Symptom-Related Distress among Indigenous Australians in Specialist End-of-Life Care: Findings from the Multi-Jurisdictional Palliative Care Outcomes Collaboration Data
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Data Source
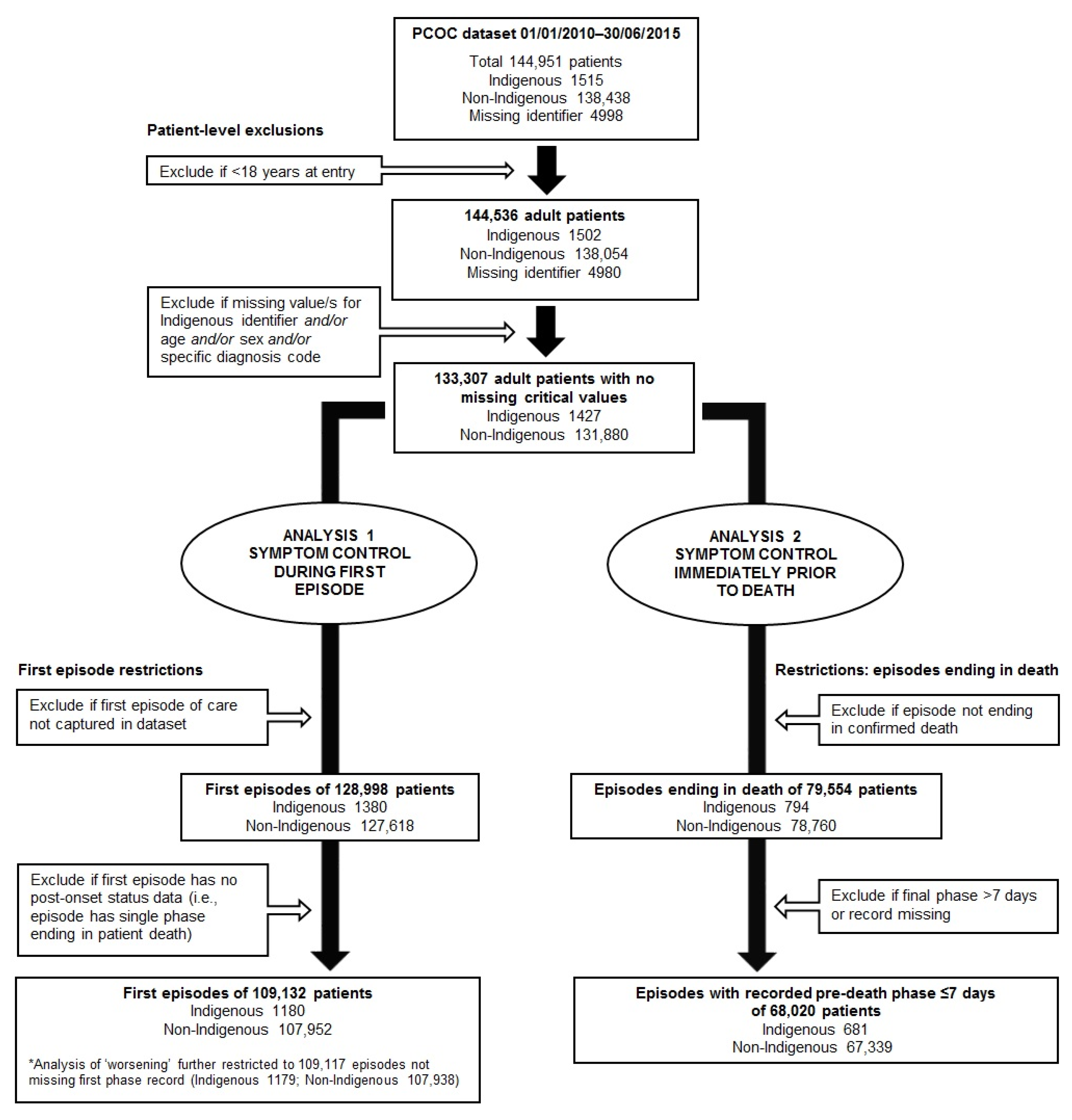
2.2. Study Participants and Episode of Care Inclusion Criteria
2.3. Study Measures
2.4. Statistical Analysis
2.5. Ethics
3. Results
3.1. Objective 1: First Episodes
3.2. Objective 2: ‘Pre-death’ Episodes
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Data Availability Statement
References
- Walling, A.M.; Asch, S.M.; Lorenz, K.A.; Roth, C.P.; Barry, T.; Kahn, K.L.; Wenger, N.S. The quality of care provided to hospitalized patients at the end of life. Arch. Intern. Med. 2010, 170, 1057–1063. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.H. Overcoming barriers in cancer pain management. J. Clin. Oncol. 2014, 32, 1727–1733. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.T.; Butow, P.N.; Costa, D.S.J.; Lovell, M.R.; Agar, M. Symptom clusters in patients with advanced cancer: A systematic review of observational studies. J. Pain Symptom Manag. 2014, 48, 411–450. [Google Scholar] [CrossRef] [PubMed]
- Moens, K.; Higginson, I.J.; Harding, R. Are there differences in the prevalence of palliative care-related problems in people living with advanced cancer and eight non-cancer conditions? A systematic review. J. Pain Symptom Manag. 2014, 48, 660–677. [Google Scholar] [CrossRef]
- Dong, S.T.; Butow, P.N.; Tong, A.; Agar, M.; Boyle, F.; Forster, B.C.; Stockler, M.; Lovell, M.R. Patients’ experiences and perspectives of multiple concurrent symptoms in advanced cancer: A semi-structured interview study. Support. Care Cancer 2016, 24, 1373–1386. [Google Scholar] [CrossRef]
- WHO Definition of Palliative Care. Available online: http://www.who.int/cancer/palliative/definition/en/ (accessed on 4 March 2020).
- Clark, K. Care at the very end-of-life: Dying cancer patients and their chosen family’s needs. Cancers 2017, 9, 11. [Google Scholar] [CrossRef]
- Aspin, C.; Brown, N.; Jowsey, T.; Yen, L.; Leeder, S. Strategic approaches to enhanced health service delivery for Aboriginal and Torres Strait Islander people with chronic illness: A qualitative study. BMC Health Serv. Res. 2012, 12, 143. [Google Scholar] [CrossRef]
- Durey, A.; Thompson, S.C.; Wood, M. Time to bring down the twin towers in poor Aboriginal hospital care: Addressing institutional racism and misunderstandings in communication. Intern. Med. J. 2012, 42, 17–22. [Google Scholar] [CrossRef]
- Department of the Prime Minister and Cabinet. Closing the Gap Report 2019; Commonwealth of Australia: Canberra, Australia, 2019.
- Vos, T.; Barker, B.; Begg, S.; Stanley, L.; Lopez, A.D. Burden of disease and injury in Aboriginal and Torres Strait Islander Peoples: The indigenous health gap. Int. J. Epidemiol. 2009, 38, 470–477. [Google Scholar] [CrossRef]
- Amery, R. Recognising the communication gap in Indigenous health care. Med. J. Aust. 2017, 207, 13–15. [Google Scholar] [CrossRef]
- Jennings, W.; Bond, C.; Hill, P.S. The power of talk and power in talk: A systematic review of Indigenous narratives of culturally safe healthcare communication. Aust. J. Prim. Health 2018, 24, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Shahid, S.; Finn, L.D.; Thompson, S.C. Barriers to participation of Aboriginal people in cancer care: Communication in the hospital setting. Med. J. Aust. 2009, 190, 574–579. [Google Scholar] [CrossRef] [PubMed]
- McGrath, P. ‘The biggest worry’: Research findings on pain management for Aboriginal peoples in Northern Territory, Australia. Rural Remote Health 2006, 6, 549. [Google Scholar] [PubMed]
- Eagar, K.; Watters, P.; Currow, D.C.; Aoun, S.M.; Yates, P. The Australian Palliative Care Outcomes Collaboration (PCOC)–measuring the quality and outcomes of palliative care on a routine basis. Aust. Health Rev. 2010, 34, 186–192. [Google Scholar] [CrossRef]
- Clapham, S.; Holloway, A. Palliative Care Outcomes Collaboration. Palliative Care Outcomes Collaboration–Clinical Manual August 2017; Australian Health Services Research Institute (AHSRI), University of Wollongong: Wollongong, Australia, 2017. [Google Scholar]
- Woods, J.A.; Newton, J.C.; Thompson, S.C.; Malacova, E.; Ngo, H.T.; Katzenellenbogen, J.M.; Murray, K.; Shahid, S.; Johnson, C.E. Indigenous compared with non-Indigenous Australian patients at entry to specialist palliative care: Cross-sectional findings from a multi-jurisdictional dataset. PLoS ONE 2019, 14, e0215403. [Google Scholar] [CrossRef]
- Mather, H.; Guo, P.; Firth, A.; Davies, J.M.; Sykes, N.; Landon, A.; Murtagh, F.E. Phase of Illness in palliative care: Cross-sectional analysis of clinical data from community, hospital and hospice patients. Palliat. Med. 2018, 32, 404–412. [Google Scholar] [CrossRef]
- Masso, M.; Allingham, S.F.; Banfield, M.; Johnson, C.E.; Pidgeon, T.; Yates, P.; Eagar, K. Palliative Care Phase: Inter-rater reliability and acceptability in a national study. Palliat. Med. 2015, 29, 22–30. [Google Scholar] [CrossRef]
- Aoun, S.M.; Monterosso, L.; Kristjanson, L.J.; McConigley, R. Measuring symptom distress in palliative care: Psychometric properties of the Symptom Assessment Scale (SAS). J. Palliat. Med. 2011, 14, 315–321. [Google Scholar] [CrossRef]
- Ekstrom, M.; Allingham, S.F.; Eagar, K.; Yates, P.; Johnson, C.; Currow, D.C. Breathlessness During the Last Week of Life in Palliative Care: An Australian Prospective, Longitudinal Study. J. Pain Symptom Manag. 2016, 51, 816–823. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Statist. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Eagar, K.; Clapham, S.P.; Allingham, S.F. Palliative care is effective: But hospital symptom outcomes superior. BMJ Support. Palliat. Care 2018. [Google Scholar] [CrossRef]
- McCarthy, E.P.; Phillips, R.S.; Zhong, Z.; Drews, R.E.; Lynn, J. Dying with cancer: Patients’ function, symptoms, and care preferences as death approaches. J. Am. Geriatr. Soc. 2000, 48, S110–S121. [Google Scholar] [CrossRef] [PubMed]
- Levenson, J.W.; McCarthy, E.P.; Lynn, J.; Davis, R.B.; Phillips, R.S. The last six months of life for patients with congestive heart failure. J. Am. Geriatr. Soc. 2000, 48, S101–S109. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Keenan, G.; Al-Masalha, F.; Lopez, K.D.; Khokar, A.; Johnson, A.; Ansari, R.; Wilkie, D.J. Current State of Pain Care for Hospitalized Patients at End of Life. Am. J. Hosp. Palliat. Care 2013, 30, 128–136. [Google Scholar] [CrossRef]
- Campbell, M.L.; Kiernan, J.M.; Strandmark, J.; Yarandi, H.N. Trajectory of Dyspnea and Respiratory Distress among Patients in the Last Month of Life. J. Palliat. Med. 2018, 21, 194–199. [Google Scholar] [CrossRef]
- McGrath, P. ‘I don’t want to be in that big city; this is my country here’: Research findings on Aboriginal peoples’ preference to die at home. Aust. J. Rural. Health 2007, 15, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Strong, J.; Nielsen, M.; Williams, M.; Huggins, J.; Sussex, R. Quiet about pain: Experiences of Aboriginal people in two rural communities. Aust. J. Rural Health 2015, 23, 181–184. [Google Scholar] [CrossRef]
- Australian Institute of Health and Welfare. Australia’s Health 2016; Australia’s Health Series No. 15. Cat. No. AUS 199; AIHW: Canberra, Australia, 2016.
- 2071.0—Census of Population and Housing: Reflecting Australia—Stories from the Census. 2016. Available online: https://www.abs.gov.au/ausstats/abs@.nsf/mf/2071.0 (accessed on 23 April 2020).
- McGrath, P.; Holewa, H.; McGrath, Z. Practical problems for Aboriginal palliative care service provision in rural and remote areas: Equipment, power and travel issues. Collegian 2007, 14, 21–26. [Google Scholar] [CrossRef]
- Diaz, A.; Whop, L.J.; Valery, P.C.; Moore, S.P.; Cunningham, J.; Garvey, G.; Condon, J.R. Cancer outcomes for Aboriginal and Torres Strait Islander Australians in rural and remote areas. Aust. J. Rural Health 2015, 23, 4–18. [Google Scholar] [CrossRef]
- Nagin, D.S.; Odgers, C.L. Group-Based Trajectory Modeling in Clinical Research. Annu. Rev. Clin. Psychol. 2010, 6, 109–138. [Google Scholar] [CrossRef]
| Characteristic | Indigenous | Non-Indigenous | |
|---|---|---|---|
| First episode analysis | |||
| N | 1180 | 107,952 | |
| Patient-level characteristics | |||
| Age (years) at entry to care, mean (SD) | 63.1 (14.3) | 72.5 (13.5) | |
| Age group at entry, n (%) | |||
| <65 years | 625 (53.0) | 28,168 (26.1) | |
| ≥65 years | 555 (47.0) | 79,784 (73.9) | |
| Sex, n (%) | |||
| Female | 604 (51.2) | 50,096 (46.4) | |
| Male | 576 (48.8) | 57,856 (53.6) | |
| Principal Diagnosis, n (%) | |||
| Cancer | 978 (82.9) | 90,089 (83.5) | |
| Other | 202 (17.1) | 17,863 (16.5) | |
| Episode/phase-level variables | |||
| Setting of care, n (%) | |||
| Inpatient overnight admission | 739 (62.6) | 64,808 (60.0) | |
| Hospital ambulatory ** | 34 (2.9) | 1755 (1.6) | |
| Community | 407 (34.5) | 41,389 (38.3) | |
| Episode duration (days) | 32.6 | 33.8 | |
| Mean | 11 | 12 | |
| Median | 1–717 | 1–1651 | |
| Range | |||
| Number of phases in episode * | 2.4 | 2.6 | |
| 2 | 2 | ||
| 1–31 | 1–139 | ||
| Pre-death episode analysis | |||
| N | 681 | 67,339 | |
| Patient-level characteristics | |||
| Age (years) at entry to care, mean (SD) | 64.2 (14.5) | 73.9 (13.4) | |
| Age group at entry, n (%) | |||
| <65 years | 346 (50.8) | 15,722 (23.3) | |
| ≥65 years | 335 (49.2) | 51,617 (76.7) | |
| Sex, n (%) | |||
| Female | 345 (50.7) | 30,848 (45.8) | |
| Male | 336 (49.3) | 36,491 (54.2) | |
| Principal Diagnosis, n (%) | |||
| Cancer | 527 (77.4) | 52,204 (77.5) | |
| Other | 154 (22.6) | 15,135 (22.5) | |
| Episode/phase-level variables | |||
| Setting of care, n (%) | |||
| Inpatient overnight admission | 561 (82.4) | 54,206 (80.5) | |
| Hospital ambulatory ** | 0 (0.0) | 53 (0.1) | |
| Community | 120 (17.6) | 13,080 (19.4) | |
| Interval between symptom measurement and death (days) | |||
| Mean | 2.1 | 2.1 | |
| Median | 1 | 1 | |
| Interquartile range | 1–3 | 1–3 | |
| Correct recognition of phase as ‘terminal’ at phase start | 521 (76.5) | 51,022 (75.8) | |
| Model 1: All Settings, Crude | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model 2: All Settings, Adjusted for Age + Sex + Principal Diagnosis | ||||||||||||
| Model 3: Subgroup Cared for in Inpatient Setting, Adjusted for Age + Sex + Principal Diagnosis | ||||||||||||
| Model 4: Subgroup Cared for in Community Setting, Adjusted for Age + Sex + Principal Diagnosis | ||||||||||||
| Symptom(s) | Model 1 | Model 2 | Model 3 | Model 4 | ||||||||
| RR | p | RR (95% CI) | RR | p | RR (95% CI) | RR | p | RR (95% CI) | RR | p | RR (95% CI) | |
| Occurrence of moderate-to-severe (SAS score 4–10) symptom/s following commencement of an episode | ||||||||||||
| Any symptom | 0.99 | 0.682 | (0.96–1.03) | 0.97 | 0.115 | (0.94–1.01) | 0.99 | 0.792 | (0.95–1.04) | 0.96 | 0.084 | (0.91–1.01) |
| ≥3 symptoms | 0.96 | 0.313 | (0.89–1.04) | 0.91 | 0.013 | (0.84–0.98) | 0.94 | 0.211 | (0.85–1.04) | 0.89 | 0.060 | (0.79–1.01) |
| Pain | 1.07 | 0.087 | (0.99–1.16) | 0.97 | 0.403 | (0.89–1.05) | 0.97 | 0.508 | (0.87–1.07) | 0.97 | 0.660 | (0.85–1.11) |
| Nausea | 0.90 | 0.231 | (0.76–1.07) | 0.79 | 0.005 * | (0.67–0.93) | 0.82 | 0.071 | (0.66–1.02) | 0.80 | 0.091 | (0.61–1.04) |
| Breathing problem | 1.02 | 0.624 | (0.93–1.13) | 1.00 | 0.925 | (0.91–1.10) | 1.00 | 0.972 | (0.89–1.13) | 1.03 | 0.732 | (0.88–1.20) |
| Bowel problems | 0.90 | 0.080 | (0.79–1.01) | 0.88 | 0.033 | (0.78–0.99) | 0.89 | 0.099 | (0.77–1.02) | 0.83 | 0.119 | (0.66–1.05) |
| Appetite problems | 0.88 | 0.007 | (0.81–0.97) | 0.87 | 0.003 * | (0.80–0.96) | 0.93 | 0.233 | (0.83–1.05) | 0.84 | 0.017 | (0.74–0.97) |
| Insomnia | 1.08 | 0.155 | (0.97–1.21) | 0.97 | 0.596 | (0.87–1.08) | 1.06 | 0.436 | (0.92–1.22) | 0.92 | 0.355 | (0.76–1.10) |
| Fatigue | 0.97 | 0.202 | (0.92–1.02) | 0.95 | 0.037 | (0.90–1.00) | 0.99 | 0.814 | (0.93–1.06) | 0.91 | 0.013 | (0.85–0.98) |
| Worsening (SAS score increment ≥ 2) of symptom/s at any point following commencement of an episode | ||||||||||||
| Any symptom | 0.95 | 0.111 | (0.90–1.01) | 0.95 | 0.069 | (0.89–1.00) | 0.96 | 0.289 | (0.88–1.04) | 0.95 | 0.244 | (0.88–1.03) |
| ≥3 symptoms | 0.97 | 0.604 | (0.86–1.09) | 0.94 | 0.319 | (0.84–1.06) | 0.98 | 0.854 | (0.83–1.16) | 0.99 | 0.859 | (0.84–1.15) |
| Pain | 0.97 | 0.655 | (0.87–1.09) | 0.95 | 0.380 | (0.85–1.07) | 0.96 | 0.618 | (0.82–1.13) | 0.99 | 0.930 | (0.85–1.16) |
| Nausea | 0.85 | 0.079 | (0.70–1.02) | 0.77 | 0.006* | (0.64–0.93) | 0.75 | 0.051 | (0.57–1.00) | 0.86 | 0.224 | (0.67–1.10) |
| Breathing problems | 0.95 | 0.412 | (0.83–1.08) | 0.94 | 0.336 | (0.82–1.07) | 0.96 | 0.665 | (0.8–1.15) | 0.96 | 0.706 | (0.78–1.18) |
| Bowel problems | 0.94 | 0.398 | (0.82–1.08) | 0.94 | 0.411 | (0.82–1.08) | 0.95 | 0.535 | (0.79–1.13) | 0.97 | 0.795 | (0.78–1.21) |
| Appetite problems | 0.94 | 0.347 | (0.83–1.07) | 0.94 | 0.303 | (0.82–1.06) | 1.04 | 0.657 | (0.87–1.24) | 0.91 | 0.304 | (0.76–1.09) |
| Insomnia | 1.05 | 0.510 | (0.91–1.20) | 1.00 | 0.984 | (0.87–1.15) | 1.11 | 0.291 | (0.92–1.34) | 0.96 | 0.665 | (0.78–1.17) |
| Fatigue | 0.94 | 0.226 | (0.84–1.04) | 0.93 | 0.204 | (0.84–1.04) | 0.91 | 0.196 | (0.78–1.05) | 1.01 | 0.932 | (0.87–1.17) |
| Model 1: All Settings, Crude | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model 2: All Settings, Adjusted for Age + Sex + Principal Diagnosis + Interval between Assessment and Death (i.e., Final Phase Length) | ||||||||||||
| Model 3: Subgroup Cared for in Inpatient Setting, Adjusted for Age + Sex + Principal Diagnosis + Interval between Assessment and Death | ||||||||||||
| Model 4: Subgroup Cared for in Community Setting, Adjusted for Age + Sex + Principal Diagnosis + Interval between Assessment and Death | ||||||||||||
| Symptom(s) | Model 1 | Model 2 | Model 3 | Model 4 | ||||||||
| RR | p | RR (95% CI) | RR | p | RR (95% CI) | RR | p | RR (95% CI) | RR | p | RR (95% CI) | |
| Any symptom | 1.00 | 0.870 | (0.94–1.05) | 0.97 | 0.400 | (0.91–1.04) | 1.03 | 0.362 | (0.96–1.11) | 0.78 | 0.001 * | (0.67–0.91) |
| ≥3 symptoms | 1.04 | 0.571 | (0.92–1.17) | 0.93 | 0.333 | (0.81–1.07) | 1.01 | 0.898 | (0.87–1.17) | 0.65 | 0.030 | (0.45–0.96) |
| Pain | 1.12 | 0.051 | (1.00–1.25) | 0.98 | 0.743 | (0.87–1.11) | 1.04 | 0.545 | (0.91–1.19) | 0.68 | 0.044 | (0.47–0.99) |
| Nausea | 0.83 | 0.195 | (0.62–1.10) | 0.72 | 0.049 | (0.52–1.00) | 0.77 | 0.137 | (0.54–1.09) | 0.53 | 0.150 | (0.22–1.26) |
| Breathing problem | 1.09 | 0.105 | (0.98–1.22) | 1.02 | 0.741 | (0.91–1.14) | 1.07 | 0.246 | (0.95–1.21) | 0.79 | 0.180 | (0.57–1.11) |
| Bowel problems | 0.84 | 0.065 | (0.69–1.01) | 0.79 | 0.035 | (0.64–0.98) | 0.81 | 0.076 | (0.65–1.02) | 0.69 | 0.213 | (0.38–1.24) |
| Appetite problems | 0.82 | 0.010 | (0.71–0.95) | 0.81 | 0.013 | (0.68–0.96) | 0.90 | 0.255 | (0.74–1.08) | 0.55 | 0.004 * | (0.37–0.83) |
| Insomnia | 1.11 | 0.256 | (0.92–1.34) | 0.97 | 0.806 | (0.79–1.21) | 0.96 | 0.777 | (0.75–1.24) | 1.02 | 0.910 | (0.68–1.55) |
| Fatigue | 0.97 | 0.426 | (0.89–1.05) | 0.95 | 0.265 | (0.86–1.04) | 1.00 | 0.984 | (0.90–1.11) | 0.80 | 0.029 | (0.66–0.98) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Woods, J.A.; Johnson, C.E.; Ngo, H.T.; Katzenellenbogen, J.M.; Murray, K.; Thompson, S.C. Symptom-Related Distress among Indigenous Australians in Specialist End-of-Life Care: Findings from the Multi-Jurisdictional Palliative Care Outcomes Collaboration Data. Int. J. Environ. Res. Public Health 2020, 17, 3131. https://doi.org/10.3390/ijerph17093131
Woods JA, Johnson CE, Ngo HT, Katzenellenbogen JM, Murray K, Thompson SC. Symptom-Related Distress among Indigenous Australians in Specialist End-of-Life Care: Findings from the Multi-Jurisdictional Palliative Care Outcomes Collaboration Data. International Journal of Environmental Research and Public Health. 2020; 17(9):3131. https://doi.org/10.3390/ijerph17093131
Chicago/Turabian StyleWoods, John A., Claire E. Johnson, Hanh T. Ngo, Judith M. Katzenellenbogen, Kevin Murray, and Sandra C. Thompson. 2020. "Symptom-Related Distress among Indigenous Australians in Specialist End-of-Life Care: Findings from the Multi-Jurisdictional Palliative Care Outcomes Collaboration Data" International Journal of Environmental Research and Public Health 17, no. 9: 3131. https://doi.org/10.3390/ijerph17093131
APA StyleWoods, J. A., Johnson, C. E., Ngo, H. T., Katzenellenbogen, J. M., Murray, K., & Thompson, S. C. (2020). Symptom-Related Distress among Indigenous Australians in Specialist End-of-Life Care: Findings from the Multi-Jurisdictional Palliative Care Outcomes Collaboration Data. International Journal of Environmental Research and Public Health, 17(9), 3131. https://doi.org/10.3390/ijerph17093131





