Children Born with Congenital Heart Defects and Growth Restriction at Birth: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Search Strategy
2.2. Study Selection
2.3. Data Extraction
2.4. Evaluation of Bias
2.5. Definitions
2.6. Statistical Analysis
3. Results
3.1. Study Characteristics
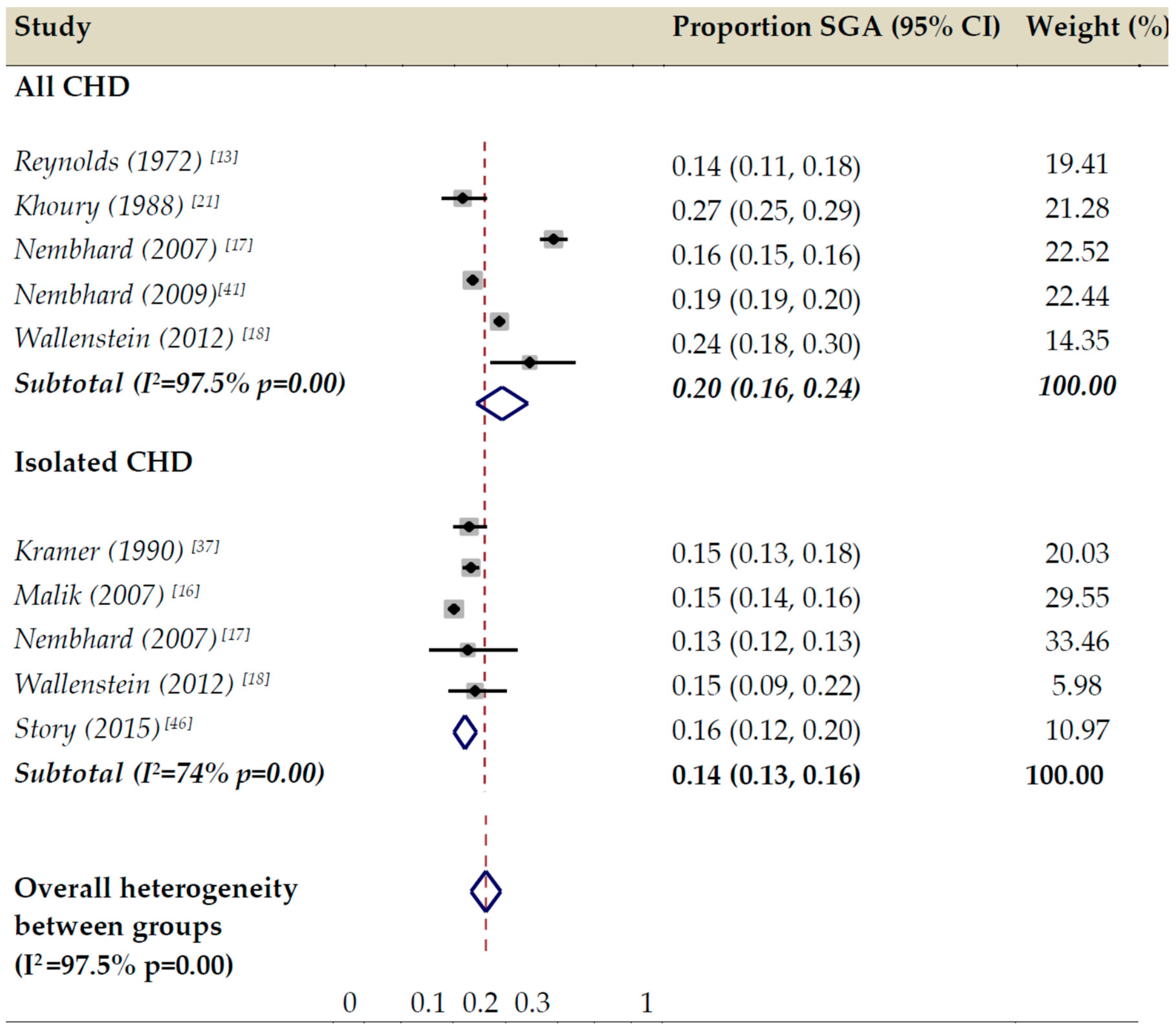
3.2. Proportion of SGA in All CHD, Isolated CHD, and Subgroups Reported by Individual Studies
3.3. Evaluation of Bias
3.4. Meta-Analysis
| Author | Country | Definition of SGA | CHD | CHD (n) | SGA (%) |
|---|---|---|---|---|---|
| Archer (2011) [23] | USA | <10th P° according to GA, maternal race, gender, and type of gestation | All | 99,786 | 21 |
| Bain (2014) [24] | USA | <10th P° according to GA, gender, race | All | 98,523 | 24 |
| Calderon (2018) [25] | France | <10th P° according to GA and gender | All | 419 | 14 |
| Cedergren (2006) * [26] | Sweden | <2SD below mean birth weight according to GA | All | 6346 | 7 |
| Isolated | 5338 | 6 | |||
| Chu (2015) [27] | USA | ICD? | All | 28,806 | 6 |
| Cnota (2013) [28] | USA | <10th P° according to GA, gender, race | HLHS | 33 | No data |
| Joelsson (2001) [29] | Sweden | Not stated | PAIVS | 84 | 14 |
| El Hassan (2008) [30] | USA | ICD | HLHS | 5720 | 3 |
| Fisher (2015) [31] | USA | Not stated | All | 235,643 | 43 |
| Gelehrter (2011) * [32] | USA | <3rd P° according to GA | HLHS | 52 | 37 |
| Jacobs (2003) * [33] | China | <-2 z score from normal mean for age and gender | Isolated | 454 | 15 |
| PA | 18 | 11 | |||
| ToF | 63 | 24 | |||
| TGV | 12 | 16 | |||
| CoAo | 20 | 20 | |||
| VSD | 86 | 12 | |||
| ASD | 31 | 23 | |||
| PS | 52 | 11 | |||
| Jones (2015) * [20] | USA | <10th P° according to GA and gender | HLHS | 16 | 31 |
| Josefsson (2011) [34] | Sweden | <-2 SD of the mean birthweight for gestational length | All | 2216 | 31 |
| Karr (1992) [35] | USA | Not stated | ToF | 125 | 21 |
| Kernell (2014) [36] | Sweden | <-2 SD of the mean birthweight for gestational length | All | 2689 | 21 |
| Khoury (1988) * [12] | USA | <10th P° according to GA, race and gender | All | 3669 | 28 |
| HLHS | 91 | 23 | |||
| CAT | 34 | 24 | |||
| ToF | 110 | 33 | |||
| TGV | 167 | 17 | |||
| CoAo | 139 | 28 | |||
| VSD | 833 | 27 | |||
| ASD | 409 | 30 | |||
| i.ASD | 26 | 11 | |||
| AVSD | 103 | 28 | |||
| Kramer (1990) * [37] | West Germany | <10P° | Isolated | 843 | 15 |
| ToF | 81 | 26 | |||
| TGV | 60 | 15 | |||
| AS | 45 | 8 | |||
| CoAo | 69 | 13 | |||
| VSD | 236 | 13 | |||
| ASD | 70 | 17 | |||
| Levin (1975) [38] | USA | Not stated | All | 37 | 43 |
| VSD | 5 | 40 | |||
| AoA | 3 | 70 | |||
| Levy (1978) * [39] | USA | <2SD below mean birth weight of control group | All | 2178 | 6 |
| HLHS | 163 | 6 | |||
| TA | 64 | 5 | |||
| TAPVR | 58 | 3 | |||
| ToF | 156 | 7 | |||
| TGV | 217 | 2 | |||
| AS | 43 | 2 | |||
| CoAo | 136 | 6 | |||
| VSD | 313 | 10 | |||
| ASD | 59 | 8 | |||
| AVSD | 107 | 8 | |||
| PS | 81 | 5 | |||
| PAIVS | 64 | 6 | |||
| Li (2009) [21] | China | Not stated | All | 274 | 5 |
| Lupo (2011) [40] | USA | <10th P° according to GA and gender | Ebstein | 175 | 19 |
| Malik (2007) * [16] | USA | <10th P° according to GA and gender | Isolated | 3395 | 15 |
| Nembhard (2009) * [41] | USA | <10th P° using race specific growth curve | All | 9645 | 19 |
| HLHS | 283 | 23 | |||
| CAT | 112 | 25 | |||
| ToF | 602 | 26 | |||
| Ebstein | 61 | 15 | |||
| TGV | 472 | 20 | |||
| CoAo | 592 | 20 | |||
| VSD | 5528 | 17 | |||
| ASD | 467 | 28 | |||
| Nembhard (2007) * [17] | USA | <10th P° using race specific growth curve | All | 12,964 | 16 |
| Isolated | 10,870 | 13 | |||
| Oyarzún (2018) [22] | Chile | Not stated | Isolated | 46 | 26 |
| Pappas (2012) [42] | USA | <10th P° | All | 110 | 27 |
| Polito (2013) [43] | Italy | <3rd P° | All | 70 | 17 |
| Reynolds (1972) * [13] | USA | <10th P° according to GA | All | 433 | 14 |
| AS | 21 | 38 | |||
| Rosenthal (1991) * [14] | USA | <10th P° according to GA | Isolated | 1299 | 12 |
| HLHS | 96 | 20 | |||
| CAT | 113 | 18 | |||
| ToF | 119 | 7 | |||
| Ebstein | 57 | 5 | |||
| TGV | 103 | 10 | |||
| CoAo | 470 | 11 | |||
| VSD | 130 | 12 | |||
| ASD | 44 | 18 | |||
| PS | 167 | 14 | |||
| Sochet (2013) [44] | USA | <10th P° according to GA | All | 230 | 25 |
| Steurer (2018) * [45] | USA | <10th P° according to GA and sex | Isolated | 6863 | 16 |
| Story (2015) * [46] | UK | <10th P° | Isolated | 308 | 16 |
| Swenson (2012) * [47] | USA | <10th P° | All | 753 | 21 |
| HLHS | 261 | 19 | |||
| TA | 38 | 16 | |||
| CAT | 28 | 21 | |||
| DROV | 54 | 24 | |||
| TAPVR | 35 | 26 | |||
| ToF | 70 | 36 | |||
| TGV | 181 | 13 | |||
| IAA | 44 | 36 | |||
| AVSD | 25 | 32 | |||
| Wallenstein (2012) * [18] | USA | <10th P° | All | 193 | 24 |
| Isolated | 129 | 15 | |||
| Wei (2015) [48] | USA | Size < 10th P° | All | 74 | 51 |
| HLHS | 11 | 30 | |||
| ToF | 12 | 70 | |||
| Ebstein | 4 | 50 | |||
| CoAo | 7 | 57 | |||
| VSD | 6 | 17 | |||
| PAIVS | 5 | 60 | |||
| Williams (2010) * [49] | USA | <10th P° according to GA | HLHS | 606 | 20 |
| TA | 114 | 30 | |||
| AVSD | 148 | 25 | |||
| PAIVS | 102 | 25 | |||
| Wollins (2001) * [19] | USA | <10th P° according to sex and GA | CoAo | 181 | 12 |
| Yu (2014) [15] | China | Not stated | All | 477 | 11 |
4. Discussion
4.1. Main Findings and Interpretations
4.2. Strengths
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CHD | Congenital heart defects |
| SGA | Small for gestational age |
| CASP | Critical Appraisal Skills Programme |
| HLHS | hypoplastic left heart syndrome |
| ToF | Tetraology of Fallot |
| TGV | transposition of great vessels |
| VSD | ventricualar septal defect |
| CoAo | coarctation of the aorta |
| AVSD | atrioventricular septal defect |
| TA | tricuspid atresia |
| CAT | common truncus arteriosus |
References
- van der Linde, D.; Konings, E.E.; Slager, M.A.; Witsenburg, M.; Helbing, W.A.; Takkenberg, J.J.; Roos-Hesselink, J.W. Birth prevalence of congenital heart disease worldwide: A systematic review and meta-analysis. J. Am. Coll. Cardiol. 2011, 58, 2241–2247. [Google Scholar] [CrossRef]
- Zimmerman, M.S.; Smith, A.G.C.; Sable, C.A.; Echko, M.M.; Wilner, L.B.; Olsen, H.E.; Atalay, H.T.; Awasthi, A.; Bhutta, Z.A.; Boucher, J.L.; et al. Global, regional, and national burden of congenital heart disease, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Child Adolesc. Health 2020, 4, 185–200. [Google Scholar] [CrossRef]
- Best, K.E.; Rankin, J. Long-term survival of individuals born with congenital heart disease: A systematic review and meta-analysis. J. Am. Heart Assoc. 2016, 5, e002846. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. Ann. Intern. Med. 2009, 151, W-65. [Google Scholar] [CrossRef] [PubMed]
- Derridj, N.; Khoshnood, B.; Salomon, L.J.; GhanchiI, A. A Systematic Review of the Prevalence of Fetal Growth Restriction (FGR) in Children Born with a Congenital Heart Defect (CHD). PROSPERO 2019 CRD42019131079. Available online: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42019131079 (accessed on 29 March 2020).
- Suri, H. Ethical Considerations of Conducting Systematic Reviews in Educational Research; Zawacki-Richter, O., Kerres, M., Bedenlier, S., Bond, M., Buntins, K., Eds.; Systematic Reviews in Educational Research; Springer VS: Wiesbaden, Germany, 2020. [Google Scholar]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Critical Appraisal Skills Programme CASP Cohort Study Checklist. Available online: https://casp-uk.net/wp-content/uploads/2018/01/CASP-Cohort-Study-Checklist_2018.pdf (accessed on 29 March 2020).
- Ego, A. Definitions: Small for gestational age and intrauterine growth retardation. J. Gynecol. Obstet. Biol. Reprod. 2013, 42, 872–894. [Google Scholar] [CrossRef]
- Barendregt, J.J.; Doi, S.A.; Lee, Y.Y.; Norman, R.E.; Vos, T. Meta-analysis of prevalence. J. Epidemiol. Community Health 2013, 67, 974–978. [Google Scholar] [CrossRef]
- Nyaga, J.; Muthuri, C.W.; Matiru, V.N.; Jefwa, J.M.; Okoth, S.A.; Wachira, P. Influence of soil fertility amendment practices on ex-situ utilisation of indigenous arbuscular mycorrhizal fungi and performance of maize and common bean in Kenyan highlands. Trop. Subtrop. Agroecosyst. 2014, 17, 129–141. [Google Scholar]
- Khoury, M.J.; Erickson, J.D.; Cordero, J.F.; McCarthy, B.J. Congenital malformations and intrauterine growth retardation: A population study. Pediatrics 1988, 82, 83–90. [Google Scholar]
- Reynolds, J.L. Intrauterine growth retardation in children with congenital heart disease—Its relation to aortic stenosis. Birth Defects Orig. Artic. Ser. 1972, 8, 143–148. [Google Scholar]
- Rosenthal, G.L.; Wilson, P.D.; Permutt, T.; Boughman, J.A.; Ferencz, C. Birth weight and cardiovascular malformations: A population-based study: The Baltimore-Washington infant study. Am. J. Epidemiol. 1991, 133, 1273–1281. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Mao, L.; Chen, S. Clinical features of early newborn infants with congenital heart disease. Zhonghua Xin Xue Guan Bing Za Zhi 2014, 42, 484–486. [Google Scholar] [PubMed]
- Malik, S.; Cleves, M.A.; Zhao, W.; Correa, A.; Hobbs, C.A. Association between congenital heart defects and small for gestational age. Pediatrics 2007, 119, e976–e982. [Google Scholar] [CrossRef] [PubMed]
- Nembhard, W.N.; Salemi, J.L.; Hauser, K.W.; Kornosky, J.L. Are there ethnic disparities in risk of preterm birth among infants born with congenital heart defects? Birth Defects Res. Part A: Clin. Mol. Teratol. 2007, 79, 754–764. [Google Scholar] [CrossRef]
- Wallenstein, M.B.; Harper, L.M.; Odibo, A.O.; Roehl, K.A.; Longman, R.E.; Macones, G.A.; Cahill, A.G. Fetal congenital heart disease and intrauterine growth restriction: A retrospective cohort study. J. Matern. -Fetal Neonatal Med. 2012, 25, 662–665. [Google Scholar] [CrossRef]
- Wollins, D.S.; Ferencz, C.; Boughman, J.A.; Loffredo, C.A. A population-based study of coarctation of the aorta: Comparisons of infants with and without associated ventricular septal defect. Teratology 2001, 64, 229–236. [Google Scholar] [CrossRef]
- Jones, H.N.; Olbrych, S.K.; Smith, K.L.; Cnota, J.F.; Habli, M.; Ramos-Gonzales, O.; Owens, K.J.; Hinton, A.C.; Polzin, W.J.; Muglia, L.J.; et al. Hypoplastic left heart syndrome is associated with structural and vascular placental abnormalities and leptin dysregulation. Placenta 2015, 36, 1078–1086. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.H.; Wang, F.Y.; Zhao, X.L.; Zhang, X.; Zhang, Y.P. Analysis of the birth defects among, 61 272 live born infants in Beijing. J. Peking Univ. Health Sci. 2009, 41, 414–417. [Google Scholar]
- Oyarzún, I.; Claveria, C.; Larios, G.; Le Roy, C. Nutritional recovery after cardiac surgery in children with congenital heart disease. Rev. Chil. Pediatr. 2018, 89, 24–31. [Google Scholar] [CrossRef]
- Archer, J.M.; Yeager, S.B.; Kenny, M.J.; Soll, R.F.; Horbar, J.D. Distribution of and mortality from serious congenital heart disease in very low birth weight infants. Pediatrics 2011, 127, 293–299. [Google Scholar] [CrossRef]
- Bain, J.; Benjamin, D.K.; Hornik, C.P.; Clark, R.; Smith, P.B. Risk of necrotizing enterocolitis in very-low-birth-weight infants with isolated atrial and ventricular septal defects. J. Perinatol. 2014, 34, 319–321. [Google Scholar] [CrossRef]
- Calderon, J.; Willaime, M.; Lelong, N.; Bonnet, D.; Houyel, L.; Ballon, M.; Goffinet, F.; Khoshnood, B. Population-based study of cognitive outcomes in congenital heart defects. Arch. Dis. Child. 2018, 103, 49–56. [Google Scholar] [CrossRef]
- Cedergren, M.I.; Källén, B.A. Obstetric outcome of 6346 pregnancies with infants affected by congenital heart defects. Eur. J. Obstet. Gynecol. Reprod. Biol. 2006, 125, 211–216. [Google Scholar] [CrossRef]
- Chu, P.Y.; Li, J.S.; Kosinski, A.S.; Hornik, C.P.; Hill, K.D. Epidemiology and Mortality of Very and Extremely Preterm Infants with Congenital Heart Defects. Circulation 2015, 132 (Suppl. 3), A12713. [Google Scholar]
- Cnota, J.F.; Hangge, P.T.; Wang, Y.; Woo, J.G.; Hinton, A.C.; Divanovic, A.A.; Michelfelder, E.C.; Hinton, R.B. Somatic growth trajectory in the fetus with hypoplastic left heart syndrome. Pediatric Res. 2013, 74, 284–289. [Google Scholar] [CrossRef]
- Joelsson, B.M.E.; Sunnegårdh, J.; Hanseus, K.; Berggren, H.; Jonzon, A.; Jögi, P.; Lundell, B. The outcome of children born with pulmonary atresia and intact ventricular septum in Sweden from 1980 to 1999. Scand. Cardiovasc. J. 2001, 35, 192–198. [Google Scholar]
- ElHassan, N.O.; Tang, X.; Gossett, J.; Zakaria, D.; Ross, A.; Kona, S.K.; Prodhan, P. Necrotizing enterocolitis in infants with hypoplastic left heart syndrome following stage, 1 palliation or heart transplant. Pediatric Cardiol. 2018, 39, 774–785. [Google Scholar] [CrossRef]
- Fisher, J.G.; Bairdain, S.; Sparks, E.A.; Khan, F.A.; Archer, J.M.; Kenny, M.; Edwards, E.M.; Soll, R.F.; Modi, B.P.; Yeager, S.; et al. Serious congenital heart disease and necrotizing enterocolitis in very low birth weight neonates. J. Am. Coll. Surg. 2015, 220, 1018–1026. [Google Scholar] [CrossRef]
- Gelehrter, S.; Fifer, C.G.; Armstrong, A.; Hirsch, J.; Gajarski, R. Outcomes of hypoplastic left heart syndrome in low-birth-weight patients. Pediatric Cardiol. 2011, 32, 1175–1181. [Google Scholar] [CrossRef]
- Jacobs, E.G.J.; Leung, M.P.; Karlberg, L.J. Birthweight distribution in southern Chinese infants with symptomatic congenital heart disease. J. Paediatr. Child Health 2003, 39, 191–196. [Google Scholar] [CrossRef]
- Josefsson, A.; Kernell, K.; Nielsen, N.E.; Bladh, M.; Sydsjö, G. Reproductive patterns and pregnancy outcomes in women with congenital heart disease—A Swedish population-based study. Acta Obstet. Gynecol. Scand. 2011, 90, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Karr, S.S.; Brenner, J.I.; Loffredo, C.; Neill, C.A.; Rubin, J.D. Tetralogy of fallot: The spectrum of severity in a regional study 1981–1985. Am. J. Dis. Children 1992, 146, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Kernell, K.; Sydsjö, G.; Bladh, M.; Nielsen, N.E.; Josefsson, A. Congenital heart disease in men–birth characteristics and reproduction: A national cohort study. BMC Pregnancy Childbirth 2014, 14, 187. [Google Scholar] [CrossRef] [PubMed]
- Kramer, H.H.; Trampisch, H.J.; Rammos, S.; Giese, A. Birth weight of children with congenital heart disease. Eur. J. Pediatrics 1990, 149, 752–757. [Google Scholar] [CrossRef]
- Levin, D.L.; Stanger, P.A.U.L.; Kitterman, J.A.; Heymann, M.A. Congenital heart disease in low birth weight infants. Circulation 1975, 52, 500–503. [Google Scholar] [CrossRef]
- Levy, R.J.; Rosenthal, A.; Fyler, D.C.; Nadas, A.S. Birthweight of infants with congenital heart disease. Am. J. Dis. Child. 1978, 132, 249–254. [Google Scholar] [CrossRef]
- Lupo, P.J.; Langlois, P.H.; Mitchell, L.E. Epidemiology of Ebstein anomaly: Prevalence and patterns in Texas 1999–2005. Am. J. Med. Genet. Part A 2011, 155, 1007–1014. [Google Scholar] [CrossRef]
- Nembhard, W.N.; Loscalzo, M.L. Fetal growth among infants with congenital heart defects by maternal race/ethnicity. Ann. Epidemiol. 2009, 19, 311–315. [Google Scholar] [CrossRef]
- Pappas, A.; Shankaran, S.; Hansen, N.I.; Bell, E.F.; Stoll, B.J.; Laptook, A.R.; Walsh, M.C.; Das, A.; Bara, R.; Hale, E.C.; et al. Outcome of extremely preterm infants (<1000 g) with congenital heart defects from the National Institute of Child Health and Human Development Neonatal Research Network. Pediatric Cardiol. 2012, 33, 1415–1426. [Google Scholar]
- Polito, A.; Piga, S.; Cogo, P.E.; Corchia, C.; Carnielli, V.; Da Frè, M.; Di Lallo, D.; Favia, I.; Gagliardi, L.; Macagno, F.; et al. Increased morbidity and mortality in very preterm/VLBW infants with congenital heart disease. Intensive Care Med. 2013, 39, 1104–1112. [Google Scholar] [CrossRef]
- Sochet, A.A.; Ayers, M.; Quezada, E.; Braley, K.; Leshko, J.; Amankwah, E.K.; Quintessenza, J.A.; Jacobs, J.P.; Dadlani, G. The importance of small for gestational age in the risk assessment of infants with critical congenital heart disease. Cardiol. Young 2013, 23, 896–904. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Steurer, M.; Burke, E.; Oltman, S.; Baer, R.; Ryckman, K.; Paynter, R.; Liang, L.; McCarthy, M.; Feuer, S.; Chambers, C.; et al. The Effect of Birth Weight on Mortality in Infants with Critical Congenital Heart Disease. J. Am. Coll. Cardiol. 2018, 71 (Suppl. 11), A629. [Google Scholar] [CrossRef]
- Story, L.; Pasupathy, D.; Sankaran, S.; Sharland, G.; Kyle, P. Influence of birthweight on perinatal outcome in fetuses with antenatal diagnosis of congenital heart disease. J. Obstet. Gynaecol. Res. 2015, 41, 896–903. [Google Scholar] [CrossRef] [PubMed]
- Swenson, A.W.; Dechert, R.E.; Schumacher, R.E.; Attar, M.A. The effect of late preterm birth on mortality of infants with major congenital heart defects. J. Perinatol. 2012, 32, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Azen, C.; Bhombal, S.; Hastings, L.; Paquette, L. Congenital heart disease in low-birth-weight infants: Effects of small for gestational age (SGA) status and maturity on postoperative outcomes. Pediatric Cardiol. 2015, 36, 1–7. [Google Scholar] [CrossRef]
- Williams, R.V.; Ravishankar, C.; Zak, V.; Evans, F.; Atz, A.M.; Border, W.L.; Levine, J.; Li, J.S.; Mahony, L.; Mital, S.; et al. Birth weight and prematurity in infants with single ventricle physiology: Pediatric heart network infant single ventricle trial screened population. Congenit. Heart Dis. 2010, 5, 96–103. [Google Scholar] [CrossRef]
- Gaudineau, A. Prevalence, risk factors, maternal and fetal morbidity and mortality of intrauterine growth restriction and small-for-gestational age. J. Gynecol. Obstet. Biol. Reprod. 2013, 42, 895–910. [Google Scholar] [CrossRef]
- Miller, S.L.; Huppi, P.S.; Mallard, C. The consequences of fetal growth restriction on brain structure and neurodevelopmental outcome. J. Physiol. 2016, 594, 807–823. [Google Scholar] [CrossRef]
- Suhag, A.; Berghella, V. Intrauterine growth restriction (IUGR): Etiology and diagnosis. Curr. Obstet. Gynecol. Rep. 2013, 2, 102–111. [Google Scholar] [CrossRef]
- Blue, G.M.; Kirk, E.P.; Sholler, G.F.; Harvey, R.P.; Winlaw, D.S. Congenital heart disease: Current knowledge about causes and inheritance. Med. J. Aust. 2012, 197, 155–159. [Google Scholar] [CrossRef]
- Puccio, G.; Giuffré, M.; Piccione, M.; Piro, E.; Rinaudo, G.; Corsello, G. Intrauterine growth restriction and congenital malformations: A retrospective epidemiological study. Ital. J. Pediatrics 2013, 39, 23. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Macgowan, C.K.; Sled, J.G.; Yoo, S.J.; Manlhiot, C.; Porayette, P.; Grosse-Wortmann, L.; Jaeggi, E.; McCrindle, B.W.; Kingdom, J.; et al. Reduced fetal cerebral oxygen consumption is associated with smaller brain size in fetuses with congenital heart disease. Circulation 2015, 131, 1313–1323. [Google Scholar] [CrossRef] [PubMed]
- Malik, S.; Cleves, M.A.; Honein, M.A.; Romitti, P.A.; Botto, L.D.; Yang, S.; Hobbs, C.A. Maternal smoking and congenital heart defects. Pediatrics 2008, 121, e810–e816. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.E.; Harmancey, R.; Stec, D.E. Lean heart: Role of leptin in cardiac hypertrophy and metabolism. World J. Cardiol. 2015, 7, 511. [Google Scholar] [CrossRef] [PubMed]
- Spiers, P.S. Does growth retardation predispose the fetus to congenital malformation? Lancet 1982, 319, 312–314. [Google Scholar] [CrossRef]
- Schulkey, C.E.; Regmi, S.D.; Magnan, R.A.; Danzo, M.T.; Luther, H.; Hutchinson, A.K.; Panzer, A.A.; Grady, M.M.; Wilson, D.B.; Jay, P.Y. The maternal-age-associated risk of congenital heart disease is modifiable. Nature 2015, 520, 230–233. [Google Scholar] [CrossRef]
- Fung, A.; Manlhiot, C.; Naik, S.; Rosenberg, H.; Smythe, J.; Lougheed, J.; Mondal, T.; Chitayat, D.; McCrindle, B.W.; Mital, S. Impact of prenatal risk factors on congenital heart disease in the current era. J. Am. Heart Assoc. 2013, 2, e000064. [Google Scholar] [CrossRef]
- Vecoli, C.; Pulignani, S.; Foffa, I.; Grazia Andreassi, M. Congenital heart disease: The crossroads of genetics, epigenetics and environment. Curr. Genom. 2014, 15, 390–399. [Google Scholar] [CrossRef]
- Chowdhury, S.; Cleves, M.A.; MacLeod, S.L.; James, S.J.; Zhao, W.; Hobbs, C.A. Maternal DNA hypomethylation and congenital heart defects. Birth Defects Res. Part A: Clin. Mol. Teratol. 2011, 91, 69–76. [Google Scholar] [CrossRef]
- Digilio, M.C.; Marino, B. What is new in genetics of congenital heart defects? Front. Pediatrics 2016, 4, 120. [Google Scholar] [CrossRef]
- Monteagudo-Sánchez, A.; Sánchez-Delgado, M.; Mora, J.R.H.; Santamaría, N.T.; Gratacós, E.; Esteller, M.; de Heredia, M.L.; Nunes, V.; Choux, C.; Fauque, P.; et al. Differences in expression rather than methylation at placenta-specific imprinted loci is associated with intrauterine growth restriction. Clin. Epigenetics 2019, 11, 35. [Google Scholar] [CrossRef] [PubMed]
- Moore-Morris, T.; van Vliet, P.P.; Andelfinger, G.; Puceat, M. Role of epigenetics in cardiac development and congenital diseases. Physiol. Rev. 2018, 98, 2453–2475. [Google Scholar] [CrossRef] [PubMed]
- Gijtenbeek, M.; Shirzada, M.R.; Ten Harkel, A.D.; Oepkes, D.; C Haak, M. Congenital heart defects in monochorionic twins: A systematic review and meta-analysis. J. Clin. Med. 2019, 8, 902. [Google Scholar] [CrossRef] [PubMed]
- Barber, E.; Weiner, E.; Feldstein, O.; Dekalo, A.; Mizrachi, Y.; Gonullu, D.C.; Bar, J.; Schreiber, L.; Kovo, M. The differences in placental pathology and neonatal outcome in singleton vs. twin gestation complicated by small for gestational age. Arch. Gynecol. Obstet. 2018, 298, 1107–1114. [Google Scholar] [CrossRef]
- Grantz, K.L.; Grewal, J.; Albert, P.S.; Wapner, R.; D’Alton, M.E.; Sciscione, A.; Grobman, W.A.; Wing, D.A.; Owen, J.; Newman, R.B.; et al. Dichorionic twin trajectories: The NICHD fetal growth studies. Am. J. Obstet. Gynecol. 2016, 215, 221.E1–221.E16. [Google Scholar] [CrossRef]
- Kibel, M.; Kahn, M.; Sherman, C.; Kingdom, J.; Zaltz, A.; Barrett, J.; Melamed, N. Placental abnormalities differ between small for gestational age fetuses in dichorionic twin and singleton pregnancies. Placenta 2017, 60, 28–35. [Google Scholar] [CrossRef]
- Matthiesen, N.B.; Henriksen, T.B.; Gaynor, J.W.; Agergaard, P.; Bach, C.C.; Hjortdal, V.E.; Østergaard, J.R. Congenital heart defects and indices of fetal cerebral growth in a nationwide cohort of 924 422 liveborn infants. Circulation 2016, 133, 566–575. [Google Scholar]
- Gordijn, S.J.; Beune, I.M.; Thilaganathan, B.; Papageorghiou, A.; Baschat, A.A.; Baker, P.N.; Silver, R.M.; Wynia, K.; Ganzevoort, W. Consensus definition of fetal growth restriction: A Delphi procedure. Ultrasound Obstet. Gynecol. 2016, 48, 333–339. [Google Scholar] [CrossRef]
- Clayton, P.E.; Cianfarani, S.; Czernichow, P.; Johannsson, G.; Rapaport, R.; Rogol, A. Management of the child born small for gestational age through to adulthood: A consensus statement of the International Societies of Pediatric Endocrinology and the Growth Hormone Research Society. J. Clin. Endocrinol. Metab. 2007, 92, 804–810. [Google Scholar] [CrossRef]
- Zeve, D.; Regelmann, M.O.; Holzman, I.R.; Rapaport, R. Small at birth, but how small? The definition of SGA revisited. Horm. Res. Paediatr. 2016, 86, 357–360. [Google Scholar] [CrossRef]
- Beune, I.M.; Bloomfield, F.H.; Ganzevoort, W.; Embleton, N.D.; Rozance, P.J.; van Wassenaer-Leemhuis, A.G.; Wynia, K.; Gordijn, S.J. Consensus based definition of growth restriction in the newborn. J. Pediatrics 2018, 196, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Hunter, J.P.; Saratzis, A.; Sutton, A.J.; Boucher, R.H.; Sayers, R.D.; Bown, M.J. In meta-analyses of proportion studies, funnel plots were found to be an inaccurate method of assessing publication bias. J. Clin. Epidemiol. 2014, 67, 897–903. [Google Scholar] [CrossRef] [PubMed]
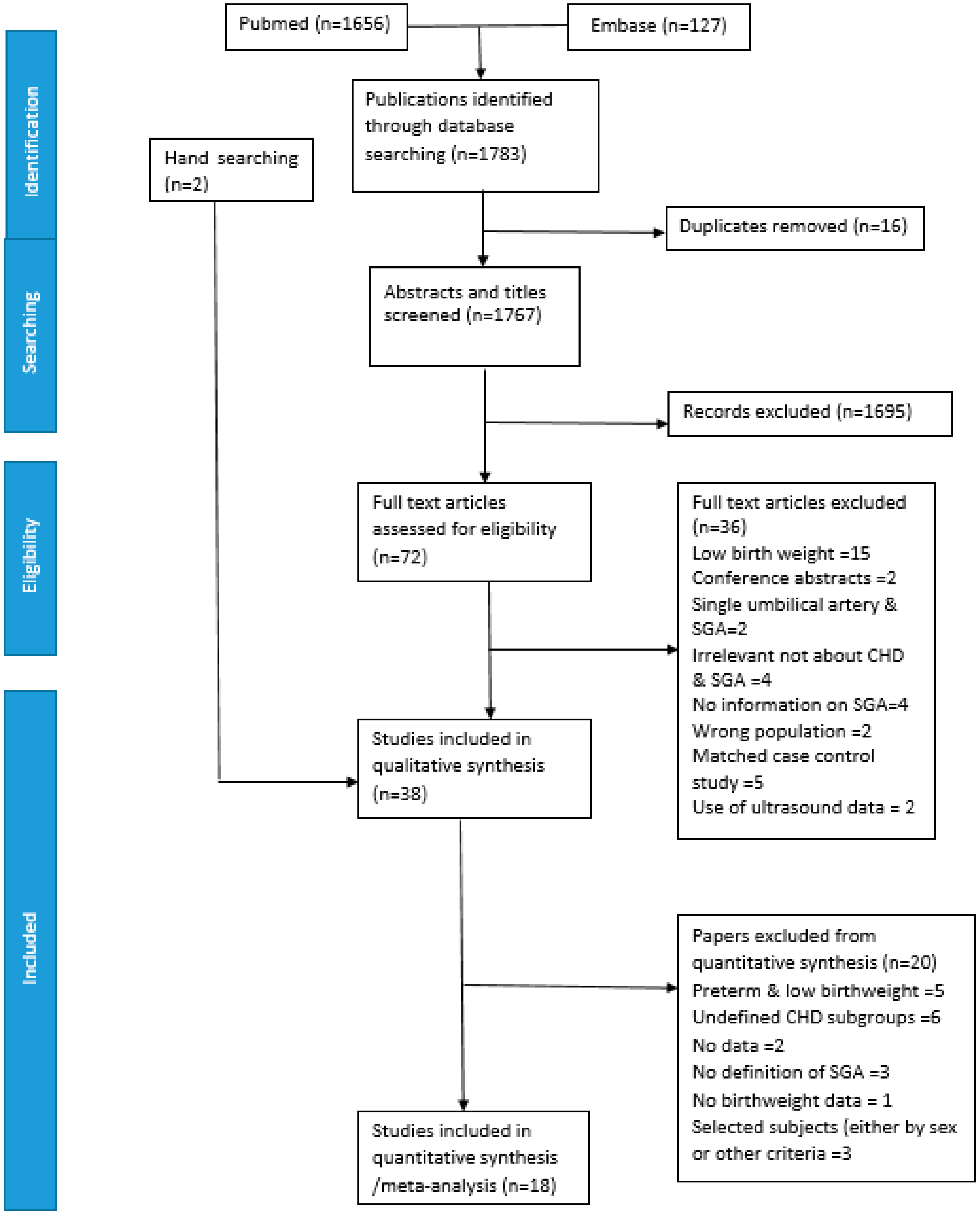
| Characteristics of Study | Number of Publications | Number of Publications in MA |
| Year of Publication (n = 38) | (n = 18) | |
| 1970–1979 | 3 (7.9%) | 2 (11.1%) |
| 1980–1989 | 1 (2.6%) | 1(5.6%) |
| 1990–1999 | 3 (7.9%) | 2 (11.1%) |
| 2000–2009 | 8 (21.1%) | 6 (33.3%) |
| 2010–2019 | 23 (60.5%) | 7 (38.9%) |
| Country (n = 38) | (n = 18) | |
| USA | 26 (68.5%) | 14 (77.8%) |
| Sweden | 4 (10.5%) | 1 (5.6%) |
| China | 3 (8%) | 1 (5.6%) |
| Italy | 1 (2.6%) | 0 |
| France | 1 (2.6%) | 0 |
| Chili | 1 (2.6%) | 0 |
| UK | 1 (2.6%) | 1 (5.6%) |
| Definition of SGA according to percentile (n = 38) | (n = 18) | |
| 10th percentile (consensus definition of SGA) | 22 (57.9%) | 14 (77.8%) |
| 3rd percentile | 7 (18.4%) | 4 (22.2%) |
| Undefined percentile | 9 (23.7%) | 0 |
| Consensus definition of SGA: 10th percentile: (n = 38) | (n = 14) | |
| No comparison | 6 (27.2%) | 4 (28.6%) |
| According to gestational age and sex | 6 (27.3%) | 4 (28.6%) |
| According to gestational age | 4 (18.2%) | 3 (21.4%) |
| According to gestational age, sex and race | 3 (13.7%) | 1 (7.1%) |
| According to gestational age and race | 2 (9.1%) | 2 (14.3%) |
| According to gestational age, race, sex, and single or multiple gestation | 1 (4.6%) | 0 |
| Birthweight data provided for SGA | 35 (92.1%) | 18 (100%) |
| Characteristics of Study | Number of Publications | Number of Publications in MA |
| SGA 1st aim of study | 17 (44.7%) | 13 (72.2%) |
| CHD | ||
| All | 23 | 8 |
| Isolated | 10 | 7 |
| CHD subtype | ||
| HLHS | 10 | 8 |
| ToF | 10 | 7 |
| CoAo | 8 | 7 |
| TGV | 7 | 7 |
| AVSD | 7 | 7 |
| ASD | 7 | 6 |
| TA | 3 | 3 |
| CAT | 3 | 3 |
| Subgroup | Author | Pooled Proportion (95% CI) | % Weight | |
|---|---|---|---|---|
| HLHS | ||||
| Total pooled result | 21 | (19–23) | ||
| Khoury (1988) [12] | 23 | (15–33) | 7.36 | |
| Nembhard (2009) [41] | 23 | (18–28) | 22.81 | |
| Williams (2010) [49] | 20 | (17–24) | 48.79 | |
| Swenson (2012) [47] | 19 | (15–24) | 21.04 | |
| ToF | ||||
| Total pooled result | 30 | (24–37) | ||
| Khoury (1988) [12] | 34 | (25–43) | 29.05 | |
| Nembhard (2009) [41] | 26 | (23–30) | 48.18 | |
| Swenson (2012) [47] | 36 | (25–48) | 22.77 | |
| TGV | ||||
| Total pooled result | 17 | (13–22) | ||
| Khoury (1988) [12] | 17 | (11–23) | 28.79 | |
| Nembhard (2009) [41] | 20 | (17–24) | 41.34 | |
| Swenson (2012) [47] | 13 | (8–18) | 29.87 | |
| VSD | ||||
| Total pooled result | 19 | (18–20) | ||
| Khoury (1988) [12] | 27 | (24–31) | 13.1 | |
| Nembhard (2009) [41] | 17 | (16–19) | 86.9 | |
| CoAo | ||||
| Total pooled result | 22 | (19–25) | ||
| Khoury (1988) [12] | 28 | (21–36) | 19.06 | |
| Nembhard (2009) [41] | 20 | (17–24) | 80.94 | |
| AVSD | ||||
| Total pooled result | 27 | (21–32) | ||
| Khoury (1988) [12] | 28 | (20–38) | 37.3 | |
| Williams (2010) [49] | 25 | (18–33) | 53.51 | |
| Swenson (2012) [47] | 32 | (15–54) | 9.19 | |
| TA | ||||
| Total pooled result | 27 | (21–35) | ||
| Williams (2010) [49] | 30 | (22–39) | 74.84 | |
| Swenson (2012) [47] | 21 | (10–37) | 25.16 | |
| CAT | ||||
| Total pooled result | 23 | (17–30) | ||
| Khoury (1988) [12] | 24 | (11–41) | 19.66 | |
| Nembhard (2009) [41] | 25 | (17–34) | 64.1 | |
| Swenson (2012) [47] | 18 | (6–37) | 16.24 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghanchi, A.; Derridj, N.; Bonnet, D.; Bertille, N.; Salomon, L.J.; Khoshnood, B. Children Born with Congenital Heart Defects and Growth Restriction at Birth: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2020, 17, 3056. https://doi.org/10.3390/ijerph17093056
Ghanchi A, Derridj N, Bonnet D, Bertille N, Salomon LJ, Khoshnood B. Children Born with Congenital Heart Defects and Growth Restriction at Birth: A Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health. 2020; 17(9):3056. https://doi.org/10.3390/ijerph17093056
Chicago/Turabian StyleGhanchi, Ali, Neil Derridj, Damien Bonnet, Nathalie Bertille, Laurent J. Salomon, and Babak Khoshnood. 2020. "Children Born with Congenital Heart Defects and Growth Restriction at Birth: A Systematic Review and Meta-Analysis" International Journal of Environmental Research and Public Health 17, no. 9: 3056. https://doi.org/10.3390/ijerph17093056
APA StyleGhanchi, A., Derridj, N., Bonnet, D., Bertille, N., Salomon, L. J., & Khoshnood, B. (2020). Children Born with Congenital Heart Defects and Growth Restriction at Birth: A Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health, 17(9), 3056. https://doi.org/10.3390/ijerph17093056






