Abstract
Electronic waste recycling can release heavy metals into the environment and cause adverse health effects. We assessed the association between exposure to heavy metals from electronic waste recycling and the prevalence of asthma in a nested case-control study of 51 subject pairs with and without asthma. House dust, airborne dust, blood, and urine were collected from residents of two neighboring sites in Ubon Ratchathani province, Thailand. Multiple electronic waste-handling activities are conducted in the first site, while the second site is mostly agricultural. Concentrations of chromium, mercury, nickel, and lead in house dust and airborne dust were higher in the electronic waste-handling site (p < 0.05), but levels of exposure were similar in subjects with and without asthma. Although we did not find an association between exposure to these metals and the prevalence of asthma, control measures should be implemented to reduce health risks from long-term exposure to heavy metals.
1. Introduction
Electronic waste (e-waste), discarded electrical and electronic equipment that is outdated, damaged, or malfunctioning, is one of the major sources of environmental and health-related problems worldwide [1,2]. It has emerged as the fastest growing waste stream due to technological advancement, fashion style, market expansion, end of product life, and misuse or lack of maintenance of electrical and electronic equipment. Since e-waste contains several toxic substances, inappropriate management can contribute to environmental contamination leading to adverse effects on human health, the environment and ecological systems [3,4]. Especially, in the informal sector, manual dismantling and open burning is commonly used for recovering valuable materials without adequate and proper personal protective equipment (PPE), which results in significant risk of exposure to toxic substances of the workers and communities [5,6]. In addition, several studies have reported levels of toxic substances such as heavy metals and persistent halogenated compounds in air, water, soil, sediment, and dust in and around e-waste sites [7,8,9,10].
The potential adverse health consequences from exposure to toxic substances in e-waste vary by substance, concentration and duration of exposure [11]. For example, lead (Pb) can affect the liver kidney, and nervous system and disrupt cognitive development [12,13,14]. Chromium (VI) can cause respiratory tract irritation, kidney and liver damage, weakened immune systems, and nasal, sinus or lung cancer. [15]. Mercury (Hg) exposure may lead to memory loss, immune toxicity, and muscle weakness [16]. Meanwhile, nickel (Ni) might contribute to dermatitis and bronchial asthma [17]. In addition, persistent halogenated compounds such as brominated flame retardants can interfere with hormone function and cause neurobehavioral effects, behavioral disorders, and cancer [16,18,19]. E-waste workers are at risk from direct exposure to toxic substances during work, whereas others may be exposed to toxic substances contaminating the environment.
Asthma is a respiratory disorder caused by chronic inflammation of the bronchi that results in airway hyperresponsiveness to stimuli such as allergens or irritants. The symptoms of asthma include shortness of breath, wheezing, chest tightness, and cough [6,20]. A wide variety of natural and man-made materials can cause sensitization of the respiratory tract as chemical allergens, including heavy metals [21,22]. Additionally, dermal absorption may also represent a relevant route through which respiratory sensitization can be achieved [23]. Exposure to lead (Pb) and mercury (Hg) has been associated with asthma via increased IgE levels [24], and more than 50% of asthma cases are hypothesized to be mediated by elevated IgE, which may also influence the association between blood levels of heavy metals [25].
Similar to other countries, the e-waste problem in Thailand has become an increasing concern. In 2013, Thailand generated 0.56 million tons of hazardous waste from households, and approximately 65% was e-waste [7], much of which went to informal sectors such as pickers, waste buyers, junk shops, recyclers, and re-processors. Inappropriate collecting, dismantling, recycling, and disposing may contribute to detrimental effects to human health and the environment, especially for the workers who carry out e-waste related activities without protective equipment [6].
The BanKok sub-district of Kuengnai district, Ubon Ratchathani province, has been involved in e-waste activities since 2011. In 2015, the Bureau of Environmental Health determined that the overall levels of heavy metals were below recommended limits [26]. Exposure to heavy metals can lead to bronchial inflammation [27,28], but a direct association to asthma has not been shown conclusively. The prevalence of asthma in BanKok has been shown to be double that of an area with no e-waste activities [29,30], perhaps owing to heavy metal exposure from activities related to e-waste.
The present study assessed the association between heavy metal exposure and the prevalence of asthma by measuring metal levels in house dust, airborne dust, and biological samples from asthmatic and non-asthmatic individuals residing in an area with e-waste activities and in a reference area without e-waste activities. The findings may contribute to the formulation of strategies for preventing adverse health effects from heavy metals in e-waste.
2. Materials and Methods
2.1. Study Sites
The study sites were the BanKok and BanKlang sub-districts of Kuengnai district, Ubon Ratchathani province, northeastern Thailand, which are located approximately 590 km from Bangkok (Figure 1). BanKok is an e-waste recycling site and is estimated to include 23 businesses and 76 households engaged in e-waste processing. BanKlang, located 2 km from BanKok, is mostly agricultural and has no e-waste activities.
Figure 1.
Map of the study sites.
2.2. Study Design and Population
We designed the investigation as a nested case-control study. The inclusion criteria were Thai nationality, age 20–70 years, resident of BanKok or BanKlang for more than 5 years, and no history of asthma (code J45 in the International Classification of Diseases, 10th revision) before 2011. A case was an asthma patient and a control was a non-asthmatic individual matched to the case by age and sex. All registered asthma cases in BanKok and BanKlang were included in this study. This study was approved by the Ethics Committee of the Faculty of Tropical Medicine, Mahidol University (approval MUTM 2017-008-01) and was conducted in accordance with the 1964 Helsinki declaration (and later amendments) or other comparable ethical standards. Written informed consent was obtained from each subject.
2.3. Environmental and Biological Sample Collection and Analysis
2.3.1. House Dust and Airborne Dust Collection and Analysis
House dust and airborne dust samples were collected from the residences of participants by purposive sampling between May and July 2017. House dust samples were collected from four sub-areas of 0.25 m2 for a total area of 1 m2 of the floor using dried and pre-weighed task wipes (Kimberly-Clark, Irving, TX, USA) and processed using the American Society for Testing and Materials (ASTM) method D6966-13 [31] with minor modifications. All subjects were asked to carry personal air samplers with a pre-weighted mixed cellulose ester membrane filter (0.8 µm pore size) that operated continuously for 8 h at a flow rate of 4 L/min to collect airborne dust in the daytime. The collected task wipes and membrane filters were kept in a resealable plastic bag, transported to the laboratory, placed in a desiccator for 24 h prior to weighing, and stored at −20 °C until analysis.
Task wipes and membrane filters were cut into small segments, digested in a microwave oven in a solution of pure concentrated nitric acid (65% HNO3), and filtered through a 0.45-µm Millipore filter paper (MilliporeSigma, Burlington, MA, USA) to obtain a clear solution. The filtrate was stored at 4 °C in polypropylene bottles for heavy metal analysis.
2.3.2. Blood and Urine Sample Collection
Whole blood was obtained by venipuncture, collected in tubes with heparin as an anticoagulant, and stored at 4 °C until analysis. A spot urine sample was collected from the first morning urine and stored at −20 °C until analysis.
2.3.3. Analysis of Heavy Metals
Concentrations of chromium (Cr), Hg, Ni, and Pb in dust samples were analyzed using inductively coupled plasma-optical emission spectrometry. Blood Pb and urinary Cr and Ni were analyzed using graphite furnace atomic absorption spectrophotometry (model Z-8200 polarized Zeeman atomic absorption spectrophotometer, Hitachi, Tokyo, Japan). Urinary Hg was determined using a mercury analyzer (NIC MA-3000, Nippon Instrument Corporation, Tokyo, Japan). A calibration curve was generated with known concentrations of each heavy metal from standard solutions. The solvents and chemicals used in this study were of analytical grade.
2.3.4. Questionnaires
We conducted interviews with participants to collect information on socio-demographic and lifestyle factors that may be related to heavy metal exposure, as well as information on the use of personal protective equipment.
2.4. Data Analysis
Data obtained from this study were analyzed using SPSS version 19 (IBM SPSS, Armonk, NY, USA). After performing a Komogorov-Smirnov test, a Student’s t-test or the chi-squared test or the Mann-Whitney U test were used to compare general characteristics and heavy metal concentrations in the samples between asthma cases and controls. The level of significance was set at α = 0.05.
3. Results
3.1. General Characteristics of Study Subjects
A total of 102 subjects (84 from BanKok and 18 from BanKlang) participated in the study. There were 51 asthma cases (42 from BanKok and 9 from BanKlang). The control group consisted of 42 residents of BanKok and 9 residents of BanKlang. The majority of subjects (60) were female. Most participants were aged 47–68 years and had a primary school education and monthly income of 1000–5699 baht. Six asthma patients reported experiencing symptoms weekly, 12 experienced symptoms monthly, and 33 experienced symptoms less frequently, such as once every 6 months (Table 1).

Table 1.
Demographic information of participants stratified by site and group.
3.2. Occupational and Environmental Factors Related to E-Waste Handling
Twenty-six asthma cases were involved in e-waste activities (Figure 2). Of the asthma patients involved in e-waste activities, eight separated the waste, four were involved in purchasing it, another four both purchased and separated e-waste, and two separated and collected it; a few did not specify their involvement (Figure 3). Most worked ≥ 5 h per day, 6 days per week, and had been involved in e-waste activities for at least 5 years. Almost a third of all asthma patients lived near an e-waste shop, 23 had family members who worked with e-waste, and 10 cooked at an e-waste site.
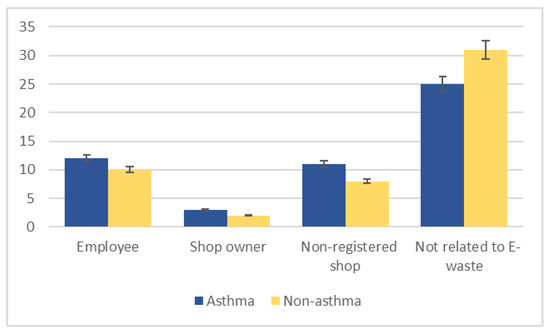
Figure 2.
Participant involvement in electronic waste (e-waste) activities, stratified by asthma status.
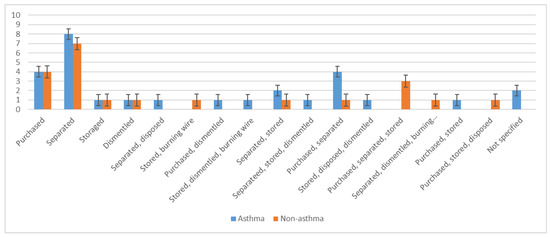
Figure 3.
Participant involvement in specific steps of electronic waste handling, stratified by asthma status.
Twenty non-asthmatic subjects were involved in e-waste activities. A third separated e-waste, four were involved in purchasing it, and others purchased or collected it (Figure 3). Similarly to the asthma cases, most worked ≥ 5 h per day, 6 days per week, and had been involved in e-waste activities for at least 5 years. Approximately a fourth of all non-asthmatic subjects resided near an e-waste shop, 13 had family members who were involved in e-waste activities, and only three cooked at an e-waste site. Fifty-six participants (25 asthma patients and 31 non-asthmatic subjects) were not involved in handling e-waste.
The e-waste items most typically handled by study participants were tabletop fans, washing machines, televisions, and cell phones (Figure 4). Asthmatic subjects were more likely to use personal protective equipment when handling e-waste, including the facility’s ventilation system (Figure 5).
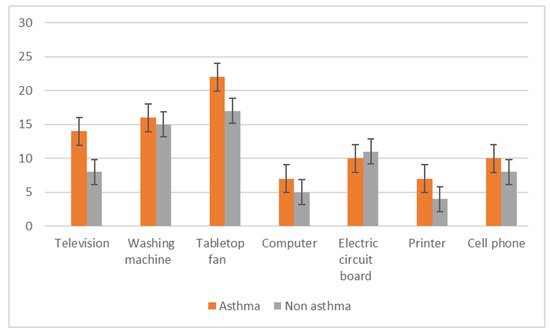
Figure 4.
Types of electronic waste items handled by study participants.
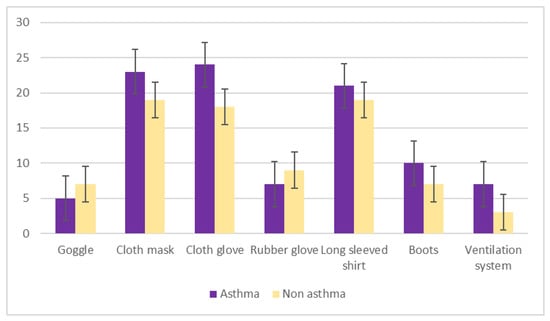
Figure 5.
Use of personal protective equipment by study participants handling electronic waste.
3.3. Heavy Metal Concentrations in House Dust and Airborne Dust of E-Waste and Non-E-Waste Related House
Average concentrations of Cr, Hg, Ni, and Pb in house dust and airborne dust samples collected from participants involved in e-waste activities were higher than concentrations in samples collected from participants who were not involved in e-waste activities (Table 2).

Table 2.
Heavy metal concentrations in house dust and airborne dust collected from study participants.
3.4. Heavy Metal Concentrations in House Dust and Airborne Dust Stratified by Village
Average concentrations of Cr, Hg, Ni, and Pb in house dust and airborne dust were higher in BanKok than in BanKlang (p < 0.05) (Table 3). No difference in metal concentrations was observed between samples collected from asthmatics and non-asthmatics (p > 0.05), with the exception of Cr concentrations in airborne dust, which were higher in the breathing zone of asthmatics (p = 0.049) (Table 4).

Table 3.
Average concentrations of chromium (Cr), mercury (Hg), nickel (Ni), and lead (Pb) in house dust and airborne dust stratified by village.

Table 4.
Average concentrations of chromium (Cr), mercury (Hg), nickel (Ni), and lead (Pb) in house dust and airborne dust stratified by asthma status.
3.5. Heavy Metal. Levels in Blood and Urine
We measured the concentrations of metals in blood and urine as markers of exposure in participants. Mean concentrations of blood Pb and urinary Cr and Ni were slightly lower in the asthma group, but the difference did not reach statistical significance. Urinary Hg levels were higher in the asthma group (Table 5).

Table 5.
Average concentrations of blood lead (Pb) and urinary mercury (Hg), nickel (Ni), and chromium (Cr) in study subjects stratified by asthma status.
Mean concentrations of urinary Cr, Hg, and Ni were higher in subjects who handled e-waste than in those who did not, but the difference did not reach statistical significance. Blood Pb levels were significantly higher in subjects who were not involved in e-waste activities (Table 6). No associations were found between use of personal protective equipment and blood Pb or urinary Cr, Hg, and Ni.

Table 6.
Average concentrations of blood lead (Pb) and urinary mercury (Hg), nickel (Ni), and chromium (Cr) in study subjects stratified by involvement in electronic waste (e-waste) activities.
Urinary Hg and Ni were higher in the BanKok group than in the BanKlang group, but blood Pb and urinary Cr were higher in the BanKlang group. However, only urinary Cr levels differed significantly between groups (Table 7).

Table 7.
Average concentrations of blood lead (Pb) and urinary mercury (Hg), nickel (Ni), and chromium (Cr) in study subjects stratified by location.
3.6. Association with Asthma
The chi-squared test found no significant differences between subject characteristics or occupational factors such as use of personal protective equipment and asthma (p > 0.05), with one exception: years of work were associated with a higher likelihood of asthma (p < 0.05) (Table 8).

Table 8.
Association of personal and occupational characteristics and asthma in study participants.
4. Discussion
Associations between exposure to environmental and industrial toxicants and increased prevalence of asthma have been reported [32]. E-waste handling and recycling activities are considered a source of heavy metals whose adverse effects may include respiratory and other conditions [33,34]. We assessed the association between environmental exposure to several metals and the prevalence of asthma in sites with and without e-waste activities. However, some participants from BanKlang, the reference site, disclosed their involvement with e-waste. We therefore analyzed e-waste-related activities at the participant level.
4.1. Heavy Metal Concentrations in Air and Dust
House dust and airborne levels of Cr, Hg, Ni, and Pb were higher in the homes and environment of subjects involved in e-waste activities. This is in accordance with the findings of several studies [10,35,36,37,38]. Metal concentrations did not vary significantly between house dust and airborne dust, perhaps because participants were not involved in activities that released airborne particulate matter.
We also found that the metal concentrations were higher in BanKok, the e-waste site, than in BanKlang, the reference area without e-waste recycling facilities. The overall concentrations of Cr, Hg, Ni, and Pb measured in this study were lower than those reported previously in China and Nigeria [39,40,41], probably because our study subjects were mostly collectors and separators of e-waste and not involved in dismantling electronic devices.
4.2. Heavy Metal. Concentrations in Blood and Urine
Higher median concentrations of blood Pb and Cr and urinary Ni have been reported in e-waste workers in Ghana [42], but we did not find significantly higher concentrations of metals in the blood and urine of e-waste workers. In contrast, we observed higher urinary Cr levels in subjects who were not involved in e-waste activities.
Moreover, we did not observe a connection between exposure to heavy metals and asthma in our cohort. This may be attributable to the low levels of heavy metals we recorded, perhaps because little dismantling of electronic devices was conducted, leading to low levels of emissions. The high humidity in ambient air during the sample collection period may have also caused dust to settle rather than remain airborne. In addition, economic downturns in the US reduced global demand for metal in 2015, leading to lower market prices and decreased e-waste recycling in Thailand [43].
A recent case-control study in a Chinese cohort of 551 asthma patients and matched controls reported that asthma prevalence in adults was positively associated with urinary levels of some metals, including Cr, and negatively associated with levels of Ni and Pb [44]. Blood Pb levels did not differ between participants from BanKok and BanKlang, suggesting either that the e-waste in BanKok did not contain appreciable levels of Pb or that the residents of BanKlang were exposed to similar levels of Pb from agricultural fertilizers.
4.3. Association with Asthma
We did not find an association between blood or urinary concentrations of metals and asthma, perhaps because our cohort was small and metal levels were low.
5. Conclusions
We measured higher concentrations of metals in the house dust and breathing zone of subjects involved in handling e-waste than in those of subjects who did not engage in e-waste activities, but blood and urinary metal levels did not follow this trend. We did not find an association between exposure to heavy metals and the prevalence of asthma, probably because of the small sample, but control measures should be implemented to reduce health risks from long-term exposure to heavy metals. In addition, the association between the exposure to heavy metals and other toxic substances such as flame retardants from e-waste and asthma prevalence in a larger population-based cohort should be carried out.
Author Contributions
Conceptualization, C.K. and S.W.; methodology, C.K., S.W., and S.L.; software, C.K. and K.T.; validation, S.L., S.W., K.T., and Y.L.; formal analysis, C.K., K.T., S.L., S.W.; investigation, C.K.; resources, C.K., S.W., and R.M.; data curation, C.K., S.W., and K.T.; original draft preparation, C.K. and S.W.; review and revision, S.W.; visualization, C.K.; supervision, S.W., K.T., Y.L., S.L., and A.P.; project administration, C.K.; funding acquisition, C.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Faculty of Tropical Medicine, Mahidol University, Research Fund for Theses.
Acknowledgments
The authors thank the villagers of BanKok and BanKlang, Kuengnai district, Ubon Ratchathani province, for participating in this research and the staff of the Department of Social and Environmental Medicine, Mahidol University, for support. The authors also thank the Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ravi, V. Evaluating the overall quality of recycling of e-waste from end of life computers. J. Clean Prod. 2012, 20, 145–151. [Google Scholar] [CrossRef]
- Kiddee, P.; Naidu, R.; Wong, M.H. Electronic waste management approaches: An overview. Waste Manag. 2013, 33, 1237–1250. [Google Scholar] [CrossRef] [PubMed]
- Hicks, C.; Dietmar, R.; Eugster, M. The recycling and disposal of electrical and electronic waste in China-legislative and market responses. Environ. Impact. Assess Rev. 2005, 25, 459–471. [Google Scholar] [CrossRef]
- Robinson, B.H. E-waste: An assessment of global production and environmental impacts. Sci. Total Environ. 2009, 408, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Perkins, D.N.; Drisse, M.N.B.; Nxele, T.; Sly, P.D. E-waste: A global hazard. Ann. Glob. Health 2014, 80, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Chareonsong, P. E-Waste Management in Thailand. In Proceedings of the 4th International E-Waste Management Network (IEMN) Workshop, Hanoi, Vietnam, 14–17 July 2014; Available online: https://www.epa.gov/sites/production/files/2014-08/documents/thailand_country_presentation.Pdf (accessed on 15 January 2016).
- Schluep, M.; Hagelueken, C.; Kuehr, R.; Magalini, F.; Maurer, C.; Meskers, C.; Mueller, E.; Wang, F. Recycling-From E-Waste to Resources; UNEP–DTIE: Paris, France, 2009. [Google Scholar]
- Luo, C.; Liu, C.; Wang, Y.; Liu, X.; Li, F.; Zhang, G.; Li, X. Heavy metal contamination in soils and vegetables near an e-waste processing site, South China. J. Hazard. Mater. 2011, 186, 481–490. [Google Scholar] [CrossRef]
- Tang, X.; Shen, C.; Shi, D.; Cheema, S.A.; Khan, M.I.; Zhang, C.; Chen, Y. Heavy metal and persistent organic compound contamination in soil from Wenling: An emerging e-waste recycling city in Taizhou area, China. J. Hazard. Mater. 2010, 173, 653–660. [Google Scholar] [CrossRef]
- Lui, M.; Huang, B.; Bi, X.; Ren, Z.; Sheng, G.; Fu, J. Heavy metals and organic compounds contamination in soil from an e-waste region in South China. Environ. Sci. Process. Impacts 2013, 15, 919–929. [Google Scholar]
- Vetrivel, P.; Devi, P.K. A Focus on E-waste: Effects on Environment and Human Health. IJNTPS 2012, 2, 47–51. [Google Scholar]
- Obeng-Gyasi, E.; Armijos, R.X.; Weigel, M.M.; Filippelli, G.; Sayegh, M.A. Hepatobiliary-related outcomes in US adults exposed to Lead. Environments 2018, 5, 46. [Google Scholar] [CrossRef]
- Harari, F.; Sallsten, G.; Christensson, A.; Petkovic, M.; Hedblad, B.; Forsgard, N.; Melander, O.; Nilsson, P.M.; Borne, Y.; Engström, G.; et al. Blod lead levels and devreased kidney function on a population-based cohort. Am. J. Kidney Dis. 2018, 72, 381–389. [Google Scholar] [CrossRef]
- Bellinger, D.C. Very low lead exposures and children’s neurodevelopment. Curr. Opin. Pediatr. 2008, 20, 172–177. [Google Scholar] [CrossRef] [PubMed]
- ATSDR. Public Statement for Chromium. Available online: http://https://www.atsdr.cdc.gov/ToxProfiles/tp7-c1-b.pdf (accessed on 3 January 2018).
- Xu, X.; Zeng, X.; Boezen, H.M.; Huo, X. E-waste environmental contamination and harm to public health in China. Front. Med. 2015, 9, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Moira, C.Y.; Stephen, L. Occupational Asthma. Am. Rev. Respir. Dis. 1986, 133, 686–703. [Google Scholar]
- Costa, L.G.; Giordano, G. Developmental neurotoxicity of polybrominated diphenyl ether (PBDE) flame retardants. Neurotoxicology 2007, 28, 1047–1067. [Google Scholar] [CrossRef] [PubMed]
- Shy, C.G.; Huang, H.L.; Chang-Chien, G.P.; Chao, H.R.; Tsou, T.C. Neurodevelopment of infants with prenatal exposure to polybrominated diphenyl esters. Bull. Environ. Contam. Toxicol. 2011, 87, 643–648. [Google Scholar] [CrossRef] [PubMed]
- Frazzoli, C.; Orisakwe, O.E.; Dragone, R.; Mantovani, A. Diagnostic health risk assessment of electronic waste on the general population in developing countries’ scenarios. Environ. Impact Assess. Rev. 2010, 30, 388–399. [Google Scholar] [CrossRef]
- Realff, M.J.; Raymond, M.; Ammons, J.C. E-waste: An opportunity. Mater. Today 2004, 7, 40–45. [Google Scholar] [CrossRef]
- Wang, J.; Cen, H.; Xia, W.; Chen, L.; Zhao, J.; Li, G. Heavy metal pollution in the surface dust from E-waste disposal place and its ecological risk assessment. Adv. Mater. Res. 2012, 347–353, 2360–2364. [Google Scholar] [CrossRef]
- Zahir, F.; Rizwi, S.J.; Haq, S.K.; Khan, R.H. Low dose mercury toxicity and human health. Environ. Toxicol. Pharmacol. 2005, 20, 351–360. [Google Scholar] [CrossRef]
- Umesh, K.; Singh, D.N. Electronic Waste: Concerns & Hazardous Threats. IJCET 2014, 4, 801–811. [Google Scholar]
- Park, S.; Lee, E.H.; Kho, Y. The association of asthma, total IgE, and blood lead and cadmium levels. J. Allergy Clin. Immuniol. 2016, 138, 1702–1703.e6. [Google Scholar] [CrossRef] [PubMed]
- Bureau of Environmental Health, Department of Health, Ministry of Public Health. Report of the Surveillance Risk Area: Case of E-Waste: BanKok Sub-Distric, Kuenagnai District, Ubon Ratchthani Province; Department of Health: Bangkok, Thailand, 2015. [Google Scholar]
- Apter, A.J. Advances in the care of adults with asthma and allergy. J. Allergy Clin. Immunol. 2008, 121, 839–844. [Google Scholar] [CrossRef] [PubMed]
- Gu, Q.; Lin, R.L. Heavy metals zinc, cadmium, and copper stimulate pulmonary sensory neurons via direct activation of TRPA1. J. Appl. Physiol. 2010, 108, 891–897. [Google Scholar] [CrossRef] [PubMed]
- BanKok Health Care Center. 43 Profile Report of Public Health Service in Heath Care Center; BanKok Health Care Center: Ubon Ratchathani, Thailand, 2016. [Google Scholar]
- BanKlang Health Care Center. 43 Profile Report of Public Health Service in Heath Care Center; BanKlang Health Care Center: Ubon Ratchathani, Thailand, 2016. [Google Scholar]
- American Society for Testing and Materials (ASTM). Standard Practice for Collection of Settled Dust Samples Using Wipe Sampling Methods for Subsequent Determination of Metals. Available online: https://www.astm.org/Standards/D6966.htm (accessed on 20 January 2016).
- Seaton, A.; Godden, D.J.; Brown, K. Increase in asthma: A more toxic environment or a more susceptible population? Thorax 1994, 49, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.L.; Wong, W.H.; Lau, Y.L. Association between air pollution and asthma admission among children in Hong Kong. Clin. Exp. Allergy 2006, 36, 1138–1146. [Google Scholar] [CrossRef]
- Patel, M.M.; Hoepner, L.; Garfinkel, R.; Chillrud, S.; Reyes, A.; Quinn, J.W.; Perera, F.; Miller, R.L. Ambient metals, elemental carbon, and wheeze and cough in New York City children through 24 months of Age. Am. J. Respir. Critl. Care Med. 2009, 180, 1107–1113. [Google Scholar] [CrossRef]
- Leung, A.O.W.; Duzgoren-Aydin, N.S.; Cheung, K.C.; Wong, M.H. Heavy metals concentrations of surface dust from e-waste recycling and its human health implications in Southeast China. Environ. Sci. Technol. 2008, 42, 2674–2680. [Google Scholar] [CrossRef]
- Adaramodu, A.A.; Osuntogun, A.O.; Ehi-Eromosele, C.O. Heavy metal concentration of surface dust present in e-waste components: The Westminister electronis market, Lagos case study. Resour. Environ. 2012, 2, 9–13. [Google Scholar] [CrossRef]
- Guo, Y.; Huang, C.J.; Zhang, H.; Dong, Q.X. Heavy metal contamination from electronic waste recycling at Guiyu, Southeastern China. J. Environ. Qual. 2009, 38, 1617–1626. [Google Scholar] [CrossRef]
- Uchida, N.; Matsukami, H.; Someya, M.; Tue, N.M.; Tuyen, L.H.; Viet, P.H.; Takahashi, S.; Tanabe, S.; Suzuki, G. Hazardous metals emissions from e-waste-processing sites in a village in northern Vietnam. Emerg. Contam. 2018, 4, 11–21. [Google Scholar] [CrossRef]
- Igharo, G.O.; Anetor, J.I.; Osibanjo, O.O.; Osadolor, H.B.; Dike, K.C. Toxic metal levels in Nigerian electronic waste workers indicate occupational metal toxicity associated with crude electronic waste management practices. Biokemistri 2015, 26, 107–113. [Google Scholar]
- Zeng, X.; Xu, X.; Zheng, X.; Reponen, T.; Chen, A.; Huo, X. Heavy metals in PM2.5 and in blood, and children’s respiratory symptoms and asthma from an e-waste recycling area. Environ. Pollut. 2016, 210, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Chen, K.H.; Yan, X.; Chen, S.J.; Hu, G.C.; Peng, X.W.; Yuan, J.G.; Mai, B.X.; Yang, Z.Y. Heavy metals in food, house dust, and water from an e-waste recycling area in South China and the potential risk to human health. Ecotoxicol. Environ. Saf. 2013, 96, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Wittsiepe, J.; Feldt, T.; Till, H.; Burchard, G.; Wilhelm, M.; Fobil, J.N. Pilot study on the internal exposure to heavy metals of informal-level electronic waste workers in Agbogbloshie, Accra, Ghana. Environ. Sci. Pollut. Res. 2017, 24, 3097–3107. [Google Scholar] [CrossRef]
- Prachachat. Inhibition of Electronics Waste Import from China, Ministry of Commerce Issued a Ban on Import. 2018. Available online: https://www.prachachat.net/economy/news-404099 (accessed on 20 December 2019). (In Thai).
- Huang, X.; Xie, J.; Cui, X.; Zhou, Y.; Wu, X.; Lu, W.; Shen, Y.; Yuan, J.; Chen, W. Association between Concentrations of Metals in Urine and Adult Asthma: A Case-Control Study in Wuhan, China. PLoS ONE 2016, 11, e0155818. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).