Abstract
Salt intake is too high for safety nowadays. The main active ion in salt is sodium. The vast majority of scientific evidence points out the importance of sodium restriction for decreasing cardiovascular risk. International Guidelines recommend a large reduction in sodium consumption to help reduce blood pressure, organ damage, and cardiovascular risk. Regulatory authorities across the globe suggest a general restriction of sodium intake to prevent cardiovascular diseases. In spite of this seemingly unanimous consensus, some researchers claim to have evidence of the unhealthy effects of a reduction of sodium intake, and have data to support their claims. Evidence is against dissenting scientists, because prospective, observational, and basic research studies indicate that sodium is the real villain: actual sodium consumption around the globe is far higher than the safe range. Sodium intake is directly related to increased blood pressure, and independently to the enlargement of cardiac mass, with a possible independent role in inducing left ventricular hypertrophy. This may represent the basis of myocardial ischemia, congestive heart failure, and cardiac mortality. Although debated, a high sodium intake may induce initial renal damage and progression in both hypertensive and normotensive subjects. Conversely, there is general agreement about the adverse role of sodium in cerebrovascular disease. These factors point to the possible main role of sodium intake in target organ damage and cardiovascular events including mortality. This review will endeavor to outline the existing evidence.
1. Introduction
Table salt is mainly sodium chloride. The active ion in cardiovascular pathophysiology is sodium. Salt and sodium are in a 2.54:1 w/w ratio, the molecular weight of sodium is 23, so 100 mEq/mmol of sodium have a weight of 2300 mg or (2.3 × 2.54) 5.84 g of salt. Sodium consumption is directly related to blood pressure. The restriction of sodium intake is reported as a mandatory non-pharmacological measure to decrease blood pressure in the international Guidelines [1,2].
The vast majority of papers in the literature show the advantages of a low sodium approach for treating hypertension [3,4,5,6,7], while a minority manages to cast doubts on the healthy effects of reduced sodium intake [8]. Dissenting scientists have been accused of designing their studies with inherent biases in their protocols [9] in order to cloud the advantages of a general lowering of dietary sodium [10].
While the favorable effects of salt (and thus sodium) restriction in the diet are substantially accepted by the scientific world, less is known about the specific effects of sodium consumption (and the possible advantages of dietary sodium lessening) on target organ damage and cardiovascular events [11].
In spite of the pioneering studies by Walter Kempner [12] in the middle of the last century, where exceptional effects were attained in terms of cardiac mass reduction with a low sodium approach (a very low one, indeed), we had to wait until the 1980s to read about the first echocardiographic reports in this field [13].
Since then, research about dietary regression of left ventricular hypertrophy (LVH) has been heavily outweighed by studies based on pharmacological approaches [14].
Similarly, the favorable effects of sodium intake restriction in related fields such as stroke prevention, proteinuria reduction, and cardiovascular prognosis improvement do not represent a large mass of published papers [11].
This review will endeavor to evaluate the healthy effects of a decrease in dietary sodium on the hard endpoints already shown in the present literature.
2. Methods
Papers were retrieved from PubMed (NCBI), ISI Web of Knowledge®/Web of Science/Science Citation Index (Clarivate), and Cochrane© databases (Wiley) by means of the usual search strings and simple Boolean operators for blood (arterial) pressure, hypertension, salt (sodium), diet (intake, consumption), left ventricular hypertrophy (mass), stroke, proteinuria, cardiovascular prevention, and cardiovascular events (coronary heart disease, unstable angina, revascularization procedures, congestive heart failure, and stroke).
The searches retrieved 968 citations when duplicates were removed (Figure 1). Moreover, 752 papers were excluded after reading their titles and abstracts, and because they were outside the scope of this study. One hundred and seventy-seven full papers were reviewed. Of these, 132 papers in journals with a certified impact factor, with at least an English abstract, and published from 1980 onwards (except for two of the earlier papers that focused on the aim of our study) were selected. Papers that had already been discussed in cited reviews were not included, and instead reviews were included. In addition to the retrieved references were the Guidelines [1,2].
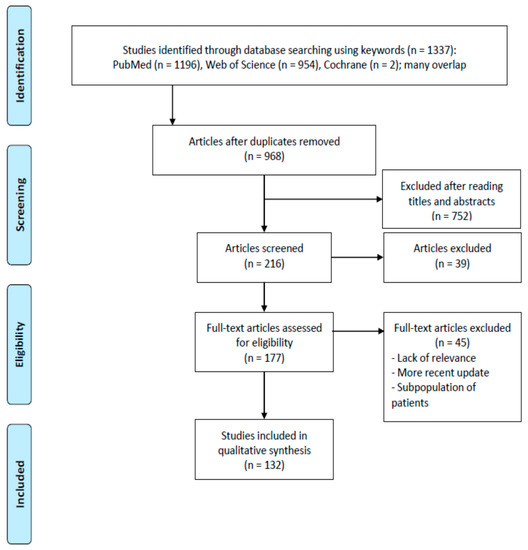
Figure 1.
PRISMA 2009 flow diagram illustrating the selection process for the articles included in this review [15]. PRISMA documents are distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction.
3. Discussion
3.1. Sodium Intake and Left Ventricular Hypertrophy
LVH is generally recognized as a primary marker of high cardiovascular risk and a potent predictor of mortality, in both normotensive and hypertensive patients [2]. LVH regression [16], or reduction [17], was shown to occur after blood pressure (BP) control as early as the beginning of the echocardiographic era [18], but the decrease in left ventricular mass (LVM) seemed already not to be entirely dependent on BP reduction [16,18]. The regression of LVH towards a normal LVM is considered to be a goal of antihypertensive treatment, because the LVH regression itself represents a marked reduction and a strong improvement in the cardiovascular risk [2].
The diet plays a key role in the reduction of the LVM. Already in the middle of the last century, a (very) low sodium approach could sensibly reduce cardiac mass in patients with malignant hypertension [12]. Thereafter, and more recently, the dietary approach, based on sodium intake reduction, showed positive results in terms of LVM decrease [3,19,20,21,22,23,24,25,26,27]. While sodium restriction has favorable effects, a high sodium diet seems to be a strong and independent predictor of LVH [24,28,29,30,31,32,33,34,35,36]. Similarly, while a successful lowering of BP may generally appear more important than the type of approach in inducing the reversal of LVH [37], an LVM reduction after diet and/or drugs seems unrelated to BP values in the view of other researchers [13]. Moreover, the regression of LVH with active drug treatment with the inhibition of the renin–angiotensin system is impaired by high sodium intake [22]. In a relatively large data set the BP-independent effect of a low sodium diet on the LVM has been observed, and a substantial LVH reversal has been shown [38]. Even in a secondary hypertension setting, the role of sodium intake plays a pivotal role in the enlargement of cardiac dimensions [39]. In spite of the clear dietary sodium involvement, it is less clear whether or not sodium intake may be linked to the LVM in terms of sodium sensitivity or sodium resistance [40]. Incidentally, sodium intake appears to be related to arrhythmias [41], or at least to an increased susceptibility to arrhythmias [42].
The results of what we have learned from the literature point to a key role for sodium intake in promoting both an increase in BP and (possibly with an independent role) an enlargement of LVM, with or without the involvement of traditional growth factors, such as the mediators of the sympathetic or the renin–angiotensin–aldosterone system, on cardiac myocytes [43,44].
The response of BP to dietary sodium shows a normal distribution across populations. Many (so-called salt-sensitive) individuals (represented mainly by women, older individuals, and black people), take advantage of sodium restriction while others (salt-resistant, i.e., men, young people, and white people) do not [45]. Pre-hypertensive and hypertensive subjects usually benefit most [45].
3.2. Sodium Intake and Cardiovascular Events
Sodium intake is linked to an increase in BP [5], to the incidence of hypertension [6], is associated with endothelial damage [46], metabolic disturbances [7,46], and markers of subclinical coronary disease [47,48]. Furthermore, a decrease in sodium intake has favorable effects on both the BP itself [49] and arterial stiffness [50].
Mechanisms linking sodium intake and BP are represented mainly by volume expansion and interactions with the renin–angiotensin–aldosterone system (RAAS), even if they are not completely understood [51]. Other research is in progress exploring the sodium–immune system connections [52,53,54]. Sodium and the immune system can impact BP through the modulation of myeloid cells as well as lymphocytes [53]. Moreover, alimentary sodium promotes endothelial dysfunction, and cytokine secretion [54]. Macrophages and endothelial growth factors may be involved as well [55]. All these events play a key role in the pathophysiology of initial cardiovascular damage [54].
Effects other than hypertension have been attributed to an exaggerated sodium intake. These include direct damage to arteries through endothelial dysfunction and reduced nitric oxide bioavailability, or by increasing vasoconstrictor peptides [55]. Other direct effects (not mediated by an increased BP) include the involvement of epithelial sodium channels in the distal nephrons [55].
These mechanisms provide some pathophysiological bases for the increased risk of stroke [56,57], stroke mortality [58], coronary heart disease mortality [57], and overall cardiovascular risk [57] associated with increased sodium consumption. Even in experimental models, a high sodium diet produces left ventricular injury and probably causes myocardial ischemia [59].
Consequently, it is not surprising to find mounting evidence linking sodium intake to a greatly increased cardiovascular and cerebrovascular risk [46,60,61,62,63].
Recently, a Dutch group expressed a word of caution, when they observed an increased risk of stroke in association with (very) low excretion rates of sodium in a large cohort of patients [64], even if many confounders had not been taken into account [65].
Finally, it is worth noting that excess sodium intake increases the overall cardiovascular mortality [66], at least since publication of the NutriCode results. This large meta-analysis of more than 100 trials showed the devastating effects of excess sodium in the diet: from 1.1 to 2.2 million annual deaths worldwide, in 2010, were attributable to dietary sodium above the WHO reference level of 2.0 g a day; this figure represents 9.5% of all cardiovascular deaths [66,67]. The majority of these deaths were due to coronary heart disease (41.7%) and to stroke (41.6%) [67].
A cornerstone of cardiovascular morbidity and mortality prevention is the implementation of sodium restriction by clinicians [68,69], both in high-risk patients and in healthy individuals [70]. Cardiovascular disease is linked to sodium intake [71,72,73], and restricting sodium consumption has favorable effects [74,75,76], while studies flawed by incorrect urinary sodium sampling show mixed or non-significant results [77,78,79], again returning to the J-shaped relationship [79].
Even in congestive heart failure, the importance of the dietary approach based on a low sodium intake should be remembered [80].
The relationships between sodium intake and BP represent the basis for the preventive effects of sodium restriction on cardiovascular morbidity and mortality [81]. Nevertheless, some doubts have been expressed about the real effects of low sodium intake on cardiovascular morbidity [82]. A further review examined 17 studies comprising more than 3000 patients evaluated for nutritional interventions in congestive heart failure (CHF), which represents an ever-increasing cardiovascular event as well as a source of cardiovascular morbidity and mortality. Although a low sodium diet may improve morbidity in this clinical setting, this research showed that a low sodium intake might be harmful [83].
Despite this evidence, many others recommend sodium restriction in CHF patients, recognizing the effects of dietary interventions on the heart and blood vessels [84,85,86], although this is not generally appreciated.
During the last few years, some reviews have addressed the pathophysiological problems connecting alimentary sodium and cardiac damage. High importance has been credited to aldosterone, parathyroid hormone, and to interstitial (tissue) sodium [87,88,89]. Finally, and more recently, many studies were scrutinized to assess the benefits of a dietary approach in CHF [83,90,91,92,93,94] with mixed results. The conclusions in this field remain unclear, and further research is required to verify the importance of dietary strategies on heart failure [91].
Cerebrovascular diseases seem more dependent on sodium intake. Current Guidelines recommend a low sodium approach to prevent cardiovascular morbidity and mortality. There is robust evidence to show that a diet that includes sodium restriction can prevent stroke [95]. Health professionals should take an active role in promoting a low sodium diet to reduce the stroke burden [96].
A large number of reviews and meta-analyses support a low sodium approach for the prevention of stroke and conversely show the relationship between high sodium intake and the prevalence of stroke. The advantages of a low sodium diet are evident in both normotensive and hypertensive subjects, with more evidence in the latter [5,97,98,99,100,101,102,103]. Similarly, notable evidence supports the link between high sodium intake and the occurrence of cerebrovascular disease [63,104,105,106,107,108,109].
The mechanisms that connect alimentary sodium to stroke have been discussed in recent years and range from vascular lesions with enhanced neurogenesis [110], to endothelial dysfunction [111], inflammation due to low parasympathetic activity [112], small vessel disease associated with high sodium consumption [113], adenosine receptor expression [114] or other protein expression, and microglia polarization [115].
3.3. Sodium Intake and Renal Impairment/Proteinuria
Updated Guidelines suggest a low sodium approach for the prevention of chronic kidney disease (CKD) or its progression. Nevertheless, even in this field discussions do exist. A recent large quantitative review [116] found that robust evidence is lacking on the association between sodium intake and CKD delay or prevention.
Other researchers [117] found high-quality studies showing adverse effects of alimentary sodium on health outcomes such as CKD.
To add fuel to the discussion, a recent meta-analysis of 11 trials involving hundreds of CKD patients [118] showed how sodium restriction induces BP reduction and proteinuria improvement in CKD patients.
Others showed how high sodium (and low water) intake have a pathogenic role in hypertension and CKD [119], whereas a few years ago [120] the importance of a low sodium approach to increase the protective effects of RAAS blockade in CKD patients was shown.
So, even with some warning, nephrologists are advised to support their patients with carefully tailored diets that are low in sodium [121]. Support for this view comes from a large prospective community-based study involving more than 8000 patients [122]. Here the authors showed a U-shaped relationship between sodium intake and the risk of CKD development, with maximum risk either in patients with very low (under 2.08 g/day) or high sodium intake (more than 4.03 g/day).
A large Cochrane review [123] concluded that sodium restriction can reduce both BP and proteinuria in CKD patients, even if long-term studies showing the direct effects of a low sodium approach on the progression to end-stage kidney disease are not available.
Short-term studies again showed that sodium reduction can help the antiproteinuric effect of RAAS blockade, but in the vast majority of CKD and renal transplant patients sodium consumption is close to that in the general population and remains high [124].
More recently, multiple dietary approaches, including sodium restriction, have been associated with lower mortality in CKD patients [125].
Dietary sodium, besides influencing fluid volumes, can induce tissue remodeling and activate the immune system [126]; this in turn may be linked to the inflammatory and fibrotic effects that have a role in the pathophysiology of kidney disease.
In this latter field, while traditional biomarkers such as creatinine appear to be decreasing in importance, new biomarkers are gaining ground in experimental studies, capable of linking sodium intake and early kidney damage [127].
4. Conclusions
A high sodium intake increases BP, overall cardiovascular adverse outcomes, and cardiovascular mortality, as well as the risk of stroke. Excess sodium induces worsening of urinary albumin excretion and enlargement of cardiac mass.
As already observed in the middle of the last century, dietary sodium can decrease survival in experimental animals [128]. Excess sodium is responsible for one cardiovascular-related death out of 10 worldwide [67]. Conversely, sodium restriction can reduce the rates of cardiovascular events by at least 25% [129].
Sodium restriction, as suggested in the literature and Guidelines, appears to be a safe measure, which significantly decreases BP [123,130], antihypertensive drug consumption [123,130], and the global cardiovascular risk [131]. Stroke prevention should be based on a dietary approach involving a decrease in dietary sodium [132]. The European Salt Action Network supported sodium reduction on a population basis, to improve the risk of cardiovascular diseases [133]. This is in agreement with the positions of scientific societies, Guidelines and WHO. Population-wide policies are now actively implementing sodium restriction measures around the globe, with good results being achieved in many cases [134], involving both upstream and downstream interventions.
Even a moderate decrease in sodium consumption can result in major successes in terms of adverse event prevention and pharmacoeconomics [135].
Author Contributions
Conceptualization, F.N. and N.M.; methodology, F.G.; investigation, M.A.; resources, M.A.; writing—original draft preparation, F.N.; writing—review and editing, N.M.; visualization, F.G.; supervision, N.M.; project administration, F.N.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.E.; Collins, K.J., Jr.; Dennison, H.C.; DePalma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W.; et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2018, 138, e426–e483. [Google Scholar] [CrossRef]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti, R.E.; Azizi, M.; Burnier, M.; Clement, D.; Coca, A.; De Simone, G.; Dominiczak, A.; et al. 2018 Practice Guidelines for the management of arterial hypertension of the European Society of Hypertension and the European Society of Cardiology: ESH/ESC Task Force for the Management of Arterial Hypertension. J. Hypertens. 2018, 36, 2284–2309. [Google Scholar] [CrossRef]
- Aburto, N.J.; Ziolkovska, A.; Hooper, L.; Elliott, P.; Cappuccio, F.P.; Meerpohl, J.J. Effect of lower sodium intake on health: Systematic review and meta-analyses. BMJ 2013, 346, f1326. [Google Scholar] [CrossRef]
- Brown, I.J.; Tzoulaki, I.; Candeias, V.; Elliott, P. Salt intakes around the world: Implications for public health. Int. J. Epidemiol. 2009, 38, 791–813. [Google Scholar] [CrossRef]
- He, F.J.; Pombo-Rodrigues, S.; MacGregor, G.A. Salt reduction in England from 2003 to 2011: Its relationship to blood pressure, stroke and ischaemic heart disease mortality. BMJ Open 2014, 4, e004549. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.M.; Arcand, J.; Leung, A.A.; Thout, S.R.; Campbell, N.R.; Webster, J. The science of salt: A regularly updated systematic review of salt and health outcomes (December 2015–March 2016). J. Clin. Hypertens. 2017, 19, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Aburto, N.J.; Das, S. Effect of Reduced Sodium Intake on Blood Pressure, Renal Function, Blood Lipids and Other Potential Adverse Effects; World Health Organization: Geneva, Switzerland, 2012. [Google Scholar]
- Mente, A.; O’Donnell, M.J.; Yusuf, S. How Robust Is the Evidence for Recommending Very Low Salt Intake in Entire Populations? J. Am. Coll. Cardiol. 2016, 68, 1618–1621. [Google Scholar] [CrossRef] [PubMed]
- National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Food and Nutrition Board; Committee to Review the Dietary Reference Intakes for Sodium and Potassium; Oria, M.; Harrison, M.; Stallings, V.A. (Eds.) Dietary Reference Intakes for Sodium and Potassium; National Academies Press (US): Washington, DC, USA, 2019. [Google Scholar] [CrossRef]
- He, F.J.; Ma, Y.; Campbell, N.R.C.; MacGregor, G.A.; Cogswell, M.E.; Cook, N.R. Formulas to Estimate Dietary Sodium Intake from Spot Urine Alter Sodium-Mortality Relationship. Hypertension 2019, 74, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.W.; Baqar, S.; Jerums, G.; Ekinci, E.I. Sodium and Its Role in Cardiovascular Disease—The Debate Continues. Front. Endocrinol. (Lausanne) 2016, 7, 164. [Google Scholar] [CrossRef]
- Kempner, W. Some effects of the rice diet treatment of the kidney disease and hypertension. Bull. N. Y. Acad. Sci. Med. 1946, 22, 358–370. [Google Scholar]
- Ferrara, L.A.; De Simone, G.; Pasanisi, F.; Mancini, M.; Mancini, M. Left ventricular mass reduction during salt depletion in arterial hypertension. Hypertension 1984, 6, 755–759. [Google Scholar] [CrossRef] [PubMed]
- Fagard, R.H.; Celis, H.; Thijs, L.; Wouters, S. Regression of left ventricular mass by antihypertensive treatment: A meta-analysis of randomized comparative studies. Hypertension 2009, 54, 1084–1091. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Bang, C.N.; Devereux, R.B.; Okin, P.M. Regression of electrocardiographic left ventricular hypertrophy or strain is associated with lower incidence of cardiovascular morbidity and mortality in hypertensive patients independent of blood pressure reduction—A LIFE review. J. Electrocardiol. 2014, 47, 630–635. [Google Scholar] [CrossRef]
- De Simone, G. Regression of LVH or reduction of left ventricular mass? Am. J. Hypertens. 2008, 21, 365–366. [Google Scholar] [CrossRef][Green Version]
- Tarazi, R.C. Regression of LVH by medical treatment: Present status and possible implications. Am. J. Med. 1983, 75, 80–86. [Google Scholar] [CrossRef]
- Kagiyama, S.; Koga, T.; Kaseda, S.; Ishihara, S.; Kawazoe, N.; Sadoshima, S.; Matsumura, K.; Takata, Y.; Tsuchihashi, T.; Iida, M. Correlation between increased urinary sodium excretion and decreased left ventricular diastolic function in patients with type 2 diabetes mellitus. Clin. Cardiol. 2009, 32, 569–574. [Google Scholar] [CrossRef]
- Schillaci, G.; Pasqualini, L.; Vaudo, G.; Lupattelli, G.; Pirro, M.; Gemelli, F.; De Sio, M.; Porcellati, C.; Mannarino, E. Effect of body weight changes on 24-hour blood pressure and left ventricular mass in hypertension: A 4-year follow-up. Am. J. Hypertens. 2003, 16, 634–639. [Google Scholar] [CrossRef]
- Fernandez, P.G.; Snedden, W.; Idikio, H.; Fernandez, D.; Kim, B.K.; Triggle, C.R. The reversal of left ventricular hypertrophy with control of blood pressure in experimental hypertension. Scand. J. Clin. Lab. Invest. 1984, 44, 711–716. [Google Scholar] [CrossRef]
- Fernandez, D.; Bolli, P.; Snedden, W.; Vasdev, S.; Fernandez, P.G. Modulation of left ventricular hypertrophy by dietary salt and inhibition of angiotensin converting enzyme. J. Hypertens. 1988, 6 (Suppl. 4), S145–S147. [Google Scholar] [CrossRef]
- Schmieder, R.E.; Grube, E.; Impelmann, V.; Rüddel, H.; Schulte, W. Determinants for myocardial hypertrophy in mild essential hypertension. The effect of sodium chloride on left-ventricular hypertrophy. Z. Kardiol. 1990, 79, 557–564. [Google Scholar] [PubMed]
- De Simone, G.; Devereux, R.B.; Camargo, M.J.; Wallerson, D.C.; Laragh, J.H. Influence of sodium intake on in vivo left ventricular anatomy in experimental renovascular hypertension. Am. J. Physiol. 1993, 264 Pt 2, H2103–H2110. [Google Scholar] [CrossRef]
- Jula, A.M.; Karanko, H.M. Effects on left ventricular hypertrophy of long-term nonpharmacological treatment with sodium restriction in mild-to-moderate essential hypertension. Circulation 1994, 89, 1023–1031. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, A.; Bentley-Lewis, R.; Jeunemaitre, X.; Adler, G.K.; Williams, J.S. Dietary sodium alters the prevalence of electrocardiogram determined left ventricular hypertrophy in hypertension. Am. J. Hypertens. 2009, 22, 669–673. [Google Scholar] [CrossRef] [PubMed]
- Schmieder, R.E.; Messerli, F.H.; Garavaglia, G.E.; Nunez, B.D. Dietary salt intake. A determinant of cardiac involvement in essential hypertension. Circulation 1988, 78, 951–956. [Google Scholar] [CrossRef] [PubMed]
- Van der Westhuizen, B.; Schutte, A.E.; Gafane-Matemane, L.F.; Kruger, R. Left ventricular mass independently associates with 24-hour sodium excretion in young masked hypertensive adults: The African-PREDICT study. Int. J. Cardiol. 2019, 276, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, S.; Djoussé, L.; Aguilar, F.G.; Martinez, E.E.; Polsinelli, V.B.; Irvin, M.R.; Arnett, D.K.; Shah, S.J. Association of Estimated Sodium Intake with Adverse Cardiac Structure and Function: From the HyperGEN Study. J. Am. Coll. Cardiol. 2017, 70, 715–724. [Google Scholar] [CrossRef]
- Blake, J.; Devereux, R.B.; Borer, J.S.; Szulc, M.; Pappas, T.W.; Laragh, J.H. Relation of obesity, high sodium intake, and eccentric left ventricular hypertrophy to left ventricular exercise dysfunction in essential hypertension. Am. J. Med. 1990, 88, 477–485. [Google Scholar] [CrossRef]
- De Simone, G.; Devereux, R.B.; Roman, M.J.; Schlussel, Y.; Alderman, M.H.; Laragh, J.H. Echocardiographic left ventricular mass and electrolyte intake predict arterial hypertension. Ann. Intern. Med. 1991, 114, 202–209. [Google Scholar] [CrossRef]
- Du Cailar, G.; Ribstein, J.; Daures, J.P.; Mimran, A. Sodium and left ventricular mass in untreated hypertensive and normotensive subjects. Am. J. Physiol. 1992, 263, H177–H181. [Google Scholar] [CrossRef]
- Beil, A.H.; Schmieder, R.E. Salt intake as a determinant of cardiac hypertrophy. Blood Press. Suppl. 1995, 2, 30–34. [Google Scholar] [PubMed]
- Langenfeld, M.R.; Schobel, H.; Veelken, R.; Weihprecht, H.; Schmieder, R.E. Impact of dietary sodium intake on left ventricular diastolic filling in early essential hypertension. Eur. Heart J. 1998, 19, 951–958. [Google Scholar] [CrossRef] [PubMed]
- Haring, B.; Wang, W.; Lee, E.T.; Jhamnani, S.; Howard, B.V.; Devereux, R.B. Effect of dietary sodium and potassium intake on left ventricular diastolic function and mass in adults ≤40 years (from the Strong Heart Study). Am. J. Cardiol. 2015, 115, 1244–1248. [Google Scholar] [CrossRef] [PubMed]
- Kupari, M.; Koskinen, P.; Virolainen, J. Correlates of left ventricular mass in a population sample aged 36 to 37 years. Focus on lifestyle and salt intake. Circulation 1994, 89, 1041–1050. [Google Scholar] [CrossRef]
- Soliman, E.Z.; Prineas, R.J. Antihypertensive Therapies and Left Ventricular Hypertrophy. Curr. Hypertens. Rep. 2017, 19, 79. [Google Scholar] [CrossRef]
- Musso, N.; Dotto, A.; Shen, Z. Left ventricular mass reduction by low sodium diet in treated hypertensive patients. Hypertension 2019, 74 (Suppl. 1), A064. [Google Scholar] [CrossRef]
- Pimenta, E.; Gordon, R.D.; Ahmed, A.H.; Cowley, D.; Leano, R.; Marwick, T.H.; Stowasser, M. Cardiac dimensions are largely determined by dietary salt in patients with primary aldosteronism: Results of a case-control study. J. Clin. Endocrinol. Metab. 2011, 96, 2813–2820. [Google Scholar] [CrossRef]
- Gerdts, E.; Lund-Johansen, P.; Omvik, P. Factors influencing left ventricular mass in salt sensitive and salt resistant essential hypertensive patients. Blood Press. 1998, 7, 223–230. [Google Scholar] [CrossRef]
- Pääkkö, T.J.W.; Perkiömäki, J.S.; Silaste, M.L.; Bloigu, R.; Huikuri, H.V.; Antero Kesäniemi, Y.; Ukkola, O.H. Dietary sodium intake is associated with long-term risk of new-onset atrial fibrillation. Ann. Med. 2018, 50, 694–703. [Google Scholar] [CrossRef]
- Marketou, M.E.; Zacharis, E.A.; Parthenakis, F.; Kochiadakis, G.E.; Maragkoudakis, S.; Chlouverakis, G.; Vardas, P.E. Association of sodium and potassium intake with ventricular arrhythmic burden in patients with essential hypertension. J. Am. Soc. Hypertens. 2013, 7, 276–282. [Google Scholar] [CrossRef]
- Fields, N.G.; Yuan, B.X.; Leenen, F.H. Sodium-induced cardiac hypertrophy. Cardiac sympathetic activity versus volume load. Circ. Res. 1991, 68, 745–755. [Google Scholar] [CrossRef] [PubMed]
- Le Corvoisier, P.; Adamy, C.; Sambin, L.; Crozatier, B.; Berdeaux, A.; Michel, J.B.; Hittinger, L.; Su, J. The cardiac renin-angiotensin system is responsible for high-salt diet-induced left ventricular hypertrophy in mice. Eur. J. Heart Fail. 2010, 12, 1171–1178. [Google Scholar] [CrossRef] [PubMed]
- Montasser, M.E.; Douglas, J.A.; Roy-Gagnon, M.H.; van Hout, C.V.; Weir, M.R.; Vogel, R.; Parsa, A.; Steinle, N.I.; Snitker, S.; Brereton, N.H.; et al. Determinants of blood pressure response to low salt intake in a healthy adult population. J. Clin. Hypertens. 2011, 13, 795–800. [Google Scholar] [CrossRef] [PubMed]
- Campino, C.; Baudrand, R.; Valdivia, C.A.; Carvajal, C.; Vecchiola, A.; Tapia-Castillo, A.; Martínez-Aguayo, A.; Garcia, H.; García, L.; Allende, F.; et al. Sodium Intake Is associated With Endothelial Damage Biomarkers and Metabolic Dysregulation. Am. J. Hypertens. 2018, 31, 1127–1132. [Google Scholar] [CrossRef]
- Kapoor, K.; Fashanu, O.; Post, W.S.; Lutsey, P.L.; Michos, E.D.; deFilippi, C.R.; McEvoy, J.W. Relation of Dietary Sodium Intake With Subclinical Markers of Cardiovascular Disease (from MESA). Am. J. Cardiol. 2019, 124, 636–643. [Google Scholar] [CrossRef]
- Joosten, M.M.; Gansevoort, R.T.; Mukamal, K.J.; Lambers Heerspink, H.J.; Geleijnse, J.M.; Feskens, E.J.; Navis, G.; Bakker, S.J.; PREVEND Study Group. Sodium excretion and risk of developing coronary heart disease. Circulation 2014, 129, 1121–1128. [Google Scholar] [CrossRef]
- He, F.J.; Li, J.; MacGregor, G.A. Effect of longer term modest salt reduction on blood pressure: Cochrane systematic review and meta-analysis of randomised trials. BMJ 2013, 346, f1325. [Google Scholar] [CrossRef]
- D’Elia, L.; Galletti, F.; La Fata, E.; Sabino, P.; Strazzullo, P. Effect of dietary sodium restriction on arterial stiffness: Systematic review and meta-analysis of the randomized controlled trials. J. Hypertens. 2018, 36, 734–743. [Google Scholar] [CrossRef]
- O’Donnell, M.; Mente, A.; Yusuf, S. Sodium intake and cardiovascular health. Circ. Res. 2015, 116, 1046–1057. [Google Scholar] [CrossRef]
- Willebrand, R.; Kleinewietfeld, M. The role of salt for immune cell function and disease. Immunology 2018, 154, 346–353. [Google Scholar] [CrossRef]
- Rucker, A.J.; Rudemiller, N.P.; Crowley, S.D. Salt, hypertension and immunity. Annu. Rev. Physiol. 2018, 80, 283–307. [Google Scholar] [CrossRef] [PubMed]
- Afsar, B.; Kuwabara, M.; Ortiz, A.; Yerlikaya, A.; Siriopol, D.; Covic, A.; Rodriguez-Iturbe, B.; Johnson, R.; Kanbay, M. Salt intake and immunity. Hypertension 2018, 72, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Robinson, A.T.; Edwards, D.G.; Farquhar, W.B. The influence of dietary salt beyond blood pressure. Curr. Hypertens. Rep. 2019, 21, 42. [Google Scholar] [CrossRef] [PubMed]
- Frisoli, T.M.; Schmieder, R.E.; Grodzicki, T.; Messerli, F.H. Salt and hypertension: Is salt dietary reduction worth the effort? Am. J. Med. 2012, 125, 433–439. [Google Scholar] [CrossRef]
- Du Cailar, G.; Mimran, A. Non-pressure related effects of dietary sodium. Curr. Hypertens. Rep. 2007, 9, 154–159. [Google Scholar] [CrossRef]
- Antonios, T.F.T.; MacGregor, G.A. Deleterious effects of salt intake other than effects on blood pressure. Clin. Exp. Pharmacol. Physiol. 1995, 22, 180–184. [Google Scholar] [CrossRef]
- Kashioulis, P.; Hammarsten, O.; Marcussen, N.; Shubbar, E.; Saeed, A.; Guron, G. High-NaCl Diet Aggravates Cardiac Injury in Rats with Adenine-Induced Chronic Renal Failure and Increases Serum Troponin T Levels. Cardiorenal Med. 2016, 6, 317–327. [Google Scholar] [CrossRef]
- Ando, K.; Kawarazaki, H.; Miura, K.; Matsuura, H.; Watanabe, Y.; Yoshita, K.; Kawamura, M.; Kusaka, M.; Kai, H.; Tsuchihashi, T.; et al. [Scientific statement] Report of the Salt Reduction Committee of the Japanese Society of Hypertension (1) Role of salt in hypertension and cardiovascular diseases. Hypertens. Res. 2013, 36, 1009–1019. [Google Scholar] [CrossRef]
- Zhao, X.; Yang, X.; Zhang, X.; Li, Y.; Zhao, X.; Ren, L.; Wang, L.; Gu, C.; Zhu, Z.; Han, Y. Dietary salt intake and coronary atherosclerosis in patients with prehypertension. J. Clin. Hypertens. (Greenwich) 2014, 16, 575–580. [Google Scholar] [CrossRef]
- Joossens, J.V.; Kesteloot, H. Trends in systolic blood pressure, 24-hour sodium excretion, and stroke mortality in the elderly in Belgium. Am. J. Med. 1991, 90, 5S–11S. [Google Scholar] [CrossRef]
- Li, X.Y.; Cai, X.L.; Bian, P.D.; Hu, L.R. High salt intake and stroke: Meta-analysis of the epidemiologic evidence. CNS Neurosci. Ther. 2012, 18, 691–701. [Google Scholar] [CrossRef] [PubMed]
- Kieneker, L.M.; Eisenga, M.F.; Gansevoort, R.T.; de Boer, R.A.; Navis, G.; Dullaart, R.P.F.; Joosten, M.M.; Bakker, S.J.L. Association of low urinary sodium excretion with increased risk of stroke. Mayo Clin. Proc. 2018, 93, 1803–1809. [Google Scholar] [CrossRef] [PubMed]
- Musso, N.; Dotto, A. Low-sodium intake: A risk factor for stroke? Mayo Clin. Proc. 2019, 94, 728–729. [Google Scholar] [CrossRef]
- Strohle, A. The ongoing sodium controversy—Between Pure and Nutricode. Int. J. Vitam. Nutr. Res. 2017, 87, 322–329. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Fahimi, S.; Singh, G.M.; Micha, R.; Khatibzadeh, S.; Engell, R.E.; Lim, S.; Danaei, G.; Ezzati, M.; Powles, J.; et al. Global sodium consumption and death from cardiovascular causes. N. Engl. J. Med. 2014, 371, 624–634. [Google Scholar] [CrossRef] [PubMed]
- Pallazola, V.A.; Davis, D.M.; Whelton, S.P.; Cardoso, R.; Latina, J.M.; Michos, E.D.; Sarkar, S.; Blumenthal, R.S.; Arnett, D.K.; Stone, N.J.; et al. A Clinician’s Guide to Healthy Eating for Cardiovascular Disease Prevention. Mayo Clin. Proc. Innov. Qual. Outcomes 2019, 3, 251–267. [Google Scholar] [CrossRef]
- Calabrese, I.; Riccardi, G. Effectiveness of Changes in Diet Composition on Reducing the Incidence of Cardiovascular Disease. Curr. Cardiol. Rep. 2019, 21, 88. [Google Scholar] [CrossRef]
- Yu, E.; Malik, V.S.; Hu, F.B. Cardiovascular Disease Prevention by Diet Modification: JACC Health Promotion Series. J. Am. Coll. Cardiol. 2018, 72, 914–926. [Google Scholar] [CrossRef]
- Mirmiran, P.; Bahadoran, Z.; Nazeri, P.; Azizi, F. Dietary sodium to potassium ratio and the incidence of hypertension and cardiovascular disease: A population-based longitudinal study. Clin. Exp. Hypertens. 2018, 40, 772–779. [Google Scholar] [CrossRef]
- Polonia, J.; Monteiro, J.; Almeida, J.; Silva, J.A.; Bertoquini, S. High salt intake is associated with a higher risk of cardiovascular events: A 7.2-year evaluation of a cohort of hypertensive patients. Blood Press. Monit. 2016, 21, 301–306. [Google Scholar] [CrossRef]
- Äijälä, M.; Malo, E.; Santaniemi, M.; Bloigu, R.; Silaste, M.L.; Kesäniemi, Y.A.; Ukkola, O. Dietary sodium intake and prediction of cardiovascular events. Eur. J. Clin. Nutr. 2015, 69, 1042–1047. [Google Scholar] [CrossRef] [PubMed]
- Morrison, A.C.; Ness, R.B. Sodium intake and cardiovascular disease. Annu. Rev. Public Health 2011, 32, 71–90. [Google Scholar] [CrossRef] [PubMed]
- Cook, N.R.; Appel, L.J.; Whelton, P.K. Lower levels of sodium intake and reduced cardiovascular risk. Circulation 2014, 129, 981–989. [Google Scholar] [CrossRef] [PubMed]
- Abreu, D.; Sousa, P.; Matias-Dias, C.; Pinto, F.J. Cardiovascular disease and high blood pressure trend analyses from 2002 to 2016: After the implementation of a salt reduction strategy. BMC Public Health 2018, 18, 722. [Google Scholar] [CrossRef]
- Welsh, C.E.; Welsh, P.; Jhund, P.; Delles, C.; Celis-Morales, C.; Lewsey, J.D.; Gray, S.; Lyall, D.; Iliodromiti, S.; Gill, J.M.R.; et al. Urinary Sodium Excretion, Blood Pressure, and Risk of Future Cardiovascular Disease and Mortality in Subjects Without Prior Cardiovascular Disease. Hypertension 2019, 73, 1202–1209. [Google Scholar] [CrossRef]
- Kalogeropoulos, A.P.; Georgiopoulou, V.V.; Murphy, R.A.; Newman, A.B.; Bauer, D.C.; Harris, T.B.; Yang, Z.; Applegate, W.B.; Kritchevsky, S.B. Dietary sodium content, mortality, and risk for cardiovascular events in older adults: The Health, Aging, and Body Composition (Health ABC) Study. JAMA Intern. Med. 2015, 175, 410–419. [Google Scholar] [CrossRef]
- Lamelas, P.M.; Mente, A.; Diaz, R.; Orlandini, A.; Avezum, A.; Oliveira, G.; Lanas, F.; Seron, P.; Lopez-Jaramillo, P.; Camacho-Lopez, P.; et al. Association of Urinary Sodium Excretion With Blood Pressure and Cardiovascular Clinical Events in 17,033 Latin Americans. Am. J. Hypertens. 2016, 29, 796–805. [Google Scholar] [CrossRef]
- Aggarwal, M.; Bozkurt, B.; Panjrath, G.; Aggarwal, B.; Ostfeld, R.J.; Barnard, N.D.; Gaggin, H.; Freeman, A.M.; Allen, K.; Madan, S.; et al. Lifestyle Modifications for Preventing and Treating Heart Failure. J. Am. Coll. Cardiol. 2018, 72, 2391–2405. [Google Scholar] [CrossRef]
- Elliott, P.; Stamler, J.; Nichols, R.; Dyer, A.R.; Stamler, R.; Kesteloot, H.; Marmot, M.; Intersalt Cooperative Research Group. Intersalt revisited: Further analyses of 24 hour sodium excretion and blood pressure within and across populations. BMJ 1996, 312, 1249–1253. [Google Scholar] [CrossRef]
- Adler, A.J.; Taylor, F.; Martin, N.; Gottlieb, S.; Taylor, R.S.; Ebrahim, S. Reduced dietary salt for the prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2014, 12, CD009217. [Google Scholar] [CrossRef]
- Abshire, M.; Jiayun, X.; Baptiste, D.; Almansa, J.R.; Jingzhi, X.; Cummings, A.; Andrews, M.J.; Dennison Himmelfarb, C. Nutritional interventions in heart failure: A systematic review of the literature. J. Card. Fail. 2015, 21, 989–999. [Google Scholar] [CrossRef] [PubMed]
- Susic, D.; Frohlich, E.D. Salt consumption and cardiovascular, renal, and hypertensive diseases: Clinical and mechanistic aspects. Curr. Opin. Lipidol. 2012, 23, 11–16. [Google Scholar] [CrossRef] [PubMed]
- He, F.J.; Burnier, M.; Macgregor, G.A. Nutrition in cardiovascular disease: Salt in hypertension and heart failure. Eur. Heart J. 2011, 32, 3073–3080. [Google Scholar] [CrossRef] [PubMed]
- Payne-Emerson, H.; Lennie, T.A. Nutritional considerations in heart failure. Nurs. Clin. N. Am. 2008, 43, 117–132. [Google Scholar] [CrossRef] [PubMed]
- Nijst, P.; Verbrugge, F.H.; Grieten, L.; Dupont, M.; Steels, P.; Tang, W.H.W.; Mullens, W. The pathophysiological role of interstitial sodium in heart failure. J. Am. Coll. Cardiol. 2015, 65, 378–388. [Google Scholar] [CrossRef] [PubMed]
- Tomaschitz, A.; Ritz, E.; Pieske, B.; Rus-Machan, J.; Kienreich, K.; Verheyen, N.; Gaksch, M.; Grübler, M.; Fahrleitner-Pammer, A.; Mrak, P.; et al. Aldosterone and parathyroid hormone interactions as mediators of metabolic and cardiovascular disease. Metabolism 2014, 63, 20–31. [Google Scholar] [CrossRef]
- Catena, C.; Colussi, G.; Sechi, L.A. Aldosterone, organ damage and dietary salt. Clin. Exp. Pharmacol. Physiol. 2013, 40, 922–928. [Google Scholar] [CrossRef]
- Aronow, W.S.; Shamliyan, T.A. Dietary Sodium Interventions to Prevent Hospitalization and Readmission in Adults with Congestive Heart Failure. Am. J. Med. 2018, 131, 365–370. [Google Scholar] [CrossRef]
- Colin-Ramirez, E.; Ezekowitz, J.A. Salt in the diet in patients with heart failure: What to recommend. Curr. Opin. Cardiol. 2016, 31, 196–203. [Google Scholar] [CrossRef]
- Butler, T. Dietary management of heart failure: Room for improvement? Br. J. Nutr. 2016, 115, 1202–1217. [Google Scholar] [CrossRef]
- DiNicolantonio, J.J.; Chatterjee, S.; O’Keefe, J.H. Dietary Salt Restriction in Heart Failure: Where Is the Evidence? Prog. Cardiovasc. Dis. 2016, 58, 401–406. [Google Scholar] [CrossRef]
- Rifai, L.; Silver, M.A. A Review of the DASH Diet as an Optimal Dietary Plan for Symptomatic Heart Failure. Prog. Cardiovasc. Dis. 2016, 58, 548–554. [Google Scholar] [CrossRef] [PubMed]
- Aaron, K.J.; Sanders, P.W. Role of dietary salt and potassium intake in cardiovascular health and disease: A review of the evidence. Mayo Clin. Proc. 2013, 88, 987–995. [Google Scholar] [CrossRef] [PubMed]
- Turlova, E.; Feng, Z. Dietary salt intake and stroke. Acta Pharmacol. Sin. 2013, 34, 8–9. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Eshak, E.S.; Liu, K.; Gero, K.; Liu, Z.; Yu, C. Age-period-cohort analysis of stroke mortality attributable to high sodium intake in China and Japan. Stroke 2019, 50, 1648–1654. [Google Scholar] [CrossRef] [PubMed]
- Newberry, S.J.; Chung, M.; Anderson, C.A.M.; Chen, C.; Fu, Z.; Tang, A.; Zhao, N.; Booth, M.; Marks, J.; Hollands, S.; et al. Sodium and Potassium Intake: Effects on Chronic Disease Outcomes and Risks [Internet]; Report No.: 18-EHC009-EF; AHRQ Comparative Effectiveness Reviews; Agency for Healthcare Research and Quality (US): Rockville, MD, USA, 2018. [Google Scholar]
- Spence, J.D. Diet for stroke prevention. Stroke Vasc. Neurol. 2018, 13, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Aigner, A.; Becher, H.; Jacobs, S.; Wilkens, L.R.; Boushey, C.J.; Le Marchand, L.; Haiman, C.A.; Maskarinec, G. Low diet quality and the risk of stroke mortality: The multiethnic cohort study. Eur. J. Clin. Nutr. 2018, 72, 1035–1145. [Google Scholar] [CrossRef]
- Brown, D.L.; Conley, K.M.; Sanchez, B.N.; Resnicow, K.; Cowdery, J.E.; Sais, E.; Murphy, J.; Skolarus, L.E.; Lisabeth, L.D.; Morgenstern, L.B. A multicomponent behavioral intervention to reduce stroke risk factor behaviors: The stroke health and risk education cluster-randomized controlled trial. Stroke 2015, 46, 2861–2867. [Google Scholar] [CrossRef]
- Hendriksen, M.A.; van Raaij, J.M.; Geleijnse, J.M.; Breda, J.; Boshuizen, H.C. Health gain by salt reduction in Europe: A modelling study. PLoS ONE 2015, 10, e0118873. [Google Scholar] [CrossRef]
- Rodriguez-Campello, A.; Jimenez-Conde, J.; Ois, A.; Cuadrado-Godia, E.; Giralt-Steinhauer, E.; Schroeder, H.; Romeral, G.; Llop, M.; Soriano-Tarraga, C.; Garralda-Anaya, M.; et al. Dietary habits in patients with ischemic stroke: A case-control study. PLoS ONE 2014, 9, e114716. [Google Scholar] [CrossRef]
- Dearborn, J.L.; Khera, T.; Peterson, M.; Shahab, Z.; Kernan, W.N. Diet quality in patients with stroke. Stroke Vasc. Neurol. 2019, 4, 154–157. [Google Scholar] [CrossRef] [PubMed]
- Arsang-Jang, S.; Mansourian, M.; Mohammadifard, N.; Khosravi, A.; Oveis-Gharan, S.; Nouri, F.; Sarrafzadegan, N. Temporal trend analysis of stroke and salt intake: A 15-year population-based study. Nutr. Neurosci. 2019, 13, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhang, J.; Li, Z.; Liu, Y.; Fan, X.; Zhang, Y.; Zhang, Y. Association of sodium intake and major cardiovascular outcomes: A dose-response meta-analysis of prospective cohort studies. BMC Cardiovasc. Disord. 2018, 18, 192. [Google Scholar] [CrossRef] [PubMed]
- Jayedi, A.; Ghomashi, F.; Zargar, M.; Shab-Bidar, S. Dietary sodium, sodium to potassium ratio, and risk of stroke: A systematic review and nonlinear dose-response meta-analysis. Clin. Nutr. 2019, 38, 1092–1100. [Google Scholar] [CrossRef] [PubMed]
- Wiley, J.; Gardener, H.; Cespedes, S.; Cheung, Y.K.; Sacco, R.L.; Elkind, M.S.V. Dietary sodium to potassium ratio and risk of stroke in a multhiethnic urban population: The northern Manhattan study. Stroke 2017, 48, 2979–2983. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.C. Dietary fats and other nutrients on stroke. Curr. Opin. Lipidol. 2013, 24, 41–48. [Google Scholar] [CrossRef]
- Cova, L.; Gelosa, P.; Mura, E.; Mauro, A.; Stramba-Badiale, M.; Michailidis, G.; Colonna, A.; El Assawy, N.; Pignieri, A.; Busca, G.; et al. Vascular and parenchymal lesions along with enhanced neurogenesis characterize the brain of asymptomatic stroke-prone spontaneous hypertensive rats. J. Hypertens. 2013, 31, 1618–1628. [Google Scholar] [CrossRef]
- Kusche-Vihrog, K.; Schmitz, B.; Brand, E. Salt controls endothelial and vascular phenotype. Pflugers Arch. 2015, 467, 499–512. [Google Scholar] [CrossRef]
- Chapleau, M.W.; Rotella, D.L.; Reho, J.J.; Rahmouni, K.; Stauss, H.M. Chronic vagal nerve stimulation prevents high-salt diet-induced endothelial dysfunction and aortic stiffening in stroke-prone spontaneously hypertensive rats. Am. J. Physiol. Heart Circ. Physiol. 2016, 311, H276–H285. [Google Scholar] [CrossRef]
- Makin, S.D.J.; Mubki, G.F.; Doubal, F.N.; Shuler, K.; Staals, J.; Dennis, M.S.; Wardlaw, J.M. Small vessel disease and dietary salt intake: Cross-sectional study and systematic review. J. Stroke Cerebrovasc. Dis. 2017, 26, 3020–3028. [Google Scholar] [CrossRef]
- Jackson, E.K.; Gillespie, D.G.; Mi, Z.; Cheng, D. Adenosine receptors influence hypertension in Dahl salt-sensitive rats. Hypertension 2018, 72, 511–521. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wang, D.; Li, X.; Jiang, Y.; Wang, C.; Zhang, Y.; Kong, Q.; Tian, C.; Dai, Y.; Zhao, W.; et al. Excess salt intake promotes M1 microglia polarization via a p38/MAPK/AR-dependent pathway after cerebral ischemia in mice. Int. Immunopharmacol. 2020, 81, 106176. [Google Scholar] [CrossRef] [PubMed]
- Nomura, K.; Asayama, K.; Jacobs, L.; Thijs, L.; Staessen, A. Renal function in relation to sodium intake: A quantitative review of the literature. Kidney Int. 2017, 92, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Arcand, J.; Wong, M.M.Y.; Santos, J.A.; Leung, A.A.; Trieu, K.; Thout, S.R.; Webster, J.; Campbell, N.R.C. More evidence that salt increases blood pressure and risk of kidney disease from the Science of Salt: A regularly updated systematic review of salt and health outcomes (April–July 2016). J. Clin. Hypertens. 2017, 19, 813–823. [Google Scholar] [CrossRef]
- Garofalo, C.; Borrelli, S.; Provenzano, M.; De Stefano, T.; Vita, C.; Chiodini, P.; Minutolo, R.; De Nicola, L.; Conte, G. Dietary salt restriction in chronic kidney disease: A meta-analysis of randomized clinical trials. Nutrients 2018, 10, 732. [Google Scholar] [CrossRef]
- Qian, Q. Salt, water and nephron: Mechanisms of action and the link to hypertension and chronic kidney disease. Nephrology 2018, 24 (Suppl. 4), 44–49. [Google Scholar] [CrossRef]
- Humalda, J.K.; Navis, G. Dietary sodium restriction: A neglected therapeutic opportunity in chronic kidney disease. Curr. Opin. Nephrol. Hypertens. 2014, 23, 533–540. [Google Scholar] [CrossRef]
- Jain, N.; Reilly, R.F. Effects of dietary interventions on incidence and progression of CKD. Nat. Rev. Nephrol. 2014, 10, 712–724. [Google Scholar] [CrossRef]
- Yoon, C.Y.; Noh, J.; Lee, J.; Kee, Y.K.; Seo, C.; Lee, M.; Cha, M.U.; Kim, H.; Park, S.; Yun, H.R.; et al. High and low sodium intakes are associated with incident chronic kidney disease in patients with normal renal function and hypertension. Kidney Int. 2018, 93, 921–931. [Google Scholar] [CrossRef]
- McMahon, E.J.; Campbell, K.L.; Bauer, J.D.; Mudge, D.W. Altered dietary salt intake for people with chronic kidney disease. Cochrane Database Syst. Rev. 2015, 2, CD0100070. [Google Scholar] [CrossRef]
- De Borst, M.H.; Navis, G. Sodium intake, RAAS-blockade and progressive renal disease. Pharmacol. Res. 2016, 107, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Rysz, J.; Franczyk, B.; Cialkowska-Rysz, A.; Gluba-Brzozka, A. The effect of diet on the survival of patients with chronic kidney disease. Nutrients 2017, 9, 495. [Google Scholar] [CrossRef] [PubMed]
- Oppelaar, J.J.; Vogt, L. Body fluid-independent effects of dietary salt consumption in chronic kidney disease. Nutrients 2019, 11, 2779. [Google Scholar] [CrossRef] [PubMed]
- Hosohata, K. Biomarkers for chronic kidney disease associated with high salt intake. Int. J. Mol. Sci. 2017, 18, 2080. [Google Scholar] [CrossRef]
- Meneely, G.R.; Ball, C.O. Experimental epidemiology of chronic sodium chloride toxicity and the protective effect of potassium chloride. Am. J. Med. 1958, 25, 713–725. [Google Scholar] [CrossRef]
- Cook, N.R.; Cutler, J.A.; Obarzanek, E.; Buring, J.E.; Rexrode KMKumanyika, S.K.; Appel, L.J.; Whelton, P.K. Long-term effects of dietary sodium reduction on cardiovascular disease outcomes: Observational follow-up of the Trials of Hypertension Prevention (TOHP). BMJ 2007, 334, 885–888. [Google Scholar] [CrossRef]
- Musso, N.; Carloni, B.; Chiusano, M.C.; Giusti, M. Simple dietary advice reduces 24-hour urinary sodium excretion, blood pressure, and drug consumption in hypertensive patients. J. Am. Soc. Hypertens. 2018, 12, 652–659. [Google Scholar] [CrossRef]
- Sanders, P.W. Vascular consequences of dietary salt intake. Am. J. Physiol. Renal Physiol. 2009, 297, F237–F243. [Google Scholar] [CrossRef]
- Spence, J.D. Nutrition and Risk of Stroke. Nutrients 2019, 11, 647. [Google Scholar] [CrossRef]
- Cappuccio, F.P.; Beer, M.; Strazzullo, P.; European Salt Action Network. Population dietary salt reduction and the risk of cardiovascular disease. A scientific statement from the European Salt Action Network. Nutr. Metab. Cardiovasc. Dis. 2018, 29, 107–114. [Google Scholar] [CrossRef]
- Hyseni, L.; Elliot-Green, A.; Lloyd-Williams, F.; Kypridemos, C.; O’Flaherty, M.; McGill, R.; Orton, L.; Bromley, H.; Cappuccio, F.P.; Capewell, S. Systematic review of dietary salt reduction policies: Evidence for an effectiveness hierarchy? PLoS ONE 2017, 12, e0177535. [Google Scholar] [CrossRef] [PubMed]
- He, F.J.; MacGregor, G.A. Role of salt intake in prevention of cardiovascular disease: Controversies and challenges. Nat. Rev. Cardiol. 2018, 15, 371–377. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).