Arsenic Exposure and Risk of Urothelial Cancer: Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Outcomes
- Mean Difference (MD) (cases vs. controls) of IA (%);
- Mean Difference (MD) (cases vs. controls) of DMA (%);
- Mean Difference (MD) (cases vs. controls) of MMA (%).
2.2. Search Strategy and Study Selection
2.3. Data Extraction
2.4. Statistical Analysis
3. Results
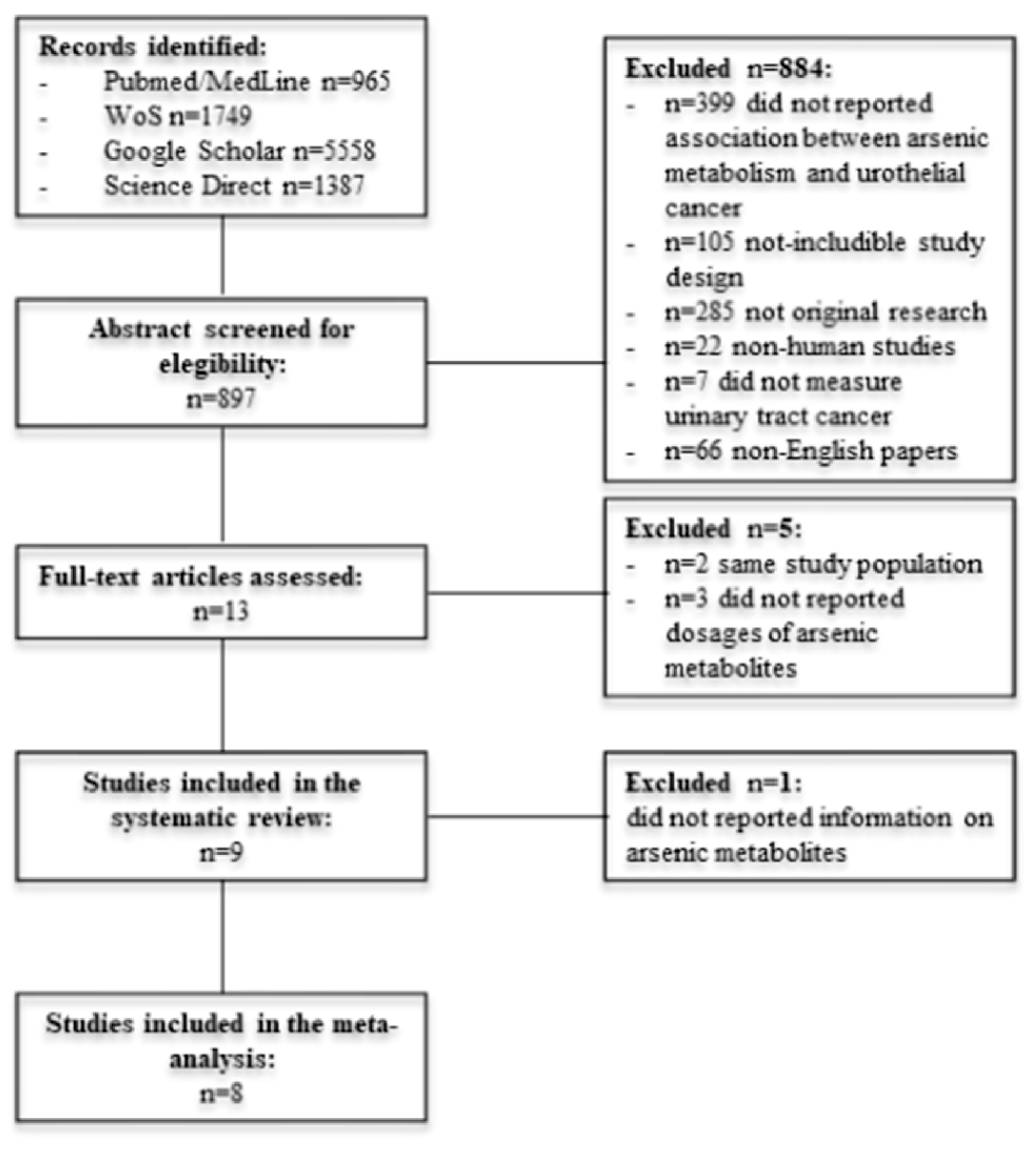
3.1. Studies Selection
3.2. Characteristics of Included Studies
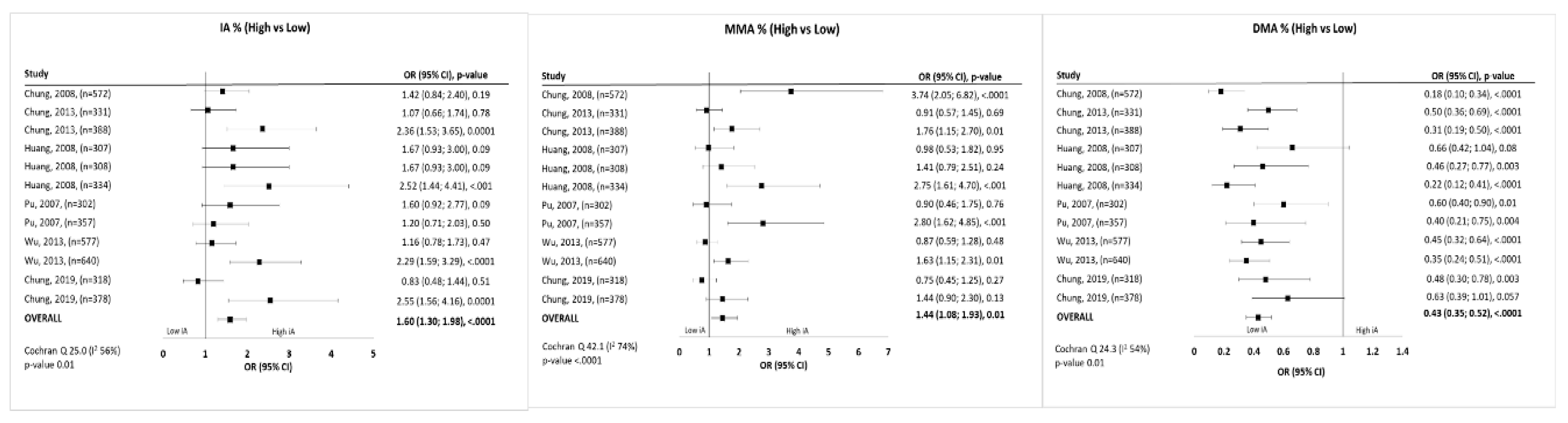
3.3. Meta-Analysis
3.4. Study Design and Quality Assessment
4. Discussion
4.1. Main Findings
4.2. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- American Cancer Society. Arsenic. 2016. Available online: http://www.cancer.org/cancer/cancercauses/othercarcinogens/intheworkplace/arsenic (accessed on 14 April 2020).
- (FDA) UF and DA. Metals>Arsenic. Available online: http://www.fda.gov/Food/FoodborneIllnessContaminants/Metals/ucm280202.htm (accessed on 14 April 2020).
- WHO. Arsenic. 2012. Available online: http://www.who.int/mediacentre/factsheets/fs372/en/ (accessed on 14 April 2020).
- WHO. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Arsenic in Drinking-Water. 2004. Available online: https://monographs.iarc.fr/wp-content/uploads/2018/06/mono84.pdf (accessed on 14 April 2020).
- Singh, N.; Kumar, D.; Sahu, A.P. Arsenic in the environment: Effects on human health and possible prevention. J. Environ. Biol. 2007, 28 (Suppl. 2), 359–365. [Google Scholar] [PubMed]
- Cantor, K.P.; Lubin, J.H. Arsenic, internal cancers, and issues in inference from studies of low-level exposures in human populations. Toxicol. Appl. Pharmacol. 2007, 222, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Mondal, D.; Banerjee, M.; Kundu, M.; Banerjee, N.; Bhattacharya, U.; Giri, A.K.; Ganguli, B.; Sen Roy, S.; Polya, D.A. Comparison of drinking water, raw rice and cooking of rice as arsenic exposure routes in three contrasting areas of West Bengal, India. Environ. Geochem. Health 2010, 32, 463–477. [Google Scholar] [CrossRef]
- Shen, H.; Niu, Q.; Xu, M.; Rui, D.; Xu, S.; Feng, G.; Ding, Y.; Li, S.; Jing, M. Factors Affecting Arsenic Methylation in Arsenic-Exposed Humans: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2016, 13, 205. [Google Scholar] [CrossRef] [PubMed]
- Saint-Jacques, N.; Parker, L.; Brown, P.; Dummer, T.J. Arsenic in drinking water and urinary tract cancers: A systematic review of 30 years of epidemiological evidence. Environ. Health 2014, 13, 44. [Google Scholar] [CrossRef]
- WHO. Arsenic, Fact Sheet n. 372. 2012. Available online: http://www.who.int/mediacentre/factsheets/fs372/en (accessed on 14 April 2020).
- Kitchin, K.T. Recent advances in arsenic carcinogenesis: Modes of action, animal model systems, and methylated arsenic metabolites. Toxicol. Appl. Pharmacol. 2001, 172, 249–261. [Google Scholar] [CrossRef]
- Luster, M.I.; Simeonova, P.P. Arsenic and urinary bladder cell proliferation. Toxicol. Appl. Pharmacol. 2004, 198, 419–423. [Google Scholar] [CrossRef]
- Dekkers, O.M.; Vandenbroucke, J.P.; Cevallos Renehan, A.G.; Altman, D.G.; Egger, M. COSMOS-E: Guidance on conducting systematic reviews and meta-analyses of observational studies of etiology. PLoS Med. 2019, 16, e1002742. [Google Scholar] [CrossRef]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef]
- Van Houwelingen, H.C.; Arends, L.R.; Stijnen, T. Advanced methods in meta-analysis: Multivariate approach and meta-regression. Stat. Med. 2002, 21, 589–624. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.J.; Huang, Y.L.; Huang, Y.K.; Wu, M.M.; Chen, S.Y.; Hsueh, Y.M.; Chen, C.J. Urinary arsenic profiles and the risks of cancer mortality: A population-based 20-year follow-up study in arseniasis-endemic areas in Taiwan. Environ. Res. 2013, 122, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.J.; Huang, C.J.; Pu, Y.S.; Su, C.T.; Huang, Y.K.; Chen, Y.T.; Hsueh, Y.M. Urinary 8-hydroxydeoxyguanosine and urothelial carcinoma risk in low arsenic exposure area. Toxicol. Appl. Pharmacol. 2008, 226, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Melak, D.; Ferreccio, C.; Kalman, D.; Parra, R.; Acevedo, J.; Pérez, L.; Cortés, S.; Smith, A.H.; Yuan, Y.; Liaw, J.; et al. Arsenic methylation and lung and bladder cancer in a case-control study in northern Chile. Toxicol. Appl. Pharmacol. 2014, 274, 225–231. [Google Scholar] [CrossRef]
- Wu, C.C.; Chen, M.C.; Huang, Y.K.; Huang, C.Y.; Lai, L.A.; Chung, C.J.; Shiue, H.S.; Pu, Y.S.; Lin, Y.C.; Han, B.C.; et al. Environmental tobacco smoke and arsenic methylation capacity are associated with urothelial carcinoma. J. Formos. Med. Assoc. 2013, 112, 554–560. [Google Scholar] [CrossRef][Green Version]
- Pu, Y.S.; Yang, S.M.; Huang, Y.K.; Chung, C.J.; Huang, S.K.; Chiu, A.W.H.; Yang, M.H.; Chen, C.J.; Hsueh, Y.M. Urinary arsenic profile affects the risk of urothelial carcinoma even at low arsenic exposure. Toxicol. Appl. Pharmacol. 2007, 218, 99–106. [Google Scholar] [CrossRef]
- Huang, Y.K.; Pu, Y.S.; Chung, C.J.; Shiue, H.S.; Yang, M.H.; Chen, C.J.; Hsueh, Y.M. Plasma folate level, urinary arsenic methylation profiles, and urothelial carcinoma susceptibility. Food Chem. Toxicol. 2008, 46, 929–938. [Google Scholar] [CrossRef]
- Steinmaus, C.; Yuan, Y.; Kalman, D.; Atallah, R.; Smith, A.H. Intraindividual variability in arsenic methylation in a U.S. populataion RN—Cancer Epidemiol. Biomark. Prev. 2005, 14, 919–924. [Google Scholar] [CrossRef]
- Chung, C.J.; Huang, C.Y.; Pu, Y.S.; Shiue, H.S.; Su, C.T.; Hsueh, Y.M. The effect of cigarette smoke and arsenic exposure on urothelial carcinoma risk is modified by glutathione S-transferase M1 gene null genotype. Toxicol. Appl. Pharmacol. 2013, 266, 254–259. [Google Scholar] [CrossRef]
- Chung, C.; Lee, H.; Chang, C.; Chang, H.; Liu, C.S.; Jung, W.T.; Liu, H.J.; Liou, S.H.; Chung, M.C.; Hsueh, Y.M. Measurement of urinary arsenic profiles and DNA hypomethylation in a case–control study of urothelial carcinoma. Arch. Toxicol. 2019, 93, 2155–2164. [Google Scholar] [CrossRef]
- Hughes, M.F.; Menache, M.; Thompson, D.J. Dose-dependent disposition of sodium arsenate in mice following acute oral exposure. Toxicol. Sci. 1994, 22, 80–89. [Google Scholar] [CrossRef]
- Mass, M.J.; Tennant, A.; Roop, B.C.; Cullen, W.R.; Styblo, M.; Thomas, D.J.; Kligerman, A.D. Methylated trivalent arsenic species are genotoxic. Chem. Res. Toxicol. 2001, 14, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Kligerman, A.D.; Doerr, C.L.; Tennant, A.H.; Harrington-Brock, K.; Allen, J.W.; Winkfield, E.; Poorman-Allen, P.; Kundu, B.; Funasaka, K.; Roop, B.C.; et al. Methylated trivalent arsenicals as candidate ultimate genotoxic forms of arsenic: Induction of chromosomal mutations but not gene mutations. Environ. Mol. Mutagen. 2003, 42, 192–205. [Google Scholar] [CrossRef]
- Jensen, T.J.; Wozniak, R.J.; Eblin, K.E.; Wnek, S.M.; Gandolfi, A.J.; Futscher, B.W. Epigenetic mediated transcriptional activation of WNT5A participates in arsenical-associated malignant transformation. Toxicol. Appl. Pharmacol. 2009, 235, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, K.; Mizoi, M.; Tachikawa, M.; Hasegawa, A.; Hoshino, M.; Okada, S. Oxidative DNA damage following exposure to dimethylarsinous iodide: The formation of cis-thymine glycol. Toxicol. Lett. 2003, 143, 145–153. [Google Scholar] [CrossRef]
- Kuo, C.C.; Moon, K.A.; Wang, S.L.; Silbergeld, E.; Navas-Acien, A. The association of arsenic metabolism with cancer, cardiovascular disease, and diabetes: A systematic review of the epidemiological evidence. Environ. Health Perspect. 2017, 125, 087001. [Google Scholar] [CrossRef]
- Gamboa-Loira, B.; Cebrián, M.E.; Franco-Marina, F.; López-Carrillo, L. Arsenic metabolism and cancer risk: A meta-analysis. Environ. Res. 2017, 156, 551–558. [Google Scholar] [CrossRef]
- Pilsner, J.R.; Liu, X.; Ahsan, H.; Ilievski, V.; Slavkovich, V.; Levy, D.; Factor-Litvak, P.; Graziano, J.H.; Gamble, M.V. Folate deficiency, hyperhomocysteinemia, low urinary creatinine, and hypomethylation of leukocyte DNA are risk factors for arsenic-induced skin lesions. Environ. Health Perspect. 2009, 117, 254–260. [Google Scholar] [CrossRef]
- Burger, M.; Catto, J.W.; Dalbagni, G.; Grossman, H.B.; Herr, H.; Karakiewicz, P.; Kassouf, W.; Kiemeney, L.A.; La Vecchia, C.; Shariat, S.; et al. Epidemiology and risk factors of urothelial bladder cancer. Eur. Urol. 2013, 63, 234–241. [Google Scholar] [CrossRef]
- Zaitsu, M.; Kawachi, I.; Takeuchi, T.; Kobayashi, Y. Alcohol consumption and risk of upper-tract urothelial cancer. Cancer Epidemiol. 2017, 48, 36–40. [Google Scholar] [CrossRef]

| Study | Year | Country | Study Design | Participants | IA% Cases (Mean) | MMA% Cases (Mean) | DMA% Cases (Mean) | Risk Estimate Contrast IA% | Risk Estimate IA% | Risk Estimate Contrast MMA% | Risk Estimate MMA% | Risk Estimate Contrast DMA% | Risk Estimate DMA% | Adjustment | Conclusion |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chung | 2008 | Taiwan | Case-Control | 572 | 7.18 | 13.19 | 79.63 | >4.32 vs. <4.32 | 1.42 | >6.1 vs. <6.1 | 3.74 | >88 vs. <88 | 0.18 | Age and gender | MMA and DMA were associated with UC |
| Chung | 2013 | Taiwan | Case-Control | 555 | 9.06 | 10.53 | 80.41 | 2.76–5.86 vs. <2.76 | 1.07 | 3.36–9.13 vs. <3.36 | 0.91 | >91.76 vs. 83.56–91.76 | 0.5 | Age and gender | IA, MMA and DMA were associated with UC |
| >5.86 vs. <2.76 | 2.36 | >9.13 vs. <3.36 | 1.76 | >91.76 vs. <86.56 | 0.31 | ||||||||||
| Chung1 | 2013 | Taiwan | Cohort | 28 | 4.22–7.87 vs. <4.22 | 2.42 | 8.34–15.31 vs. <8.34 | 0.57 | >85.8 vs. 76.13–85.8 | 1.43 | Age, gender, education and smoking habits | IA, MMA and DMA were associated with UC | |||
| >7.86 vs. <4.22 | 3.53 | >15.32 vs. <8.34 | 1.77 | >85.8 vs. >76.13 | 0.33 | ||||||||||
| Huang | 2008 | Taiwan | Case-Control | 659 | 1.5–3.69 vs<1.49 | 1.67 | 0.9–5.89 vs. <0.89 | 0.98 | 81.9–89.19 vs. <81.89 | 0.66 | Age, gender, educational attainment, smoking status, and alcohol consumption | IA, MMA and DMA were associated with UC | |||
| 3.70–6.29 vs. <1.49 | 1.67 | 5.9–10.89 vs. <0.89 | 1.41 | 89.20–94.39 vs. <81.89 | 0.46 | ||||||||||
| >6.30 vs. <1.49 | 2.52 | >10.90 vs. <0.89 | 2.75 | >94.40 vs. <81.89 | 0.22 | ||||||||||
| Pu | 2007 | Taiwan | Case-Control | 490 | 5.9 | 9.9 | 84.2 | 2.5–5.2 vs. <2.4 | 1.6 | 3.1–9.2 vs. <3 | 0.9 | 85.1–92.5 vs. <85 | 0.6 | Age, gender, education, parents ethnicity, alcohol and pesticides exposure | IA, MMA and DMA were associated with UC |
| > 5.3 vs. <2.4 | 1.2 | >9.3 vs. <3 | 2.8 | >92.6 vs. <85 | 0.4 | ||||||||||
| Melak | 2014 | Chile | Case-Control | 464 | 10.4 | 11.2 | 81.9 | >12.5 vs. <12.5 | 1.41 | Age, gender, and smoking | MMA was associated with UC | ||||
| Steinmaus | 2005 | USA | Case-Control | 81 | 13.3 | 13.7 | 73 | Age, gender, education, pesticide exposure and smoking habits | MMA increased UC risk | ||||||
| Wu | 2013 | Taiwan | Case-Control | 933 | 9.71 | 10.55 | 79.74 | 2.61–5.7 vs. <2.61 | 1.16 | 3.04–8.87 vs. <3.04 | 0.87 | 84.72–92.50 vs. <84.72 | 0.45 | Age, gender, education and alcohol consumption | IA, MMA and DMA were associate with UC |
| >5.8 vs. <2.61 | 2.29 | >8.88 vs. <3.04 | 1.63 | >92.51 vs. <84.72 | 0.35 | ||||||||||
| Chung | 2019 | Taiwan | Case-Control | 534 | 15.1 | 12.47 | 73.33 | Q1 vs. Q2 Q1 vs. Q3 | 0.83 2.55 | Q1 vs. Q2 Q1 vs. Q3 | 0.75 1.44 | Q1 vs. Q2 Q1 vs. Q3 | 0.48 0.63 | Age, gender, education, pesticides exposure and smoking habits | IA, MMA and DMA were associated with UC |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Giovanni, P.; Di Martino, G.; Scampoli, P.; Cedrone, F.; Meo, F.; Lucisano, G.; Romano, F.; Staniscia, T. Arsenic Exposure and Risk of Urothelial Cancer: Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2020, 17, 3105. https://doi.org/10.3390/ijerph17093105
Di Giovanni P, Di Martino G, Scampoli P, Cedrone F, Meo F, Lucisano G, Romano F, Staniscia T. Arsenic Exposure and Risk of Urothelial Cancer: Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health. 2020; 17(9):3105. https://doi.org/10.3390/ijerph17093105
Chicago/Turabian StyleDi Giovanni, Pamela, Giuseppe Di Martino, Piera Scampoli, Fabrizio Cedrone, Francesca Meo, Giuseppe Lucisano, Ferdinando Romano, and Tommaso Staniscia. 2020. "Arsenic Exposure and Risk of Urothelial Cancer: Systematic Review and Meta-Analysis" International Journal of Environmental Research and Public Health 17, no. 9: 3105. https://doi.org/10.3390/ijerph17093105
APA StyleDi Giovanni, P., Di Martino, G., Scampoli, P., Cedrone, F., Meo, F., Lucisano, G., Romano, F., & Staniscia, T. (2020). Arsenic Exposure and Risk of Urothelial Cancer: Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health, 17(9), 3105. https://doi.org/10.3390/ijerph17093105








