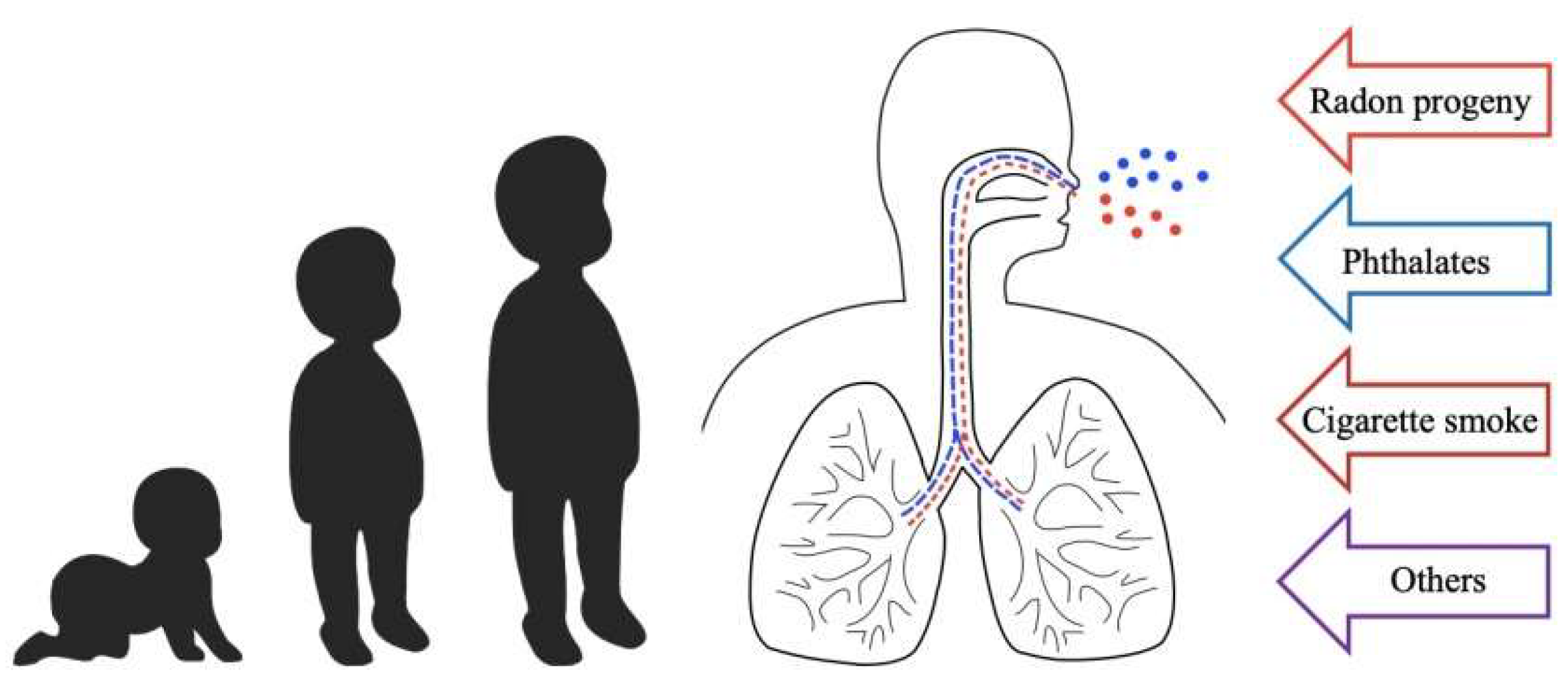
Multiple Stressor Effects of Radon and Phthalates in Children: Background Information and Future Research
Abstract
1. Introduction to Multiple Stressor Effect
2. Background Information on Radon, Cancer Risk and Dosimetry
2.1. Radon and Its Progeny
2.2. Cancer Risk
2.3. Radon Dosimetry
2.3.1. Morphometry
- (i)
- extrathoracic (ET) region;
- (ii)
- bronchial (BB) region which consisted of trachea and bronchi;
- (iii)
- bronchiolar (bb) region which consisted of bronchioles and terminal bronchioles;
- (iv)
- alveolar interstitial (AI) region which consisted of respiratory bronchioles, alveolar ducts and sacs with their alveoli, and interstitial connective tissue.
2.3.2. Respiratory Physiology
2.3.3. Radiation Biology
2.3.4. Deposition of Aerosols in Human Respiratory Tract
2.3.5. Clearance Model
2.3.6. Weighting the Doses
2.4. Background for Focus in Present Review
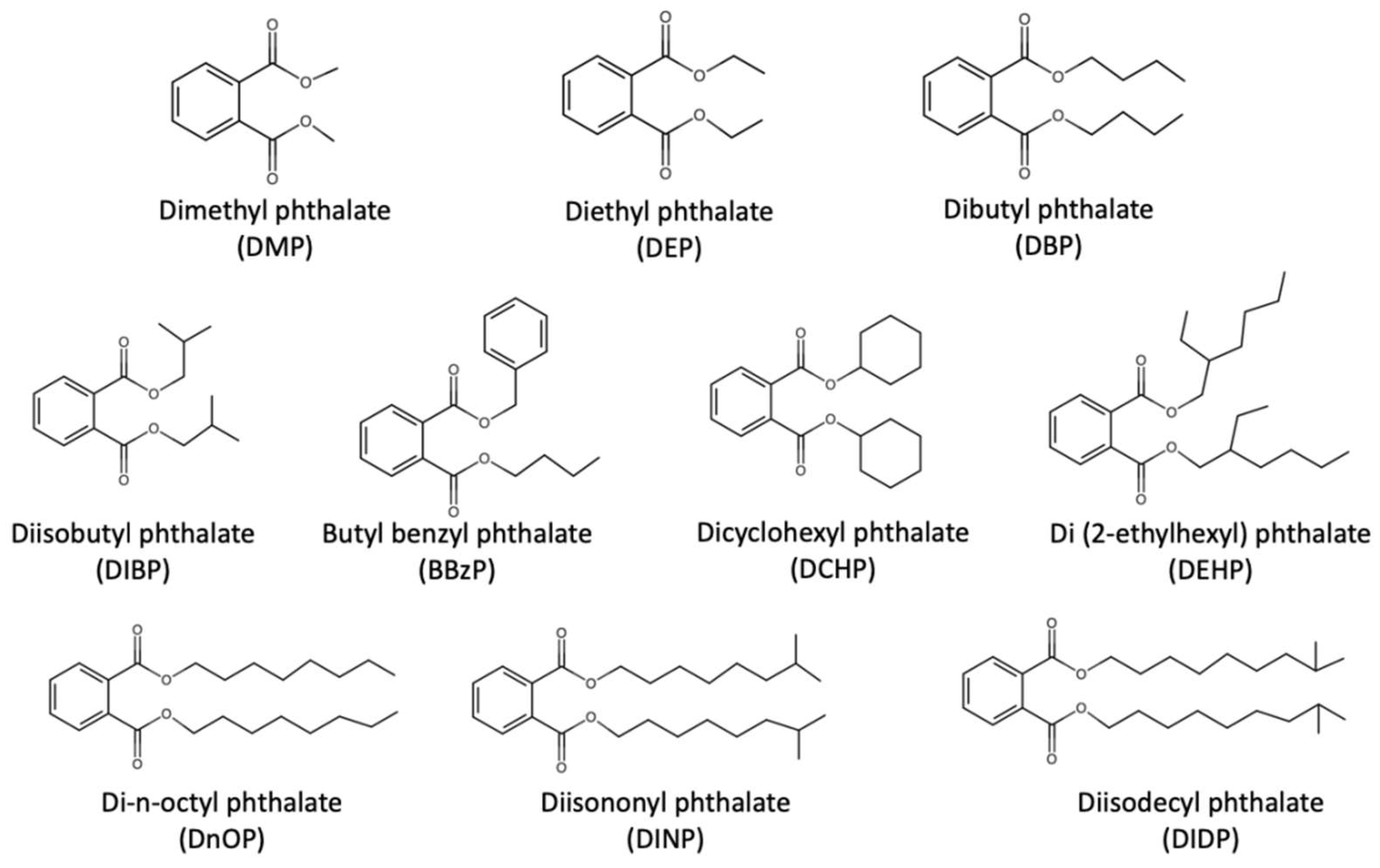
3. Background Information on Phthalates and Effects on Human Respiratory Health
4. Lung Cancer Risk of Combined Exposure to Indoor Radon and Phthalates in Children
5. Future Research Directions
5.1. Computational Modeling and Micro-Dosimetric Studies
5.2. Biological Studies
5.2.1. Effect-Specific Micro-Dosimetric Studies
5.2.2. Radiobiological Effects of α-Particles
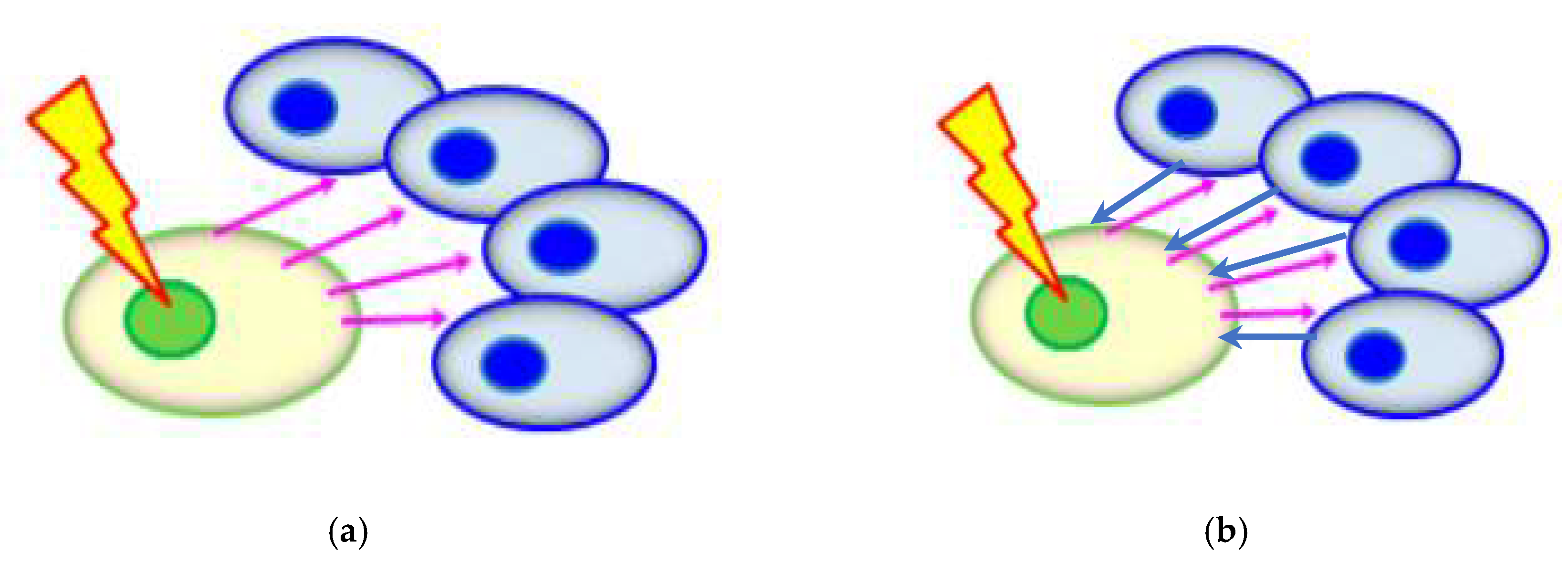
5.2.3. Potential Influence from Non-Targeted Effects
5.2.4. Biological Effects of Phthalates
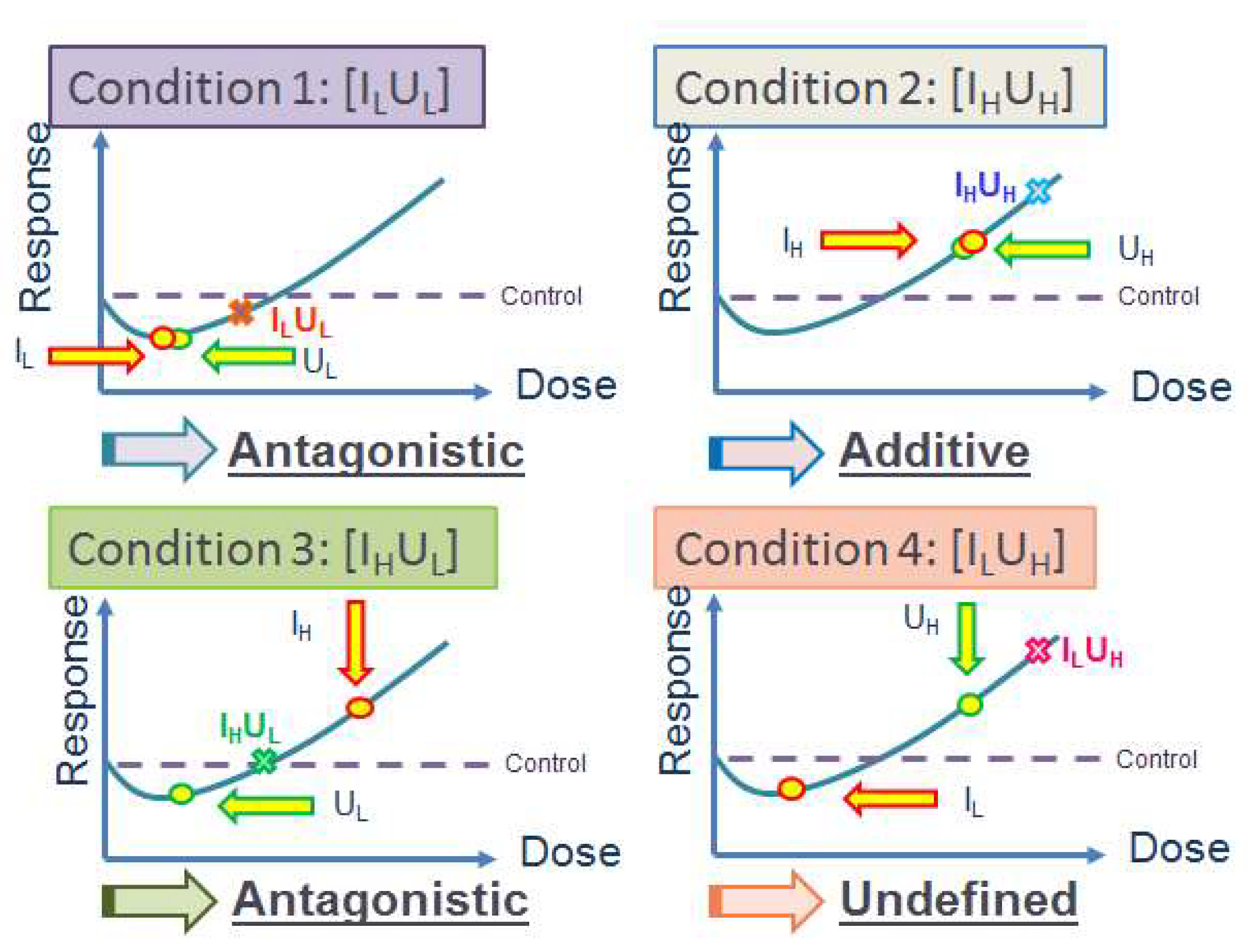
5.2.5. Multiple Stressor Effect of Radon (α-Particles) and Phthalates
6. Summary and Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Sexton, K.; Hattis, D. Assessing cumulative health risks from exposure to environmental mixtures—Three fundamental questions. Environ. Health Perspect. 2007, 115, 825–832. [Google Scholar] [CrossRef]
- Salbu, B.; Rosseland, B.O.; Oughton, D.H. Multiple stressors—A challenge for the future. J. Environ. Monit. 2005, 7, 539. [Google Scholar]
- Salbu, B.; Denbeigh, J.; Smith, R.W.; Heier, L.S.; Teien, H.C.; Rosseland, B.O.; Oughton, D.; Seymour, C.B.; Mothersill, C. Environmentally relevant mixed exposures to radiation and heavy metals induce measurable stress responses in Atlantic salmon. Environ. Sci. Technol. 2008, 42, 3441–3446. [Google Scholar] [CrossRef] [PubMed]
- Mothersill, C.; Salbu, B.; Heier, L.S.; Teien, H.C.; Denbeigh, J.; Oughton, D.; Rosseland, B.O.; Seymour, C.B. Multiple stressor effects of radiation and metals in salmon (Salmo salar). J. Environ. Radioact. 2007, 96, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Mothersill, C.; Smith, R.W.; Heier, L.S.; Teien, H.C.; Land, O.C.; Seymour, C.B.; Oughton, D.; Salbu, B. Radiation-induced bystander effects in the Atlantic salmon (Salmo salar L.) following mixed exposure to copper and aluminum combined with low-dose gamma radiation. Radiat. Environ. Biophys. 2014, 53, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Olsvik, P.A.; Heier, L.S.; Rosseland, B.O.; Teien, H.C.; Salbu, B. Effects of combined γ-irradiation and metal (Al + Cd) exposures in Atlantic salmon (Salmo salar). J. Environ. Radioact. 2010, 101, 230–236. [Google Scholar] [CrossRef]
- Vanhoudt, N.; Vandenhove, H.; Real, A.; Bradshaw, C.; Stark, K. A review of multiple stressor studies that include ionising radiation. Environ. Pollut. 2012, 168, 177–192. [Google Scholar] [CrossRef]
- Heier, L.S.; Teien, H.C.; Oughton, D.; Tollefsen, K.E.; Olsvik, P.A.; Posseland, B.O.; Lind, O.C.; Farmen, E.; Skipperud, L.; Salbu, B. Sublethal effects in Atlantic salmon (Salmo salar) exposed to mixtures of copper, aluminium and gamma radiation. J. Environ. Radioact. 2013, 121, 33–42. [Google Scholar] [CrossRef]
- Carpenter, D.O.; Arcaro, K.; Spink, D.C. Understanding the human health effects of chemical mixtures. Environ. Health Perspect. 2002, 110, 25–42. [Google Scholar] [CrossRef]
- Hertzberg, R.C.; Teuschler, L.K. Evaluating quantitative formulas for dose-response assessment of chemical mixtures. Environ. Health Perspect. 2002, 110, 965–970. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency (US EPA). Framework for Cumulative Risk Assessment, EPA/600/P02/001F; US EPA: Washington, DC, USA, 2003. [Google Scholar]
- Yu, K.N.; Tung, M.M.T.; Choi, V.W.Y.; Cheng, S.H. Alpha radiation exposure decreases apoptotic cells in Zebrafish embryos subsequently exposed to the chemical stressor, Cd. Environ. Sci. Pollut. Res. 2012, 19, 3831–3839. [Google Scholar] [CrossRef] [PubMed]
- Choi, V.W.Y.; Ng, C.Y.P.; Kong, M.K.Y.; Cheng, S.H.; Yu, K.N. Adaptive response to ionizing radiation induced by cadmium in zebrafish embryos. J. Radiol. Prot. 2013, 33, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.Y.P.; Choi, V.W.Y.; Lam, A.C.L.; Cheng, S.H.; Yu, K.N. Multiple stressor effect in Zebrafish embryos from simultaneous exposures to ionizing radiation and cadmium. J. Radiol. Prot. 2013, 33, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.Y.P.; Pereira, S.; Cheng, S.H.; Adam-Guillermin, C.; Garnier-Laplace, J.; Yu, K.N. Combined effects of depleted uranium and ionising radiation on Zebrafish embryos. Radiat. Prot. Dosim. 2015, 167, 311–315. [Google Scholar] [CrossRef]
- Ng, C.Y.P.; Pereira, S.; Cheng, S.H.; Adam-Guillermin, C.; Garnier-Laplace, J.; Yu, K.N. Combined effects of alpha particles and depleted uranium on Zebrafish (Danio rerio) embryos. J. Radiat. Res. 2016, 57, 343–355. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Baldwin, L.A. Defining hormesis. Hum. Exp. Toxicol. 2001, 21, 91–97. [Google Scholar] [CrossRef]
- Yu, K.N.; Guan, Z.J.; Stokes, M.J.; Young, E.C.M. The assessment of natural radiation dose committed to the Hong Kong people. J. Environ. Radioact. 1992, 17, 31–48. [Google Scholar] [CrossRef]
- National Research Council. Health Effects of Exposure to Radon: BEIR VI; The National Academies Press: Washington, DC, USA, 1999. [Google Scholar] [CrossRef]
- Hofmann, W.; Crawford-Brown, D.J.; Ménache, M.G.; Martonen, T.B. Carcinogenic risk of non-uniform alpha particle irradiation in the lungs: Radon progeny effects at bronchial bifurcations. Radiat. Prot. Dosim. 1991, 38, 91–97. [Google Scholar] [CrossRef]
- International Commission on Radiological Protection (ICRP). Human Respiratory Tract Model for Radiological Protection. ICRP Publication 66. Ann. of ICRP 24 (1–3); Pergamon Press: Oxford, UK, 1994. [Google Scholar]
- Zock, C.; Porstendörfer, J.; Reineking, A. The influence of biological and aerosol parameters of inhaled short-lived radon decay products on human lung dose. Radiat. Prot. Dosim. 1996, 63, 197–206. [Google Scholar] [CrossRef]
- Porstendörfer, J. Radon: Measurements related to dose. Environ. Int. 1996, 22, 563–583. [Google Scholar] [CrossRef]
- Harley, N.H.; Cohen, B.S.; Robins, E.S. The variability in radon decay product bronchial dose. Environ. Int. 1996, 22, 959–964. [Google Scholar] [CrossRef]
- Hofmann, W.; Mainelis, G.; Mohamed, A.; Balásházy, I.; Vaupotic, J.; Kobal, I. Comparison of different modeling approaches in current lung dosimetry models. Environ. Int. 1996, 22, 965–976. [Google Scholar] [CrossRef]
- Nikezic, D.; Yu, K.N.; Cheung, T.T.K.; Haque, A.K.M.M.; Vucic, D. Effects of different lung morphometry models on the calculated dose conversion factor from Rn progeny. J. Environ. Radioac. 2000, 47, 263–277. [Google Scholar] [CrossRef]
- Nikezic, D.; Yu, K.N. Absorbed fraction of alpha particles emitted in bifurcation regions of the human tracheo-bronchial tree. Radiat. Environ. Biophys. 2003, 42, 49–53. [Google Scholar] [CrossRef]
- Nikezic, D.; Novakovic, B.; Yu, K.N. Absorbed fraction of radon progeny in human bronchial airways with bifurcation geometry. Int. J. Radiat. Biol. 2003, 79, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.N.; Lau, B.M.F.; Nikezic, D. Assessment of environmental radon hazard using human respiratory tract models. J. Hazard. Mater. 2006, 132, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Sinnaeve, J.; Clemente, G.; O’Riordan, M. The emergence of natural radiation. Radiat. Prot. Dosim. 1984, 7, 15–17. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Radon and Health. Available online: https://www.who.int/news-room/fact-sheets/detail/radon-and-health (accessed on 26 February 2020).
- Darby, S.; Hill, D.; Auvinen, A.; Baysson, H.; Bochicchio, F.; Deo, H.; Falk, F.; Forastiere, F.; Hakama, M.; Heid, I.; et al. Radon in homes and risk of lung cancer: Collaborative analysis of individual data from 13 European case-control studies. BMJ 2005, 330. [Google Scholar] [CrossRef]
- Atather, J.W. Dosimetric and epidemiological approaches to accessing radon doses—Can the differences be reconciled? Radiat. Prot. Dosim. 2004, 112, 487–492. [Google Scholar] [CrossRef]
- United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR). Sources and Effects of Ionizing Radiation, United Nations Scientific Committee on the Effects of Atomic Radiation, UNSCEAR 1993 Report to the General Assembly with Scientific Annexes; United Nations: New York, NY, USA, 1993. [Google Scholar]
- United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR). Sources and Effects of Ionizing Radiation, United Nations Scientific Committee on the Effects of Atomic Radiation, UNSCEAR 2000 Report to the General Assembly with Scientific Annexes; United Nations: New York, NY, USA, 2000. [Google Scholar]
- Sethi, T.K.; El-Ghamry, M.N.; Kloecker, G.H. Radon and lung cancer. Clin. Adv. Hematol. Oncol. 2012, 10, 157–164. [Google Scholar]
- Brooks, A.L.; Eberlein, P.E.; Couch, L.A.; Boecker, B.B. The role of dose-rate on risk from internally-deposited radionuclides and the potential need to separate dose-rate effectiveness factor (DREF) from the dose and dose-rate effectiveness factor (DDREF). Health Phys. 2009, 97, 458–469. [Google Scholar] [CrossRef] [PubMed]
- Cuttler, J.M.; Feinendegen, L.E. Commentary on inhaled 239PuO2 in dogs—A prophylaxis against lung cancer? Dose-Response 2015, 13. [Google Scholar] [CrossRef]
- Hall, E.J.; Giaccia, A.J. Radiobiology for the Radiologist, 8th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2018. [Google Scholar]
- Han, W.; Yu, K.N. Ionizing radiation, DNA double strand break and mutation. In Advances in Genetics Research Volume 4; Urbano, K.V., Ed.; Nova Science Publishers: New York, NY, 5USA, 2010; Chapter 7; pp. 197–210. [Google Scholar]
- Ttuta-Popa, L.A.; Hofmann, W.; Cosma, C. Prediction of lung cancer risk for radon exposures based on cellular alpha particle hits. Radiat. Prot. Dosim. 2011, 145, 218–223. [Google Scholar] [CrossRef]
- National Research Council. Cells of origin for lung cancer. In Comparative Dosimetry of Radon in Mines and Homes; The National Academies Press: Washington, DC, USA, 1991; pp. 166–193. [Google Scholar]
- Sutherland, K.D.; Berns, A. Cell of origin of lung cancer. Mol. Oncolo. 2010, 4, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Neuberger, J.S.; Gesell, T.F. Childhood cancer, radon and gamma radiation. Lancet 2002, 360, 1437–1438. [Google Scholar] [CrossRef]
- Collier, C.G.; Strong, J.C.; Humphreys, J.A.; Timpson, N.; Baker, S.T.; Eldred, T.; Cobb, L.; Papworth, D.; Haylock, R. Carcinogenicity of radon/radon decay product inhalation in rats—Effect of dose, dose rate and unattached fraction. Int. J. Radiat. Biol. 2005, 81, 631–647. [Google Scholar] [CrossRef] [PubMed]
- Chameaud, J.; Perraud, R.; LaFuma, J.; Masse, R.; Pradel, J. Lesions and lung cancers induced in rats by inhaled radon 222 at various equilibriums with radon daughters. In Experimental Lung Cancer Carcinogenesis and Bioassays, 1st ed.; Karde, E., Park, J., Eds.; Springer-Verlag: Berlin, Germany, 1974; pp. 411–421. [Google Scholar]
- Morlier, J.P.; Morin, M.; Monchaux, G.; Fritsch, P.; Pineau, J.F.; Chameaud, J.; Lafuma, J.; Masse, R. Lung cancer incidence after exposure of rats to low doses of radon: Influence of dose rate. Radiat. Prot. Dosim. 1994, 56, 93–97. [Google Scholar] [CrossRef]
- Archer, V.E.; Wagoner, J.K.; Lundin, F.E. Lung cancer among uranium miners in the United States. Health Phys. 1973, 25, 351. [Google Scholar] [CrossRef]
- International Commission on Radiological Protection (ICRP). Protection Against Radon-222 at Home and at Work, ICRP Publication 65, Ann. ICRP 23(2); Pergamon Press: Oxford, UK, 1993. [Google Scholar]
- Lázár, I.; Tóth, E.; Marx, G.; Cziegler, I.; Köteles, G.J. Effects of residential radon on cancer incidence. J. Radioanal. Nucl. Chem. 2003, 258, 519–524. [Google Scholar] [CrossRef]
- Krewski, D.; Lubin, J.H.; Zielinski, J.M.; Alavanja, M.; Catalan, V.S.; Field, R.W.; Klotz, J.B.; Letourneau, E.G.; Lynch, C.F.; Lyon, J.I.; et al. Residential radon and risk of lung cancer: A combined analysis of 7 North American case-control studies. Epidemiology 2005, 16, 137–145. [Google Scholar] [CrossRef]
- Barros-Dios, J.M.; Ruano-Ravina, A.; Pérez-Ríos, M.; Castro-Bernárdez, M.; Abal-Arca, J.; Tojo-Castro, M. Residential radon exposure, histologic types, and lung cancer risk. A case-control study in Galicia, Spain. Cancer Epidem. Biomar. 2012, 21, 951–958. [Google Scholar] [CrossRef] [PubMed]
- Fornalski, K.W.; Adams, R.; Allison, W.; Corrice, L.E.; Cuttler, J.M.; Davey, C.; Dorbrzynski, L.; Esposito, V.J.; Feinendegen, L.E.; Gomez, L.S.; et al. The assumption of radon-induced cancer risk. Cancer Causes Control 2015, 26, 1517–1518. [Google Scholar] [CrossRef] [PubMed]
- Cohen, B.L. Test of the linear no-threshold theory of radiation carcinogenesis for inhaled radon decay products. Health Phys. 1995, 68, 157–174. [Google Scholar] [CrossRef] [PubMed]
- Becker, K. Health effects of high radon environments in Central Europe: Another test for the LNT hypothesis? Nonlinearity Biol. Toxicol. Med. 2003, 1, 3–35. [Google Scholar] [CrossRef]
- Thompson, R.E.; Nelson, D.F.; Popkin, J.H.; Popkin, Z. Case-control study of lung cancer risk from residential radon exposure in Worcester County, Massachusetts. Health Phys. 2008, 94, 228–241. [Google Scholar] [CrossRef]
- Dobrzynski, L.; Fornalski, K.W.; Reszcynska, J. Meta-analysis of thirty-two case-control and two ecological radon studies of lung cancer. J. Radiat. Res. 2018, 59, 149–163. [Google Scholar] [CrossRef]
- Hofmann, W.; Heistracher, T.; Balásházy, I. Deposition patterns of inhaled radon decay products in human bronchial airway bifurcations. Environ. Int. 1996, 22, S935–S940. [Google Scholar] [CrossRef]
- Hofmann, W.; Winkler-Heil, R. Cellular dose distributions of inhaled radon progeny among different lobes of the human lung. Radiat. Prot. Dosimetry 2020, ncz 304. [Google Scholar] [CrossRef]
- Nikezic, D.; Yu, K.N. Alpha hit frequency due to radon decay products in human lung cells. Int. J. Radiat. Biol. 2001, 77, 559–565. [Google Scholar]
- Birchall, A.; Bailey, M.R.; James, A.C. LUDEP: A lung dose evaluation program. Radiat. Prot. Dosim. 1991, 38, 167–174. [Google Scholar] [CrossRef]
- Marsh, J.W.; Birchall, A. Sensitivity analysis of the weighted equivalent lung dose per unit exposure from radon progeny. Radiat. Prot. Dosim. 2000, 87, 167–178. [Google Scholar] [CrossRef]
- International Commission on Radiological Protection (ICRP). Guide for the Practical Application of the ICRP Human Respiratory Tract Model, ICRP Supporting Guidance 3. Ann. of ICRP 32 (1–2); Pergamon Press: Oxford, UK, 2002. [Google Scholar]
- National Research Council. Health Risks from Exposure to Low Levels of Ionizing Radiation: BEIR VII Phase 2; The National Academies Press: Washington, DC, USA, 2006. [Google Scholar] [CrossRef]
- United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR). Effects of Ionizing Radiation, United Nations Scientific Committee on the Effects of Atomic Radiation, UNSCEAR 2006 Report to the General Assembly with Scientific Annexes, Volume II Scientific Annexes C, D and E; United Nations: New York, NY, USA, 2009. [Google Scholar]
- United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR). Sources and Effects of Ionizing Radiation, United Nations Scientific Committee on the Effects of Atomic Radiation, UNSCEAR 2008 Report to the General Assembly with Scientific Annexes, Volume I Scientific Annexes A and B; United Nations: New York, NY, USA, 2010. [Google Scholar]
- United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR). Sources, Effects and Risks of Ionizing Radiation, United Nations Scientific Committee on the Effects of Atomic Radiation, UNSCEAR 2017 Report to the General Assembly with Scientific Annexes; United Nations: New York, NY, USA, 2018. [Google Scholar]
- Mims, J.W. Asthma: Definitions and pathophysiology. Int. Forum Allergy Rhinol. 2015, 5, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Lee, U.; Kim, E. Combined effect of alpha particles and cigarette smoke on human lung epithelial cells in vitro. Int. Radiat. Biol. 2019, 95, 1276–1286. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Ji, Y.; Su, Z.; Liu, M.; Tong, J.; Ge, C.; Chen, T.; Li, J. Aberrant DNA methylation in radon and or cigarette smoke induced malignant transformation in BEAS 2B human lung cell line. J. Toxicol. Env. Heal. 2017, 80, 1321–1330. [Google Scholar] [CrossRef]
- Axelson, O. Occupational and environmental exposures to radon: Cancer risks. Annu. Rev. Publ. Health 1991, 12, 235–255. [Google Scholar] [CrossRef]
- Kamrin, M.A. Phthalate risks, phthalate regulation, and public health: A review. J. Toxicol. Environ. Health B 2009, 12, 157–174. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, H.; Kannan, K. A review of biomonitoring of phthalate exposures. Toxics 2019, 7, 21. [Google Scholar] [CrossRef]
- Bornehag, C.; Lundgren, B.; Weschler, C.J.; Sigsgaard, T.; Hagerhed-Engman, L.; Sundell, J. Phthalates in Indoor Dust and Their Association with Building Characteristics. Environ. Health Perspect. 2005, 113, 1399–1404. [Google Scholar] [CrossRef]
- Miao, Y.M.; Wang, R.; Lu, C.; Zhao, J.; Deng, Q. Lifetime cancer risk assessment for inhalation exposure to di(2-ethylhexyl) phthalate (DEHP). Environ. Sci. Pollut. Res. 2017, 24, 312–320. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency (US EPA). Priority Pollutant List. Available online: https://www.epa.gov/sites/production/files/2015-09/documents/priority-pollutant-list-epa.pdf (accessed on 18 February 2020).
- Canadian Council of Ministers of the Environment (CCME). Canadian Water Quality Guidelines for the Protection of Aquatic Life: Summary Table. Available online: https://www.ccme.ca/en/resources/canadian_environmental_quality_guidelines/ (accessed on 26 February 2020).
- World Health Organization (WHO). WHO Guidelines for Drinking-water Quality, 3th ed.; WHO: Geneva, Switzerland, 2004; Volume 1. [Google Scholar]
- Högberg, J.; Hanberg, A.; Berglund, M.; Skerfving, S.; Remberger, M.; Calafat, A.M.; Filipsson, A.F.; Jansson, B.; Johansson, N.; Appelgren, M.; et al. Phthalate diesters and their metabolites in human breast milk, blood or serum, and urine as biomarkers of exposure in vulnerable populations. Environ. Health Perspect. 2008, 116, 334–339. [Google Scholar] [CrossRef]
- Silva, M.J.; Samandar, E.; Preau, J.L., Jr.; Reidy, J.A.; Needham, L.L.; Calafat, A.M. Quantification of 22 phthalate metabolites in human urine. J. Chromatogr. B 2007, 806, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Alomirah, H.; Cho, H.S.; Minh, T.B.; Mohd, M.A.; Nakata, H.; Kannan, K. Occurrence of phthalate metabolites in human urine from several Asian countries. Environ. Sci. Technol. 2011, 45, 3138–3144. [Google Scholar] [CrossRef] [PubMed]
- Reddy, B.S.; Rozati, R.; Reddy, B.V.; Raman, N. General gynaecology: Association of phthalate esters with endometriosis in Indian women. Int. J. Gynaecol. Obstet. 2006, 113, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Duty, S.M.; Silva, M.J.; Barr, D.B.; Brock, J.W.; Ryan, L.; Chen, Z.; Herrick, R.F.; Christiani, D.C.; Hauser, R. Phthalate exposure and human semen parameters. Epidemiology 2003, 14, 269–277. [Google Scholar] [CrossRef]
- Joensen, U.N.; Frederiksen, H.; Jesen, M.B.; Lauritsen, M.P.; Olesen, I.A.; Lassen, T.H.; Andersson, A.M.; Jørgensen, N. Phthalate excretion pattern and testicular function: A study of 881 healthy Danish men. Environ. Health Perspect. 2012, 120, 1397–1403. [Google Scholar] [CrossRef]
- James-Todd, T.; Stahlhut, R.; Meeker, J.D.; Powell, S.G.; Hauser, R.; Huang, T.; Rich-Edwards, J. Urinary phthalate metabolite concentrations and diabetes among women in the national health and nutrition examination survey (NHANES) 2001–2008. Environ. Health Perspect. 2012, 120, 1307–1313. [Google Scholar] [CrossRef]
- Yaghjyan, L.; Sites, S.; Ruan, Y.; Chang, S.H. Associations of urinary phthalates with body mass index, waist circumference and serum lipids among females: National health and nutrient examination survey 1999–2004. In. J. Obes. 2015, 39, 994–1000. [Google Scholar] [CrossRef]
- López-Carrillo, L.; Hernández-Ramírez, R.U.; Calafat, A.M.; Torres-Sánchez, L.; Galván-Portillo, M.; Needham, L.L.; Ruiz-Ramos, R.; Cebrián, M.E. Exposure to phthalates and breast cancer risk in Northern Mexico. Environ. Health Perspect. 2010, 118, 539–544. [Google Scholar] [CrossRef]
- Hoppin, J.A.; Jaramillo, R.; London, S.J.; Bertrlsen, R.J.; Salo, P.M.; Sandler, D.P.; Zeldin, D.C. Phthalate exposure and allergy in the U.S. population: Results from NHANES 2005–2006. Environ. Health Perspect. 2013, 121, 1129–1134. [Google Scholar] [CrossRef]
- Braun, J.M.; Sathyanarayana, S.; Hauser, R. Phthalate exposure and children’s health. Curr. Opin. Pediatr. 2013, 25, 247–254. [Google Scholar] [CrossRef]
- European Union (EU). Commission Regulation (EU) 2018/2005. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32018R2005 (accessed on 18 February 2020).
- United States Consumer Product Safety Commission (US CPSC). Consumer Product Safety Improvement Act of 2008. Available online: https://www.govinfo.gov/content/pkg/PLAW-110publ314/pdf/PLAW-110publ314.pdf (accessed on 18 February 2020).
- Canada Consumer Product Safety Act (CCPSA). Phthalate Regulations (SOR/2016–188). Available online: https://laws-lois.justice.gc.ca/eng/regulations/SOR-2016-188/page-1.html (accessed on 18 February 2020).
- International Agency for Research on Cancer (IARC). IARC Monographs on the Identification of Carcinogenic Hazards to Humans. Available online: https://monographs.iarc.fr/agents-classified-by-the-iarc/ (accessed on 19 February 2020).
- Soto, A.M.; Sonnenschein, C. Environmental causes of cancer: Endocrine disruptors as carcinogens. Nat. Rev. Endocrinol. 2010, 6, 363–370. [Google Scholar] [CrossRef] [PubMed]
- David, R.M.; Moore, R.E.; Finney, D.C.; Guest, D. Chronic toxicity of di (2-ethylhexyl) phthalate in rats. Toxicol. Sci. 2000, 55, 433–443. [Google Scholar] [CrossRef] [PubMed]
- David, R.M.; Moore, R.E.; Finney, D.C.; Guest, D. Chronic toxicity of di (2-ethylhexyl) phthalate in mice. Toxicol. Sci. 2000, 58, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Voss, C.; Zerban, H.; Bannasch, P.; Berger, M.R. Lifelong exposure to di (2-ethylhexyl) phthalate induces tumors in liver and testes of Sprague-Dawley rats. Toxicology 2005, 206, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Jaakkola, J.J.K.; Oie, L.; Nafstad, P.; Botten, G.; Samuelsen, S.O.; Magnus, P. Interior surface materials in the home and the development of bronchial obstruction in young children in Oslo, Norway. Am. J. Public Health 1999, 89, 188–192. [Google Scholar] [CrossRef]
- Oie, L.; Botten, G.; Magnus, P.; Jaakkola, J.J.K. Ventilation in homes and bronchial obstruction in young children. Epidemiology 1999, 10, 294–299. [Google Scholar] [CrossRef]
- Jaakkola, J.J.K.; Verkasalo, P.K.; Jaakkola, N. Plastic wall materials in the home and respiratory health in young children. Am. J. Public Health 2000, 90, 797–799. [Google Scholar]
- Bornehag, C.G.; Sundell, J.; Hagerhed-Engmman, L.; Sigsggard, T.; Jason, S.; Aberg, N.; DBH Study Group. Dampness at home and its association with airway, nose, and skin symptoms among 10,851 preschool children in Sweden a cross- sectional study. Indoor Air 2005, 15, 48–55. [Google Scholar] [CrossRef]
- Larsson, M.; Hägerhed-Engman, L.; James, P.; Lundin, F.; Janson, S.; Sumdell, J.; Bornehag, C.G. PVC—As flooring material—And its association with incident asthma in a Swedish child cohort study. Indoor Air 2010, 20, 494–501. [Google Scholar] [CrossRef]
- Shu, H.; Jönsson, B.A.; Nånberg, E.; Bornehag, C.G. PVC flooring at home and development of asthma among young children in Sweden, a 10-year follow-up. Indoor Air 2014, 24, 227–235. [Google Scholar] [CrossRef]
- Jaakkola, J.J.K.; Parise, H.; Kislitsin, V.; Lebedava, N.I.; Spengier, J.D. Asthma, wheezing, and allergies in Russian schoolchildren in relation to new surface materials in the home. Am. J. Public Health 2004, 94, 560–562. [Google Scholar] [CrossRef] [PubMed]
- Bamai, Y.A.; Shibata, E.; Araki, A.; Kanazawa, A.; Morimoto, K.; Nakayama, K.; Tanaka, M.; Takigawa, T.; Yoshimura, T.; Chikara, H.; et al. Exposure to house dust phthalates in relation to asthma and allergies in both children and adults. Sci. Total Environ. 2014, 485–486, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Bornehag, C.G.; Sundell, J.; Weschler, C.J.; Sigsgaard, T.; Kundgren, B.; Hasselgren, M.; Hägerhed-Engman, L. The association between asthma and allergic symptoms in children and phthalates in house dust—A nested case-control study. Environ. Health Perspect. 2004, 112, 1393–1397. [Google Scholar] [CrossRef]
- Kolarik, B.; Naydenov, K.; Larsson, M.; Bornehag, C.G.; Sundell, J. The association between phthalates in dust and allergic diseases among Bulgarian children. Environ. Health Perspect. 2008, 116, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Callesen, M.; Bekö, G.; Weschler, C.J.; Sigsgaard, T.; Jensen, T.K.; Clausen, G.; Toftum, J.; Norberg, L.A.; Host, A. Associations between selected allergens, phthalates, nicotine, polycyclic aromatic hydrocarbons, and bedroom ventilation and clinically confirmed asthma, rhino conjunctivitis, and atopic dermatitis in preschool children. Indoor Air 2014, 24, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Bertelsen, R.J.; Carlsen, K.C.L.; Calafat, A.M.; Hoppin, J.A.; Håland, G.; Mowinckel, P.; Caelsen, K.H.; Løvik, M. Urinary biomarkers for phthalates associated with asthma in Norwegian children. Environ. Health Perspect. 2013, 121, 251–256. [Google Scholar] [CrossRef]
- Callesen, M.; Bekö, G.; Weschler, C.J.; Langer, S.; Brive, L.; Clausen, G.; Toftum, J.; Sigsgaard, T.; Høst, A.; Jensen, T.K. Phthalate metabolites in urine and asthma, allergic rhinoconjunctivitis and atopic dermatitis in preschool children. Int. J. Hygie. Environ. Health 2014, 217, 645–652. [Google Scholar] [CrossRef]
- Jaakkola, J.J.K.; Knight, T.L. The role of exposure to phthalates from polyvinyl chloride products in the development of asthma and allergies: A systematic review and meta-analysis. Environ. Health Perspect. 2008, 116, 845–853. [Google Scholar] [CrossRef]
- Hsu, N.Y.; Lee, C.C.; Wang, J.Y.; Li, Y.C.; Chang, H.W.; Chen, C.Y.; Bornehag, C.G.; Wu, P.C.; Sundell, J.; Su, H.J. Predicted risk of childhood allergy, asthma, and reported symptoms using measured phthalate exposure in dust and urine. Indoor Air 2012, 22, 186–199. [Google Scholar] [CrossRef]
- Tran, T.M.; Kannan, K. Occurrence of phthalate diesters in particulate and vapor phases in indoor air and implication for human exposure in Albany, New York, USA. Arch. Environ. Contam Toxicol. 2015, 68, 489–499. [Google Scholar] [CrossRef]
- Just, A.C.; Whyatt, R.M.; Miller, R.L.; Rundle, A.G.; Chen, Q.; Calafat, A.M.; Divjan, A.; Rossa, M.J.; Zhang, H.; Perera, F.P.; et al. Children’s urinary phthalate metabolites and fractional exhaled nitric oxide in an urban cohort. Am. J. Respir. Crit. Care Med. 2012, 186, 830–837. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, J.C. DEHP: Genotoxicity and potential carcinogenic mechanisms—A review. Mutat. Res. 2012, 82–157. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, M.; Liu, J.; Ni, J.; Jiao, Y.; Bai, C. Up regulation of IL-6 is involved in di (2-ethylhexyl) phthalate (DEHP) induced migration and invasion of non small cell lung cancer (NSCLC) cells. Biomed. Pharmacother. 2017, 89, 1037–1044. [Google Scholar] [CrossRef] [PubMed]
- Jepsen, K.F.; Abildtrup, A.; Larsan, S.T. Monophthalates promote IL-6 and IL-8 production in the human epithelial cell line A549. Toxicol. In Vitro. 2004, 18, 265–269. [Google Scholar] [CrossRef]
- Ma, Y.; Guo, Y.; Wu, S.; Lv, Z.; Zhang, Q.; Xie, X.; Ke, Y. Analysis of toxicity effects of Di-(2-ethylhexyl) phthalate exposure on human bronchial epithelial 16HBE cells. Cytotechnology 2018, 70, 119–128. [Google Scholar] [CrossRef]
- Zöchbauer-Müller, S.; Minna, J.D.; Gazdar, A.F. Aberrant DNA methylation in lung cancer biological and clinical implications. Oncologist 2002, 7, 451–457. [Google Scholar] [CrossRef]
- Rivas, I.; Fussell, J.C.; Kelly, F.J.; Querol, X. Indoor sources of air pollutants. In Indoor Air Pollution, 1st ed.; Harrison, R.M., Hester, R.E., Eds.; Royal Society of Chemistry: Croydon, UK, 2019. [Google Scholar]
- Hartley, M.; Sasser, E. Indoor Pollution: Types, Risks, and Federal Policies; (Air, Water and Soil Pollution Science and Technology Series); Nova Science: New York, NY, USA, 2019. [Google Scholar]
- Moya, J.; bearer, C.F.; Etzel, R.A. Children’s behavior and physiology and how it affects exposure to environmental contaminants. Pediatrics 2004, 113, 996–1006. [Google Scholar]
- Nikezic, D.; Yu, K.N.; Vucic, D. Absorbed fraction and dose conversion coefficients of alpha particles for radon dosimetry. Phys. Med. Biol. 2001, 46, 1963–1974. [Google Scholar] [CrossRef]
- Nikezic, D.; Yu, K.N. Distributions of specific energy in sensitive layers of human respiratory tract. Radiat. Res. 2002, 157, 92–98. [Google Scholar] [CrossRef]
- Nikezic, D.; Haque, A.K.M.M.; Yu, K.N. Absorbed dose delivered by alpha particles calculated in cylindrical geometry. J. Environ. Radioact. 2002, 60, 293–305. [Google Scholar] [CrossRef]
- Nikezic, D.; Yu, K.N. Alpha particle lineal energy spectra for the human lung. Int. J. Radiat. Biol. 2002, 78, 605–609. [Google Scholar] [CrossRef] [PubMed]
- Hoppin, J.A.; Ulmer, R.; London, S.J. Phthalate exposure and pulmonary function. Environ. Health Perspect. 2004, 112, 571–574. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.Y.; Tsai, M.S.; Chen, M.H.; Ng, S.; Hsieh, C.J.; Lin, C.C.; Lu, F.L.; Hsieh, W.S.; Chen, P.C. Children exposure to phthalates and pulmonary function. Sci. Total Environ. 2017, 615, 1282–1289. [Google Scholar] [CrossRef]
- Kim, K.N.; Lee, M.R.; Choi, Y.H.; Lee, B.E.; Hong, Y.C. Association between phthalate exposure and lower lung function in an urban elderly population: A reported-measures longitudinal study. Environ. Int. 2018, 113, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Park, H.Y.; Kim, J.H.; Lim, Y.H.; Bae, S.; Hong, T.C. Influence of genetic polymorphisms on the association between phthalate exposure and pulmonary function in the elderly. Environ. Res. 2013, 122, 18–24. [Google Scholar] [CrossRef]
- Cakmak, S.; Dales, R.E.; Hebbern, C.; Saravanabhavan, G. The association between urinary phthalates and lung function. J. Occup. Environ. Med. 2014, 56, 376–381. [Google Scholar] [CrossRef]
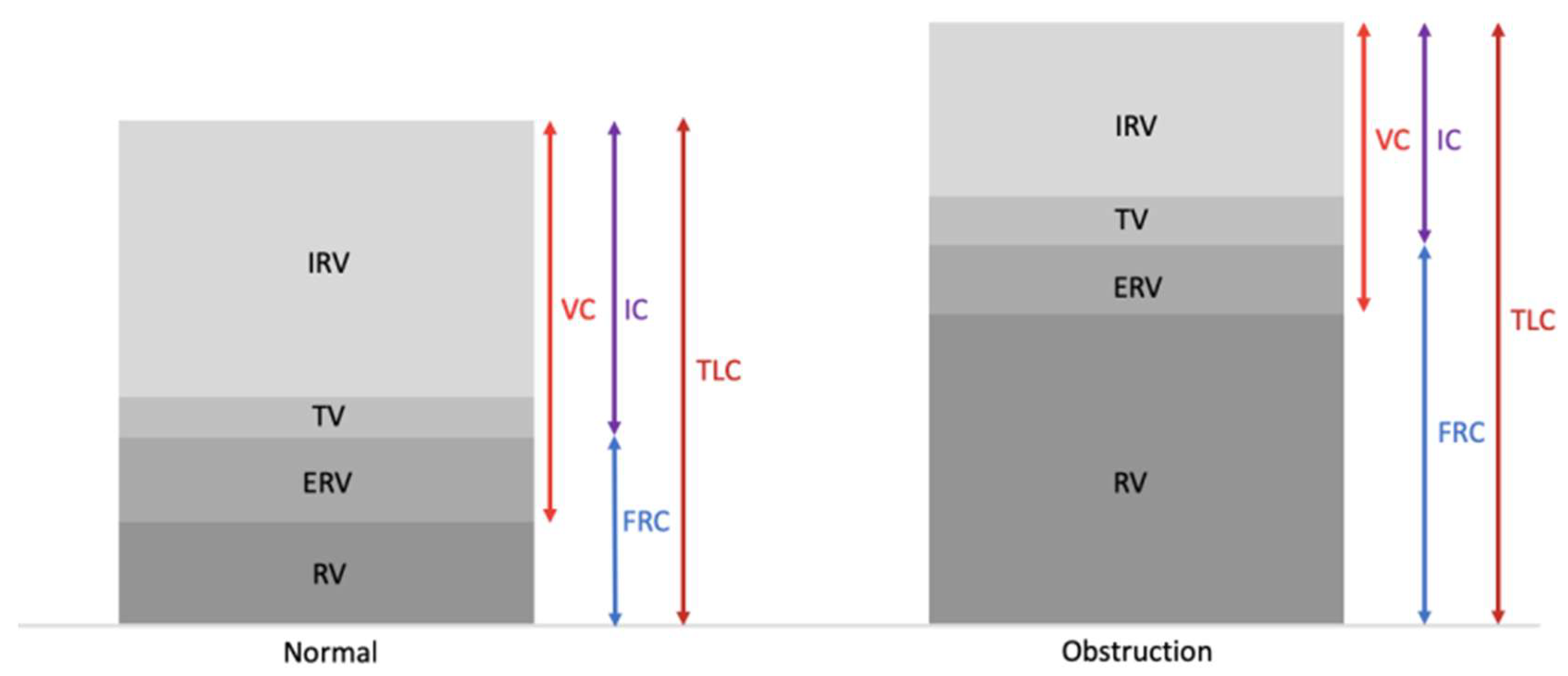
- Ranu, H.; Wilde, M.; Madden, B. Pulmonary function tests. Ulster. Med. J. 2011, 80, 84–90. [Google Scholar]
- Dykstra, B.J.; Scanlon, P.D.; Kester, M.M.; Beck, K.C.; Enright, P.L. Lung volumes in 4774 patients with obstructive lung disease. Chest 1999, 115, 68–74. [Google Scholar] [CrossRef]
- Shin, T.R.; Oh, Y.M.; Park, J.H.; Lee, K.S.; Oh, S.; Kang, D.R.; Sheen, S.; Seo, J.B.; Yoo, K.H.; Lee, J.H.; et al. The prognostic value of residual volume/total lung capacity in patients with chronic obstructive pulmonary disease. J. Korean Med. Sci. 2015, 30, 1459–1465. [Google Scholar] [CrossRef]
- Ruppel, G.L. What is the clinical value of lung volumes? Respir. Care 2012, 57, 26–38. [Google Scholar] [CrossRef]
- Zaman, M.; Mahmood, S.; Altayeh, A. Low inspiratory capacity to total lung capacity ratio is a risk factor for chronic obstructive pulmonary disease exacerbation. Am. J. Med. Sci. 2010, 339, 411–414. [Google Scholar] [CrossRef] [PubMed]
- Celli, B.R.; Decramer, M.; Lystig, T.; Kesten, S.; Tashkin, D.P. Longitudinal inspiratory capacity changes in chronic obstructive pulmonary disease. Respir. Res. 2012, 13. [Google Scholar] [CrossRef] [PubMed]
- Lutfi, M.F. The physiological basis and clinical significance of lung volume. Multidiscip. Respir. Med. 2017, 12. [Google Scholar] [CrossRef] [PubMed]
- Nikezic, D.; Haque, A.K.M.M.; Yu, K.N. Effects of different deposition models on the calculated dose conversion factors from 222Rn progeny. J. Environ. Radioact. 2002, 61, 305–318. [Google Scholar] [CrossRef]
- Crawford-Brown, D.J.; Hofmann, W. An effect-specific track length model for radiations of intermediate to high LET. Radiat. Res. 1991, 126, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, W.; Menache, M.G.; Crawford-Brown, D.J.; Caswell, R.S.; Karam, L.R. Modeling energy deposition and cellular radiation effects in human bronchial epithelium by radon progeny alpha particles. Health Phys. 2000, 78, 377–393. [Google Scholar] [CrossRef]
- Crawford-Brown, D.J.; Hofmann, W. Correlated hit probability and cell transformation in an effect-specific length model applied to in vitro alpha irradiation. Radiat. Environ. Biophys. 2001, 40, 317–323. [Google Scholar] [CrossRef]
- Lau, B.M.F.; Nikezic, D.; Yu, K.N. Killing of target cells due to radon progeny in the human lung. Radiat. Protect. Dosim. 2006, 122, 534–536. [Google Scholar] [CrossRef]
- Lehnert, S. Biomolecular Action of Ionizing Radiation; Taylor and Francis: New York, NY, USA, 2007. [Google Scholar]
- Chauhen, V.; Howland, M. Gene expression responses in human lung fibroblasts exposed to alpha particle radiation. Toxicol. In Vitro 2014, 28, 1222–1229. [Google Scholar] [CrossRef]
- Chauhan, V.; Howland, M.; Mendenhall, A.; O’Hara, S.; Stocki, T.J.; McNamee, J.P.; Wilkins, R.C. Effects of alpha particle radiation on gene expression in human pulmonary epithelial cells. Int. J. Hyg. Envir. Heal. 2012, 215, 522–535. [Google Scholar] [CrossRef]
- Lou, T.; Xiang, X.; Wu, D. Transformation of human bronchial epithelial cells BEP2D induced by 238Pu α-particles. Chin. J. Lung Cancer 2000, 3, 428–431. [Google Scholar]
- Hei, T.K.; Piao, C.Q.; Willey, J.C.; Thomas, S.; Hall, E.J. Malignant transformation of human bronchial epithelial cells by radon-simulated α-particle. Carcinogenesis 1994, 15, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Hooker, A.M.; Bhat, M.; Day, T.K.; Lane, J.M.; Swinburne, S.J.; Morley, A.A.; Sykes, P.J. The linear no threshold model does not hold for low-dose ionizing radiation. Radiat. Res. 2004, 162, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Choi, V.W.Y.; Yum, E.H.W.; Konishi, T.; Oikawa, M.; Cheng, S.H.; Yu, K.N. Triphasic low-dose response in zebrafish embryos irradiated by microbeam protons. J. Radiat. Res. 2012, 53, 475–481. [Google Scholar] [CrossRef]
- Kong, E.Y.; Cheng, S.H.; Yu, K.N. Biphasic and triphasic dose responses in zebrafish embryos to low-dose 150 kV X-rays with different hardness. J. Radiat. Res. 2016, 57, 363–369. [Google Scholar] [CrossRef]
- Nagasawa, H.; Little, J.B. Induction of sister chromatid exchanges by extremely low doses of alpha-particles. Cancer Res. 1992, 52, 6394–6396. [Google Scholar]
- Mothersill, C.; Seymour, C. Radiation induced bystander effects: Past history and future directions. Radiat. Res. 2001, 155, 759–767. [Google Scholar] [CrossRef]
- Mothersill, C.; Seymour, C. Radiation induced bystander effects—Implications for cancer. Nat. Rev. 2004, 4, 158–164. [Google Scholar]
- Goldberg, Z.; Lehnert, B.E. Radiation induced effects in unirradiated cells: A review and implications in cancer. Int. J. Oncol. 2002, 21, 337–349. [Google Scholar] [CrossRef]
- Little, J.B. Cellular radiation effects and the bystander response. Mutat. Res. 2006, 597, 113–118. [Google Scholar] [CrossRef]
- Morgan, W.F.; Sowa, M.B. Non-targeted bystander effects induced by ionizing radiation. Mutat. Res. 2007, 616, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Hei, T.K.; Zhou, H.; Ivanov, V.N.; Hong, M.; Lieberman, H.B.; Brenner, D.J.; Amundson, S.A.; Geard, C.R. Mechanism of radiation induced bystander effects: A unifying model. J. Pharm. Pharmacol. 2008, 60, 943–950. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yu, K.N.; Hou, J.; Liu, Q.; Han, W. Radiation-induced bystander effect: Early process and rapid assessment. Cancer Lett. 2015, 356, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Shareef, M.M.; Cui, N.; Burikhanov, R.; Gupta, S.; Satishkumar, S.; Shajahan, S.; Mohiuddin, M.; Rangnekar, V.M.; Ahmed, M.M. Role of tumor necrosis factor-alpha and TRAIL in high-dose radiation-induced bystander signaling in lung adenocarcinoma. Cancer Res. 2007, 67, 11811–11820. [Google Scholar] [CrossRef] [PubMed]
- Iyer, R.; Lehnert, B.E.; Svensson, R. Factors underlying the cell growth-related bystander responses to alpha particles. Cancer Res. 2000, 60, 1290–1298. [Google Scholar]
- Chou, C.H.; Chen, P.J.; Lee, P.H.; Cheng, A.L.; Hsu, H.C.; Cheng, J.C. Radiation-induced hepatitis B virus reactivation in liver mediated by the bystander effect from irradiated endothelial cells. Clin. Cancer Res. 2007, 13, 851–857. [Google Scholar] [CrossRef]
- Facoetti, A.; Ballarini, F.; Cherubini, R.; Gerardi, S.; Nano, R.; Ottolenghi, A.; Prise, K.M.; Trott, K.R.; Zilio, C. Gamma ray-induced bystander effect in tumour glioblastoma cells: A specific study on cell survival, cytokine release and cytokine receptors. Radiat. Prot. Dosim. 2006, 122, 271–274. [Google Scholar] [CrossRef]
- Han, W.; Wu, L.; Chen, S.; Bao, L.; Zhang, L.; Jiang, E.; Zhao, Y.; Xu, A.; Hei, T.K.; Yu, Z. Constitutive nitric oxide acting as a possible intercellular signaling molecule in the initiation of radiation-induced DNA double strand breaks in non-irradiated bystander cells. Oncogene 2007, 26, 2330–2339. [Google Scholar] [CrossRef]
- Shao, C.; Folkard, M.; Prise, K.M. Role of TGF-β1 and nitric oxide in the bystander response of irradiated glioma cells. Oncogene 2008, 27, 434–440. [Google Scholar] [CrossRef]
- Matsumoto, H.; Hayashi, S.; Hatashita, M.; Ohnishi, K.; Shioura, H.; Ohtsubo, T.; Kitai, R.; Ohnishi, T.; Kano, E. Induction of radioresistance by a nitric oxide-mediated bystander effect. Radiat. Res. 2001, 155, 387–396. [Google Scholar] [CrossRef]
- Shao, C.; Furusawa, Y.; Kobayashi, Y.; Funayama, T.; Wada, S. Bystander effect induced by counted high-LET particles in confluent human fibroblasts: A mechanistic study. FASEB J. 2003, 17, 1422–1427. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, A.; Goodwin, E.H.; Bailey, S.M.; Marrone, B.L.; Lehnert, B.E. Alpha-particle-induced sister chromatid exchange in normal human lung fibroblasts: Evidence for an extranuclear target. Radiat. Res. 1996, 145, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Belyakov, O.V.; Malcolmson, A.M.; Folkard, M.; Prise, K.M.; Michael, B.D. Direct evidence for a bystander effect of ionizing radiation in primary human fibroblasts. Br. J. Cancer 2001, 84, 674–679. [Google Scholar] [CrossRef] [PubMed]
- Azzam, E.I.; Toledo, D.S.M.; Dooding, T.; Little, J.B. Intercellular communication is involved in the bystander regulation of gene expression in human cells exposed to very low fluences of alpha particles. Cancer Res. 1998, 150, 497–504. [Google Scholar] [CrossRef]
- Hickman, A.W.; Jaramillo, R.J.; Lechner, J.F.; Johnson, N.F. α-particle-induced p53 protein expression in a rat lung eptthelial cell strain. Cancer Res. 1994, 54, 5797–5800. [Google Scholar]
- Brenner, D.J.; Sachs, R.K. Domestic radon risks may be dominated by bystander effects—But the risks are unlikely to be greater than we thought. Health Phys. 2003, 85, 103–108. [Google Scholar] [CrossRef]
- Chen, S.; Zhao, Y.; Han, W.; Chiu, S.K.; Zhu, L.; Wu, L.; Yu, K.N. Rescue effects in radiobiology: Unirradiated bystander cells assist irradiated cells through intercellular signal feedback. Mutat. Res. 2011, 706, 59–64. [Google Scholar] [CrossRef]
- Lam, R.K.K.; Fung, Y.K.; Han, W.; Yu, K.N. Rescue effects: Irradiated cells helped by unirradiated bystander cells. Int. J. Mol. Sci. 2015, 16, 2591–2609. [Google Scholar] [CrossRef]
- Yu, K.N. Radiation-induced rescue effect. J. Radiat. Res. 2019, 60, 163–170. [Google Scholar] [CrossRef]
- He, M.; Dong, C.; Xie, Y.; Li, J.; Yuan, D.; Bai, Y.; Shao, C. Reciprocal bystander effect between α-irradiated macrophage and hepatocyte is mediated by cAMP through a membrane signaling pathway. Mutat. Res. 2014, 763–764, 1–9. [Google Scholar] [CrossRef]
- Lam, R.K.K.; Fung, Y.K.; Han, W.; Li, L.; Chiu, S.K.; Cheng, S.H.; Yu, K.N. Modulation of NF-κB in rescued irradiated cells. Radiat. Prot. Dosimetry 2015, 167, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Lam, R.K.K.; Han, W.; Yu, K.N. Unirradiated cells rescue cells exposed to ionizing radiation: Activation of NF-κB pathway in irradiated cells. Mutat. Res. 2015, 782, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, H.; Hayashi, S.; Hatashita, M.; Shioura, H.; Ohtsubo, T.; Kitai, R.; Ohnishi, T.; Yukawa, O.; Furusawa, Y.; Kano, E. Induction of radioresistance to accelerated carbon-ion beams in recipient cells by nitric oxide excreted from irradiated donor cells of human glioblastoma. Int. J. Radiat. Biol. 2000, 76, 1649–1657. [Google Scholar] [CrossRef] [PubMed]
- Shao, C.; Furusawa, Y.; Aoki, M.; Matsumoto, H.; Ando, K. Nitric oxide-mediated bystander effect induced by heavy-ions in human salivary gland tumour cells. Int. J. Radiat. Biol. 2002, 78, 837–844. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, H.; Hamada, N.; Takahashi, A.; Kobayashi, Y.; Ohnishi, T. Vanguards of paradigm shift in radiation biology: Radiation-induced adaptive and bystander responses. J. Radiat. Res. 2007, 48, 97–106. [Google Scholar] [CrossRef]
- Matsumoto, H.; Tomita, M.; Otsuka, K.; Hatashita, M. A new paradigm in radioadaptive response developing from microbeam research. J. Radiat. Res. 2009, 50, 67–79. [Google Scholar] [CrossRef]
- Matsumoto, H.; Tomita, M.; Otsuka, K.; Hatashita, M.; Hamada, N. Nitric oxide is a key molecule serving as a bridge between radiation-induced bystander and adaptive responses. Curr. Mol. Pharmacol. 2011, 4, 126–134. [Google Scholar] [CrossRef]
- Tomita, M.; Maeda, M.; Kobayashi, K.; Matsumoto, H. Dose response of soft X-Ray-induced bystander cell killing affected by p53 status. Radiat. Res. 2013, 179, 200–207. [Google Scholar] [CrossRef]
- Maeda, M.; Kobayashi, K.; Matsumoto, H.; Usami, N.; Tomita, M. X-ray-induced bystander responses reduce spontaneous mutations in V79 cells. J. Radiat. Res. 2013, 54, 1043–1049. [Google Scholar] [CrossRef]
- Kong, E.Y.; Cheng, S.H.; Yu, K.N. Induction of autophagy and interleukin 6 secretion in bystander cells: Metabolic cooperation for radiation-induced rescue effect? J. Radiat. Res. 2018, 59, 129–140. [Google Scholar] [CrossRef]
- Pathikonda, S.; Cheng, S.H.; Yu, K.N. Role of PARP1 regulation in radiation-induced rescue effect. J. Radiat. Res. 2020. [Google Scholar] [CrossRef]
- Mothersill, C.; Seymour, C. Radiation-induced bystander effects and adaptive responses—The Yin and Yang of low dose radiobiology? Mutat. Res. 2004, 568, 121–128. [Google Scholar] [CrossRef]
- Olivieri, G.; Bodycote, J.; Wolf, S. Adaptive response of human lymphocytes to low concentrations of radioactive thymidine. Science 1984, 223, 594–597. [Google Scholar] [CrossRef] [PubMed]
- Wolff, S. The adaptive response in radiobiology: Evolving insights and implications. Environ. Health Perspect. 1998, 106, 277–283. [Google Scholar] [PubMed]
- Fornalski, K.W. Radiation adaptive response and cancer: From the statistical physics point of view. Phys. Rev. E 2019, 99. [Google Scholar] [CrossRef] [PubMed]
- Preston, R.J. Bystander effects, genomic instability, adaptive response, and cancer risk assessment for radiation and chemical exposures. Toxicol. Appl. Pharmacol. 2005, 207, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Ko, M.; Lao, X.Y.; Kapadia, R.; Elmore, E.; Redpath, J.L. Neoplastic transformation in vitro by low doses of ionizing radiation: Role of adaptive response and bystander effects. Mutat. Res. 2006, 597, 11–17. [Google Scholar] [CrossRef]
- Schwartz, J.L. Variability: The common factor linking low dose-induced genomic instability, adaption and bystander effects. Mutat. Res. 2007, 616, 196–200. [Google Scholar] [CrossRef]
- Choi, V.W.Y.; Lam, R.K.K.; Chong, E.Y.W.; Cheng, S.H.; Yu, K.N. Designing experimental setup and procedures for studying alpha-particle-induced adaptive response in zebrafish embryos in vivo. Nucl. Instrum. Meth. B 2010, 268, 651–656. [Google Scholar] [CrossRef]
- Choi, V.W.Y.; Cheng, S.H.; Yu, K.N. Radioadaptive response induced by alpha-particle-induced stress communicated in vivo between zebrafish Embryos. Environ. Sci. Technol. 2010, 44, 8829–8834. [Google Scholar] [CrossRef]
- Choi, V.W.Y.; Wong, M.Y.P.; Cheng, S.H.; Yu, K.N. Dosimetric study of radioadaptive response of zebrafish embryos using PADC-film substrates. Radiat. Meas. 2011, 46, 1795–1798. [Google Scholar] [CrossRef]
- Choi, V.W.Y.; Konishi, T.; Oikawa, M.; Iso, H.; Cheng, S.H.; Yu, K.N. Adaptive response in zebrafish embryos induced using microbeam protons as priming dose and X-ray photons as challenging dose. J. Radiat. Res. 2010, 51, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Choi, V.W.Y.; Konishi, T.; Oikawa, M.; Cheng, S.H.; Yu, K.N. The threshold number of protons to induce an adaptive response in zebrafish embryos. J. Radiol. Prot. 2013, 33, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Choi, V.W.Y.; Ng, C.Y.P.; Kobayashi, A.; Konishi, T.; Oikawa, M.; Cheng, S.H.; Yu, P.K.N. Roles of nitric oxide in adaptive response induced in zebrafish embryos in vivo by microbeam protons. J. Radiat. Res. 2014, 55, 114. [Google Scholar] [CrossRef]
- Choi, V.W.Y.; Ng, C.Y.P.; Kobayashi, A.; Konishi, T.; Oikawa, M.; Cheng, S.H.; Yu, P.K.N. Exogenous carbon monoxide suppresses adaptive response induced in zebrafish embryos in vivo by microbeam protons. J. Radiat. Res. 2014, 55, 115. [Google Scholar] [CrossRef]
- Rusyn, I.; Corton, J.C. Mechanistic considerations for human relevance of cancer hazard of di (2-ethylhexyl) phthalate. Mutat. Res. 2012, 750, 141–158. [Google Scholar] [CrossRef]
- Kleinsasser, N.H.; Kastenbauer, E.R.; Weissacher, H.; Muenzenrieder, R.K.; Harréus, U.A. Phthalates demonstrate genotoxicity on human mucosa of the upper aerodigestive tract. Environ. Mol. Mutagen. 2000, 35, 9–12. [Google Scholar] [CrossRef]
- Kim, I.Y.; Han, S.Y.; Moon, A. Phthalate inhibit tamoxifen-induced apoptosis in MCF-7 human breast cancer cells. J. Toxicol. Environ. Health 2004, 67, 2025–2035. [Google Scholar] [CrossRef]
- Chen, F.P.; Chien, M.H. Lower concentrations of phthalates induce proliferation in human breast cancer cells. Climacteric 2014, 17, 377–384. [Google Scholar] [CrossRef]
- Tomita, I.; Nakamura, Y.; Aoki, N.; Inui, N. Mutagenic/carcinogenic potential of DEHP and MEHP. Environ. Health Perspect. 1982, 45, 119–125. [Google Scholar] [CrossRef]
- Kim, J.H. Di (2-ethylhexyl) phthalate promotes lung cancer cell line A549 progression via Wnt/β-catenin signalling. J. Toxicol. Sci. 2019, 44, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Klimisch, H.J.; Gamer, A.O.; Hellwig, J.; Kaufmann, W.; Jäckh, R. Di (2-ethylhexyl) phthalate: A short-term repeated inhalation toxicity study including fertility assessment. Food Chem. Toxicol. 1992, 30, 915–919. [Google Scholar] [CrossRef]
- Liang, Z.J.; Wu, Q.P.; Chen, B.T.; Lin, Z.; Lin, J.; Chen, S.Q. Postnatal hyperoxia or DEHP exposure leads to growth restriction and delayed lung development in newborn rats. Pediatr. Neontol. 2018, 59, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.Q.; Chen, J.N.; Cai, X.H.; Chen, G.R.; Gao, Y.; Ge, R.S.; Wu, H.S.; Lin, Z.L.; Lin, J. Perinatal exposure to di-(2-ethylhexyl) phthalate leads to restricted growth and delayed lung maturation in newborn rats. J. Perinat. Med. 2010, 38, 515–521. [Google Scholar] [CrossRef]
- Andrade, J.; Grande, S.W.; Talsness, C.E.; Grote, K. A dose-response study following in utero and lactational exposure to di-(2-ethylhexyl)-phthalate (DEHP): Non-monotonic dose-response and low dose effects on rat brain aromatase activity. Toxicology 2006, 227, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Yuen, B.B.H.; Qiu, A.B.; Chen, B.H. Transient exposure to environmentally realistic concentrations of di-(2-ethylhexyl)-phthalate during sensitive windows of development impaired larval survival and reproduction success in Japan medaka. Toxicol. Rep. 2020, 7, 200–208. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwan, W.S.; Nikezic, D.; Roy, V.A.L.; Yu, K.N. Multiple Stressor Effects of Radon and Phthalates in Children: Background Information and Future Research. Int. J. Environ. Res. Public Health 2020, 17, 2898. https://doi.org/10.3390/ijerph17082898
Kwan WS, Nikezic D, Roy VAL, Yu KN. Multiple Stressor Effects of Radon and Phthalates in Children: Background Information and Future Research. International Journal of Environmental Research and Public Health. 2020; 17(8):2898. https://doi.org/10.3390/ijerph17082898
Chicago/Turabian StyleKwan, W. S., D. Nikezic, Vellaisamy A. L. Roy, and K. N. Yu. 2020. "Multiple Stressor Effects of Radon and Phthalates in Children: Background Information and Future Research" International Journal of Environmental Research and Public Health 17, no. 8: 2898. https://doi.org/10.3390/ijerph17082898
APA StyleKwan, W. S., Nikezic, D., Roy, V. A. L., & Yu, K. N. (2020). Multiple Stressor Effects of Radon and Phthalates in Children: Background Information and Future Research. International Journal of Environmental Research and Public Health, 17(8), 2898. https://doi.org/10.3390/ijerph17082898



_Kwan_Ngok_Yu.png)
