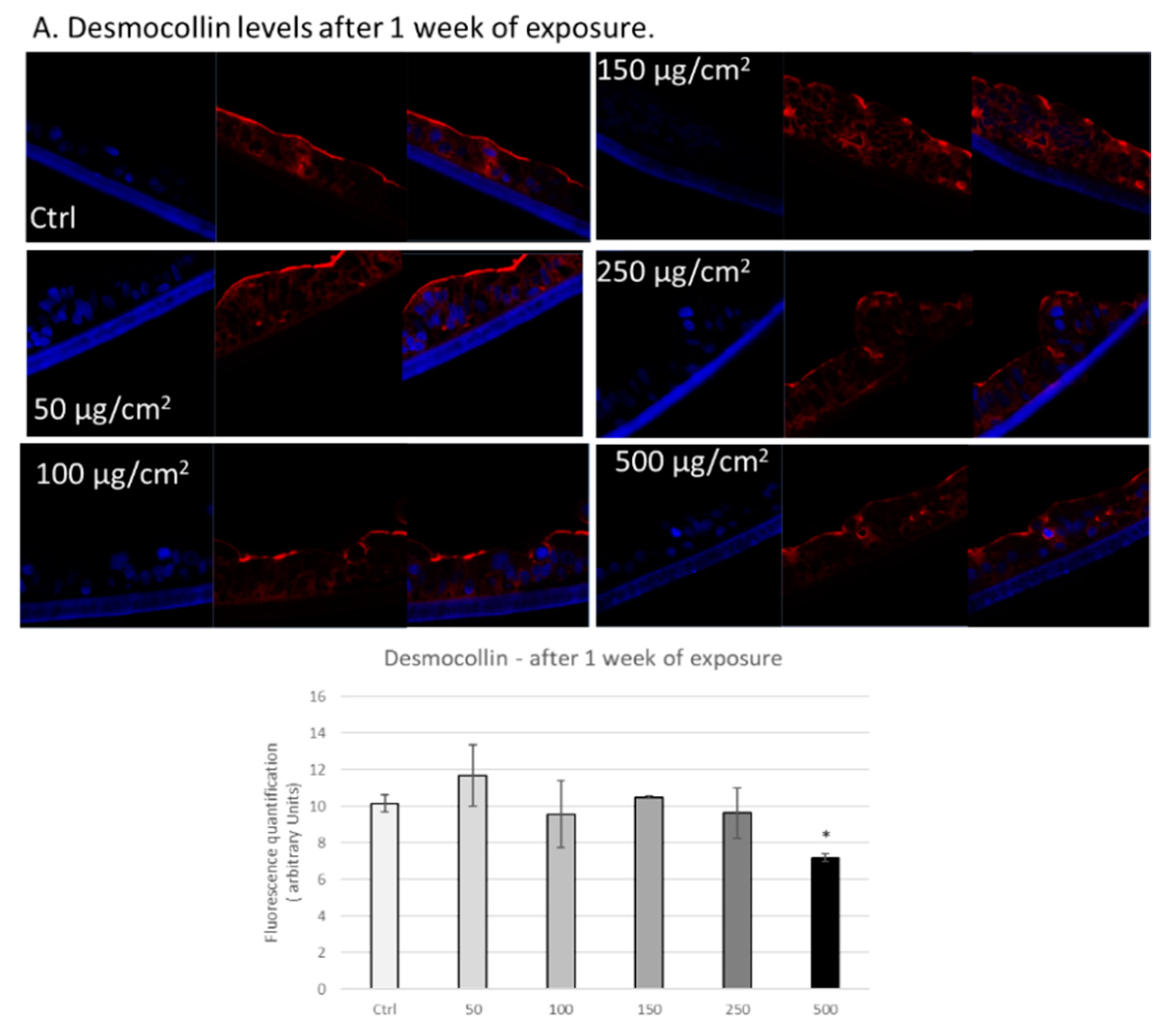
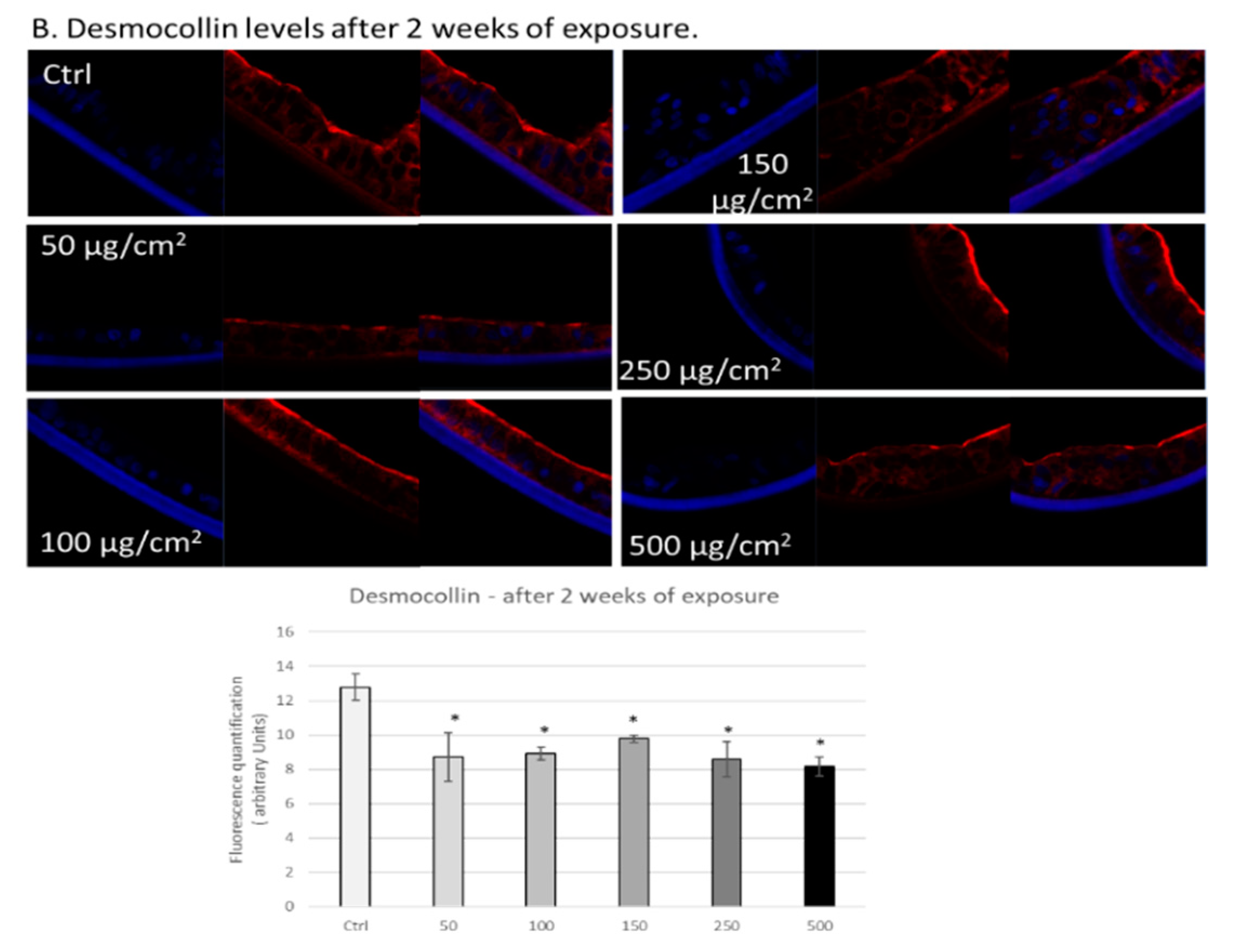
3.3. PM Exposure of 3D Human Intestinal Tissues Decreases Levels of Tight Junction Proteins and Desmocollin
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Burnett:, R.; Chen, H.; Szyszkowicz, M.; Fann, N.; Hubbell, B.; Pope, C.A.; Apte, J.S.; Brauer, M.; Cohen, A.; Weichenthal, S.; et al. Global estimates of mortality associated with long-term exposure to outdoor fine particulate matter. Proc. Natl. Acad. Sci. USA 2018, 115, 9592–9597. [Google Scholar] [CrossRef] [PubMed]
- Lelieveld, J.; Klingmüller, K.; Pozzer, A.; Pöschl, U.; Fnais, M.; Daiber, A.; Münzel, T. Cardiovascular disease burden from ambient air pollution in Europe reassessed using novel hazard ratio functions. Eur. Heart J. 2019, 40, 1590–1596. [Google Scholar] [CrossRef] [PubMed]
- Brook, R.D.; Rajagopalan, S.; Pope, C.A., III; Brook, J.R.; Bhatnagar, A.; Diez-Roux, A.V.; Holguin, F.; Hong, Y.; Luepker, R.V.; Mittleman, M.; et al. Particulate matter air pollution and cardiovascular disease: An. update to the scientific statement from the American Heart Association. Circulation 2010, 121, 2331–2378. [Google Scholar] [CrossRef] [PubMed]
- Schaumann, F.; Borm, P.J.; Herbrich, A.; Knoch, J.; Pitz, M.; Schins, R.P.; Luettig, B.; Hohlfeld, J.M.; Heinrich, J.; Krug, N. Metal−rich ambient particles (particulate matter 2.5) cause airway inflammation in healthy subjects. Am. J. Respir. Crit. Care Med. 2004, 170, 898–903. [Google Scholar] [CrossRef] [PubMed]
- Vierkotter, A.; Schikowski, T.; Ranft, U.; Sugiri, D.; Matsui, M.; Krämer, U.; Krutmann, J. Airborne particle exposure and extrinsic skin aging. J. Investig. Dermatol. 2010, 130, 2719–2726. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Chen, X.; Xu, Y.; Wu, W.; Tang, W.; Chen, Z.; Li, X.; Ji, G.Y.; Peng, J.W.; Jiang, Q.; et al. Gut microbiota partially mediates the effects of fine particulate matter on type 2 diabetes: Evidence from a population-based epidemiological study. Environ. Int. 2019, 130, 104882. [Google Scholar] [CrossRef] [PubMed]
- Nel, A.; Xia, T.; Mädler, L.; Li, N. Toxic potential of materials at the nanolevel. Science 2006, 311, 622–627. [Google Scholar] [CrossRef]
- Mukherjee, A.; Agrawal, M. World air particulate matter: Sources, distribution and health effects. Environ. Chem. Lett. 2017, 15, 283–309. [Google Scholar] [CrossRef]
- Kelly, F.J.; Fussell, J.C. Size, source and chemical composition as determinants of toxicity attributable to ambient particulate matter. Atmos. Environ. 2012, 60, 504–526. [Google Scholar] [CrossRef]
- Dominici, F.; Peng, R.D.; Bell, M.L.; Pham, L.; McDermott, L.; Zeger, S.L.; Samet, J.M. Fine particulate air pollution and hospital admission for cardiovascular and respiratory diseases. JAMA 2006, 295, 1127–1134. [Google Scholar] [CrossRef]
- Pope III, C.A.; Cohen, A.J.; Burnett, R.T. Cardiovascular Disease and Fine Particulate Matter: Lessons and Limitations of an Integrated Exposure-Response Approach. Circ. Res. 2018, 122, 1645–1647. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, G.G.; Hubbard, J.; Korzenik, J.; Sands, B.E.; Panaccione, R.; Ghosh, S.; Villeneuve, P.J. The inflammatory bowel diseases and ambient air pollution: A novel association. Am. J. Gastroenterol. 2010, 105, 2412–2419. [Google Scholar] [CrossRef] [PubMed]
- Lomer, M.C.; Hutchinson, C.; Volkert, S.; Greenfield, S.M.; Catterall, A.; Thompson, R.P.; Powell, J.J. Dietary sources of inorganic microparticles and their intake in healthy subjects and patients with Crohn’s disease. Br. J. Nutr. 2004, 92, 947–955. [Google Scholar] [CrossRef] [PubMed]
- Lomer, M.C.; Thompson, R.P.; Powell, J.J. Fine and ultrafine particles of the diet: Influence on the mucosal immune response and association with Crohn’s disease. Proc. Nutr. Soc. 2002, 61, 123–130. [Google Scholar] [CrossRef] [PubMed]
- De Verdier, M.G.; Plato, N.; Steineck, G.; Peters, J.M. Occupational exposures and cancer of the colon and rectum. Am. J. Ind. Med. 1992, 22, 291–303. [Google Scholar] [CrossRef]
- Gubéran, E.; Usel, M.; Raymond, L.; Bolay, J.; Fioretta, G.; Puissant, J. Increased risk for lung cancer and for cancer of the gastrointestinal tract among Geneva professional drivers. Br. J. Ind. Med. 1992, 49, 337–344. [Google Scholar]
- Semmler, M.; Seitz, J.; Erbe, F.; Mayer, P.; Heyder, J.; Oberdörster, G.; Kreyling, W.G. Long-term clearance kinetics of inhaled ultrafine insoluble iridium particles from the rat lung, including transient translocation into secondary organs. Inhal. Toxicol. 2004, 16, 453–459. [Google Scholar] [CrossRef]
- Semmler-Behnke, M.; Takenaka, S.; Fertsch, S.; Wenk, A.; Seitz, J.; Mayer, P.; Oberdörster, G.; Kreyling, W.G. Efficient elimination of inhaled nanoparticles from the alveolar region: Evidence for interstitial uptake and subsequent reentrainment onto airways epithelium. Environ. Health Perspect. 2007, 115, 728–733. [Google Scholar] [CrossRef]
- Lee, S.H. Intestinal permeability regulation by tight junction: Implication on inflammatory bowel diseases. Intest. Res. 2015, 13, 11–18. [Google Scholar] [CrossRef]
- Balda, M.S.; Matter, K. Tight junctions and the regulation of gene expression. Biochim. Biophys. Acta (BBA)—Biomembr. 2009, 1788, 761–767. [Google Scholar] [CrossRef]
- Delva, E.; Tucker, D.K.; Kowalczyk, A.P. The desmosome. Cold Spring Harb Perspect Biol. 2009, 1, a002543. [Google Scholar] [CrossRef] [PubMed]
- Garrod, D.; Chidgey, M. Desmosome structure, composition and function. Biochim. Biophys. Acta 2008, 1778, 572–587. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, A.P.; Green, K.J. Structure, function, and regulation of desmosomes. Prog. Mol. Biol. Transl. Sci. 2013, 116, 95–118. [Google Scholar] [PubMed]
- Ungewiß, H.; Vielmuth, F.; Suzuki, S.T.; Maiser, A.; Harz, H.; Leonhardt, H.; Kugelmann, D.; Schlegel, N.; Waschke, J. Desmoglein 2 regulates the intestinal epithelial barrier via p38 mitogen-activated protein kinase. Sci. Rep. 2017, 7, 6329. [Google Scholar]
- Li, D.; Cong, Z.; Yang, C.; Zhu, X. Inhibition of LPS-induced Nox2 activation by VAS2870 protects alveolar epithelial cells through eliminating ROS and restoring tight junctions. Biochem. Biophys. Res. Commun. 2020, 524, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Wu, H.; Wang, X.; He, J.; He, S.; Yin, Y. Resveratrol Attenuates Oxidative Stress-Induced Intestinal Barrier Injury through PI3K/Akt-Mediated Nrf2 Signaling Pathway. Oxid. Med. Cell Longev. 2019, 7591840. [Google Scholar] [CrossRef]
- Han, X.; Fink, M.P.; Delude, R.L. Proinflammatory cytokines cause NO*-dependent and -independent changes in expression and localization of tight junction proteins in intestinal epithelial cells. Shock 2003, 19, 229–237. [Google Scholar] [CrossRef]
- Mutlu, E.A.; Engen, P.A.; Soberanes, S.; Urich, D.; Forsyth, C.B.; Nigdelioglu, R.; Chiarella, S.E.; Radigan, K.A.; Gonzalez, A.; Jakate, S.; et al. Particulate matter air pollution causes oxidant-mediated increase in gut permeability in mice. Part. Fibre. Toxicol. 2011, 8, 19. [Google Scholar] [CrossRef]
- Li, R.; Yang, J.; Saffari, A.; Jacobs, J.; Baek, K.I.; Hough, G.; Zhou, B. Ambient Ultrafine Particle Ingestion Alters Gut Microbiota in Association with Increased Atherogenic Lipid Metabolites. Sci. Rep. 2017, 7, 42906. [Google Scholar] [CrossRef]
- Balimane, P.V.; Chong, S. Cell culture-based models for intestinal permeability: A critique. Drug Discov. Today 2005, 10, 335–343. [Google Scholar] [CrossRef]
- Aldhous, M.C.; Shmakov, A.N.; Bode, J.; Ghosh, S. Characterization of conditions for the primary culture of human small intestinal epithelial cells. Clin. Exp. Immunol. 2001, 125, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.; Costa, J.; Sarmento, B.; Araújo, F. Cell-based in vitro models for intestinal permeability studies. In Concepts and Models for Drug Permeability Studies; Woodhead Publishing: Sawston/Cambridge, UK, 2016; pp. 57–81. [Google Scholar]
- Koval, M.; Ward, C.; Findley, M.K.; Roser-Page, S.; Helms, M.N.; Roman, J. Extracellular matrix influences alveolar epithelial claudin expression and barrier function. Am. J. Respir. Cell Mol. Biol. 2010, 42, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.L.A.; Vieira-Silva, S.; Liston, A.; Raes, J. How informative is the mouse for human gut microbiota research? Dis. Models Mech. 2015, 8, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Ayehunie, S.; Stevens, Z.; Landry, T.; Klausner, M.; Hayden, P.; Letasiova, S. Novel 3-D human small inestinal tissue model to assess drug permeation, inflammation, and wound healing. Toxicol. Lett. 2014, 229. [Google Scholar] [CrossRef]
- Vignal, C.; Pichavant, M.; Alleman, L.Y.; Djouina, M.; Dingreville, F.; Perdrix, E.; Body-Malapel, M. Effects of urban coarse particles inhalation on oxidative and inflammatory parameters in the mouse lung and colon. Part. Fibre Toxicol. 2017, 14, 46. [Google Scholar] [CrossRef]
- Cervellati, F.; Benedusi, M.; Manarini, F.; Woodby, B.; Russo, M.; Valacchi, G.; Pietrogrande, M.C. Proinflammatory properties and oxidative effects of atmospheric particle components in human keratinocytes. Chemosphere 2020, 240, 124746. [Google Scholar] [CrossRef]
- Ferrara, F.; Pambianchi, E.; Pecorelli, A.; Woodby, B.; Therrien, J.P.; Lila, M.A.; Valacchi, G. Redox regulation of cutaneous inflammasome by ozone exposure. Free Radic. Biol. Med. 2019. [Google Scholar] [CrossRef]
- Pecorelli, A.; Woodby, B.; Prieux, R.; Valacchi, G. Involvement of 4-hydroxy−2-nonenal in pollution-induced skin damage. Biofactors 2019, 45, 536–547. [Google Scholar] [CrossRef]
- Itoh, M.; Yonemura, S.; Nagafuchi, A.; Tsukita, S.; Tsukita, S. A 220-kD undercoat-constitutive protein: Its specific localization at cadherin-based cell-cell adhesion sites. J. Cell Biol. 1991, 115, 1449–1462. [Google Scholar] [CrossRef][Green Version]
- Stevenson, B.R.; Siliciano, J.D.; Mooseker, M.S.; Goodenough, D.A. Identification of ZO−1: A high molecular weight polypeptide associated with the tight junction (zonula occludens) in a variety of epithelia. J. Cell Biol. 1986, 103, 755–766. [Google Scholar] [CrossRef]
- Fanning, A.S.; Jameson, B.J.; Jesaitis, L.A.; Anderson, J.M. The tight junction protein ZO−1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton. J. Biol. Chem. 1998, 273, 29745–29753. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Wang, L.; Moreno-Vinasco, L.; Lang, G.D.; Siegler, J.H.; Mathew, B.; Natarajan, V. Particulate matter air pollution disrupts endothelial cell barrier via calpain-mediated tight junction protein degradation. Part. Fibre. Toxicol. 2012, 9, 35. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.M.; Van Itallie, C.M. Tight junctions and the molecular basis for regulation of paracellular permeability. Am. J. Physiol. 1995, 269 (4 Pt 1), G467–G475. [Google Scholar] [CrossRef] [PubMed]
- Kirschner, N.; Kirschner, N.; Rosenthal, R.; Furuse, M.; Moll, I.; Fromm, M.; Brandner, J.M. Contribution of tight junction proteins to ion, macromolecule, and water barrier in keratinocytes. J. Investig. Dermatol. 2013, 133, 1161–1169. [Google Scholar] [CrossRef]
- Gunzel, D.; Yu, A.S. Claudins and the modulation of tight junction permeability. Physiol. Rev. 2013, 93, 525–569. [Google Scholar] [CrossRef]
- Anderson, J.M.; Van Itallie, C.M. Physiology and function of the tight junction. Cold Spring Harb. Perspect. Biol. 2009, 1, a002584. [Google Scholar] [CrossRef]
- Altemose, B.; Robson, M.G.; Kipen, H.M.; Strickland, P.O.; Meng, Q.; Gong, J.; Zhang, J. Association of air pollution sources and aldehydes with biomarkers of blood coagulation, pulmonary inflammation, and systemic oxidative stress. J. Exp. Scie. Environ. Epidemiol. 2017, 27, 244–250. [Google Scholar] [CrossRef]
- Kish, L.; Hotte, N.; Kaplan, G.G.; Vincent, R.; Tso, R.; Gänzle, M.; Madsen, K.L. Environmental particulate matter induces murine intestinal inflammatory responses and alters the gut microbiome. PLoS ONE 2013, 8, e62220. [Google Scholar] [CrossRef]
- Li, R.; Navab, K.; Hough, G.; Daher, N.; Zhang, M.; Mittelstein, D.; Lee, K.; Pakbin, P.; Saffari, A.; Bhetraratana, M.; et al. Effect of exposure to atmospheric ultrafine particles on production of free fatty acids and lipid metabolites in the mouse small intestine. Environ. Health Perspect. 2015, 123, 34–41. [Google Scholar] [CrossRef]
- Magnani, N.D.; Muresan, X.M.; Belmonte, G.; Cervellati, F.; Sticozzi, C.; Pecorelli, A.; Miracco, C.; Marchini, T.; Evelson, P.; Valacchi, G. Skin Damage Mechanisms Related to Airborne Particulate Matter Exposure. Toxicol. Sci. 2016, 149, 227–236. [Google Scholar] [CrossRef]
- Chelakkot, C.; Ghim, J.; Ryu, S.H. Mechanisms regulating intestinal barrier integrity and its pathological implications. Exp. Mol. Med. 2018, 50, 103. [Google Scholar] [CrossRef] [PubMed]
- Hamidov, Z.; Altendorf-Hofmann, A.; Chen, Y.; Settmacher, U.; Petersen, I.; Knösel, T. Reduced expression of desmocollin 2 is an independent prognostic biomarker for shorter patients survival in pancreatic ductal adenocarcinoma. J. Clin. Pathol. 2011, 64, 990–994. [Google Scholar] [CrossRef] [PubMed]
- Shigetomi, K.; Ikenouchi, J. Regulation of the epithelial barrier by post-translational modifications of tight junction membrane proteins. J. Biochem. 2018, 163, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Al-Sadi, R.; Guo, S.; Ye, D.; Rawat, M.; Ma, T.Y. TNF-alpha Modulation of Intestinal Tight Junction Permeability Is Mediated by NIK/IKK-alpha Axis Activation of the Canonical NF-kappaB Pathway. Am. J. Pathol. 2016, 186, 1151–1165. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, K.E.; Turner, J.R. Myosin light chain kinase: Pulling the strings of epithelial tight junction function. Ann. N. Y. Acad. Sci. 2012, 1258, 34–42. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Woodby, B.; Schiavone, M.L.; Pambianchi, E.; Mastaloudis, A.; N. Hester, S.; M. Wood, S.; Pecorelli, A.; Valacchi, G. Particulate Matter Decreases Intestinal Barrier-Associated Proteins Levels in 3D Human Intestinal Model. Int. J. Environ. Res. Public Health 2020, 17, 3234. https://doi.org/10.3390/ijerph17093234
Woodby B, Schiavone ML, Pambianchi E, Mastaloudis A, N. Hester S, M. Wood S, Pecorelli A, Valacchi G. Particulate Matter Decreases Intestinal Barrier-Associated Proteins Levels in 3D Human Intestinal Model. International Journal of Environmental Research and Public Health. 2020; 17(9):3234. https://doi.org/10.3390/ijerph17093234
Chicago/Turabian StyleWoodby, Brittany, Maria Lucia Schiavone, Erika Pambianchi, Angela Mastaloudis, Shelly N. Hester, Steven M. Wood, Alessandra Pecorelli, and Giuseppe Valacchi. 2020. "Particulate Matter Decreases Intestinal Barrier-Associated Proteins Levels in 3D Human Intestinal Model" International Journal of Environmental Research and Public Health 17, no. 9: 3234. https://doi.org/10.3390/ijerph17093234
APA StyleWoodby, B., Schiavone, M. L., Pambianchi, E., Mastaloudis, A., N. Hester, S., M. Wood, S., Pecorelli, A., & Valacchi, G. (2020). Particulate Matter Decreases Intestinal Barrier-Associated Proteins Levels in 3D Human Intestinal Model. International Journal of Environmental Research and Public Health, 17(9), 3234. https://doi.org/10.3390/ijerph17093234





