The Intensity of Simulated Grazing Modifies Costs and Benefits of Physiological Integration in a Rhizomatous Clonal Plant
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site and Plant Species
2.2. Experimental Design
2.3. Measurements
2.4. Compensation Index Calculation and Physiological Integration Cost–Benefit Analysis
2.5. Statistical Analysis
3. Results
3.1. Biomass Accumulation and Allocation
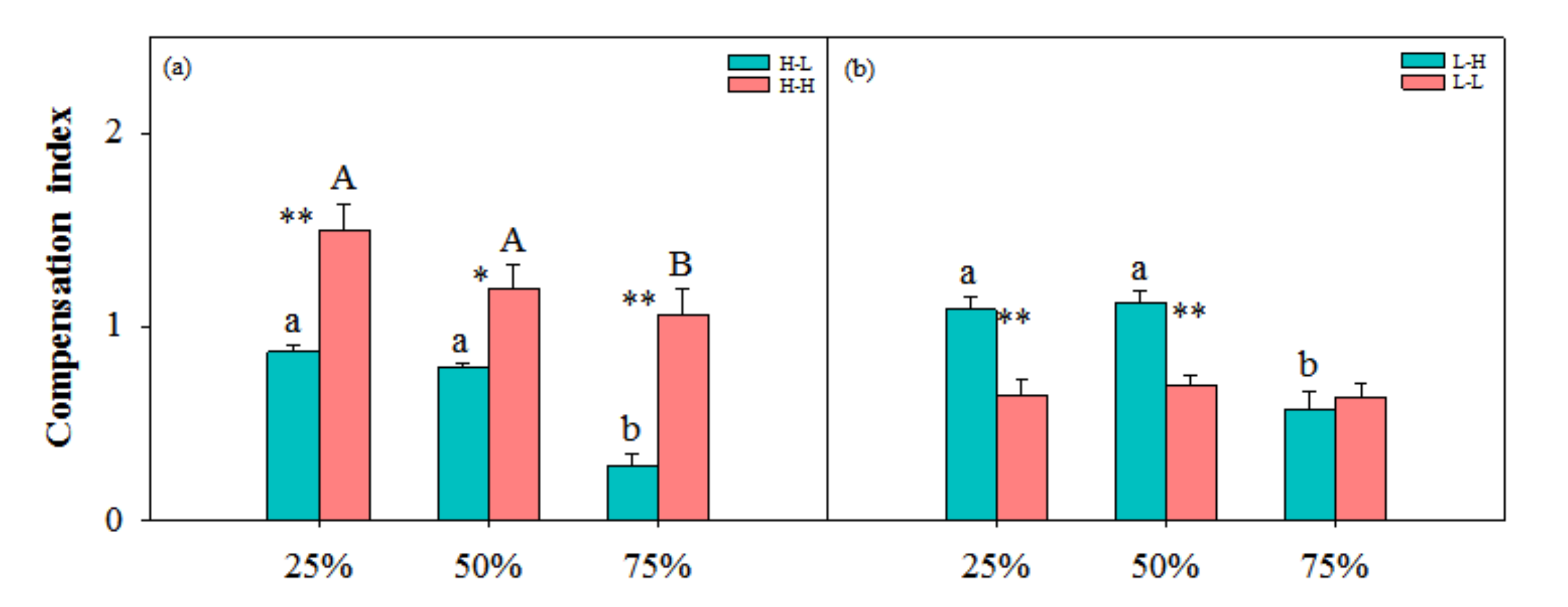
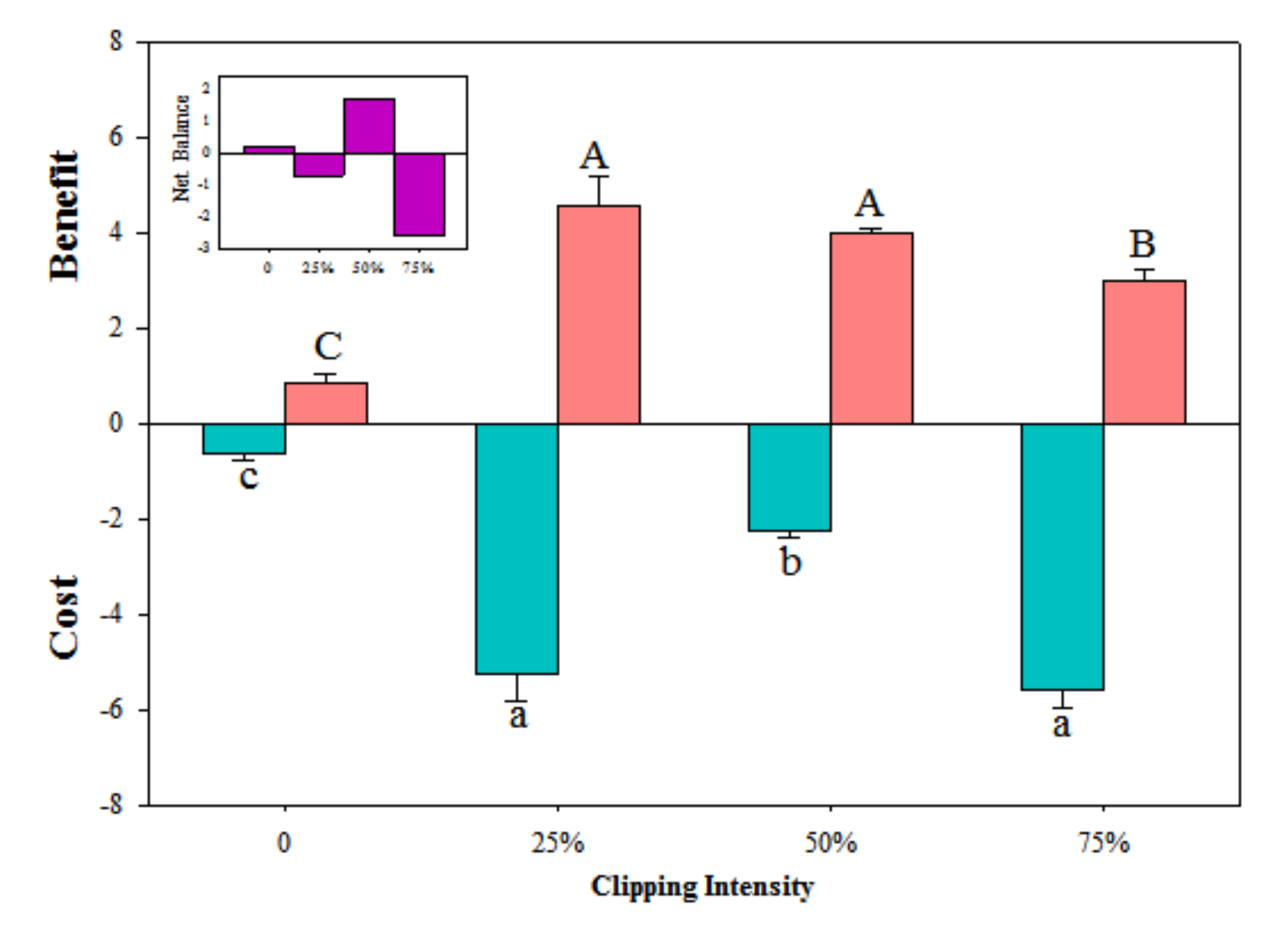
3.2. Compensation Index and Cost–Benefit of Physiological Integration
4. Discussion
4.1. Nutrient and Physiological Integration Affect Plant Response to Herbivory
4.2. Trade-off between Benefits and Costs of Physiological Integration-Determined Compensatory Growth
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alpert, P. Nutrient sharing in natural clonal fragments of Fragaria chiloensis. J. Ecol. 1996, 84, 395–406. [Google Scholar] [CrossRef]
- Bittebiere, A.K.; Benot, M.L.; Mony, C. Clonality as a key but overlooked driver of biotic interactions in plants. Perspect. Plant Ecol. 2020, 43, 125510. [Google Scholar] [CrossRef]
- Stuefer, J.F.; Hutchings, M.J. Environmental heterogeneity and clonal growth: A study of the capacity for reciprocal translocation in Glechoma hederacea L. Oecologia 1994, 100, 302–308. [Google Scholar] [CrossRef] [PubMed]
- De Kroons, H.; Hutchings, M. Morphological plasticity in clonal plants: The foraging concepts reconsidered. J. Ecol. 1995, 83, 143–152. [Google Scholar] [CrossRef]
- Song, Y.B.; Yu, F.H.; Keser, L.; Dawson, W.; Fischer, M.; Dong, M.; Van Kleunen, M. United we stand, divided we fall: A meta-analysis of experiments on clonal integration and its relationship to invasiveness. Oecologia 2013, 171, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Touchette, B.; Moody, J.; Byrne, C.; Marcus, S. Water integration in the clonal emergent hydrophyte, Justicia americana: Benefits of acropetal water transfer from mother to daughter ramets. Hydrobiologia 2013, 702, 83–94. [Google Scholar] [CrossRef]
- Wang, D.L.; Du, J.; Zhang, B.T.; Ba, L.; Hodgkinson, K. Grazing intensity and phenotypic plasticity in the clonal grass Leymus chinensis. Rangeland Ecol. Manag. 2017, 70, 740–747. [Google Scholar] [CrossRef]
- Goldberg, D.E.; Batzer, E.; Elgersma, K.; Martina, J.; Klimešová, J. Allocation to clonal growth: Critical questions and protocols to answer them. Perspect. Plant Ecol. 2020, 43, 125511. [Google Scholar] [CrossRef]
- Van Groenendael, J.L.K.; Klimešová, J.; Hendriks, R.J.J.H. Comparative ecology of clonal plants. Philos. Trans. R. Soc. B. 1996, 351, 1331–1339. [Google Scholar] [CrossRef]
- Hartnett, D.C.; Bazzaz, F.A. Physiological integration among intraclonal ramets in Solidago canadensis. Ecology 1983, 64, 779–788. [Google Scholar] [CrossRef]
- Salzman, A.G.; Parker, M.A. Neighbors ameliorate local salinity stress for a rhizomatous plant in a heterogeneous environment. Oecologia 1985, 65, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Alpert, P. Nitrogen sharing among ramets increases clonal growth in Fragaria chiloensis. Ecology 1991, 72, 69–80. [Google Scholar] [CrossRef]
- Evans, J.P.; Whitney, S. Clonal integration across a salt gradient by a nonhalophyte, Hydrocotyle bonariensis (Apiaceae). Am. J. Bot. 1992, 79, 1344–1347. [Google Scholar] [CrossRef]
- Hutchings, M.J.; De Kroon, H. Foraging in plants: The role of morphological plasticity in resource acquisition. Adv. Ecol. Res. 1994, 25, 159–238. [Google Scholar] [CrossRef]
- Vallejo-Marin, M.; Dorken, M.E.; Barrett, S.C.H. The ecological and evolutionary consequences of clonality for plant mating. Annu. Rev. Ecol. Evol. Syst. 2010, 41, 193–213. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, D.L.; Xing, F.; Liu, J.S.; Wang, L. Combined effects of resource heterogeneity and simulated herbivory on plasticity of clonal integration in a rhizomatous perennial herb. Plant Biol. 2014, 16, 774–782. [Google Scholar] [CrossRef]
- Rodríguez, J.; Calbi, M.; Roiloa, S.R.; González, L. Herbivory induced non-local responses of the clonal invader Carpobrotus edulis are not mediated by clonal integration. Sci. Total Environ. 2018, 633, 1041–1050. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, D.; Ba, L.; Bai, Y.; Liu, B. Interactions between herbivory and resource availability on grazing tolerance of Leymus chinensis. Environ. Exp. Bot. 2008, 63, 113–122. [Google Scholar] [CrossRef]
- Mcnaughton, S.J. Compensatory plant growth as a response to herbivory. Oikos 1983, 40, 329–336. [Google Scholar] [CrossRef]
- Van Staalduinen, M.A.; Anten, N.P.R. Differences in the compensatory growth of two co-occurring grass species in relation to water availability. Oecologia 2005, 146, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Detling, J.K.; Dyer, M.I.; Winn, D.T. Net photosynthesis, root respiration, and regrowth of Bouteloua gracilis following simulated grazing. Oecologia 1979, 41, 127–134. [Google Scholar] [CrossRef]
- Anten, N.P.R.; Ackerly, D.D. Canopy-level photosynthetic compensation after defoliation in a tropical understory palm. Funct. Ecol. 2001, 15, 252–262. [Google Scholar] [CrossRef]
- Briske, D.D.; Boutton, T.W.; Wang, Z. Contribution of flexible allocation priorities to herbivory tolerance in C4 perennial grasses: An evaluation with 13C labeling. Oecologia 1996, 105, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Chen, S.P.; Lin, G.H. Compensatory growth responses to clipping defoliation in Leymus chinensis (Poaceae) under nutrient addition and water deficiency conditions. Plant Ecol. 2008, 196, 85–99. [Google Scholar] [CrossRef]
- Hay, M.J.M.; Newton, P.C.D. Effect of severity of defoliation on the viability of reproductive and vegetative axillary buds of Trifolium repens L. Ann. Bot. 1996, 78, 117–123. [Google Scholar] [CrossRef][Green Version]
- Liu, H.D.; Yu, F.H.; He, W.M.; Chu, Y.; Dong, M. Clonal integration improves compensatory growth in heavily grazed ramet populations of two inland-dune grasses. Flora – Morphol. Distrib. Funct. Ecol. Plants 2009, 204, 298–305. [Google Scholar] [CrossRef]
- Anten, N.P.R.; Martinez-Ramos, M.; Ackerly, D.D. Defoliation and growth in an understory palm: Quantifying the contributions of compensatory responses. Ecology 2003, 84, 2905–2918. [Google Scholar] [CrossRef]
- Coughenour, M.B.; Detling, J.K.; Bamberg, I.E.; Mugambi, M.M. Production and nitrogen response of the African dwarf shrub Indigofera spinosa to defoliation and water limitation. Oecologia 1990, 83, 546–552. [Google Scholar] [CrossRef]
- Xu, L.; Yu, F.H.; Drunen, E.V.; Schieving, F.; Dong, M.; Anten, N.P.R. Trampling, defoliation and physiological integration affect growth, morphological and mechanical properties of a root-suckering clonal tree. Ann. Bot. 2012, 109, 1001–1008. [Google Scholar] [CrossRef]
- Roiloa, S.R.; Retuerto, R. Responses of the clonal Fragaria vesca to microtopographic heterogeneity under different water and light conditions. Environ. Exp. Bot. 2007, 61, 1–9. [Google Scholar] [CrossRef]
- Roiloa, S.R.; Rodríguez-Echeverría, S.; De La Peña, E.; Freitas, H. Physiological integration increases the survival and growth of the clonal invader Carpobrotus edulis. Biol. Invasions 2010, 12, 1815–1823. [Google Scholar] [CrossRef]
- You, W.H.; Yu, D.; Xie, D.; Han, C.M.; Liu, C.H. The invasive plant Alternanthera philoxeroides benefits from clonal integration in response to defoliation. Flora 2014, 209, 666–673. [Google Scholar] [CrossRef]
- Schmid, B.; Puttick, G.M.; Burgess, K.H.; Bazzaz, F.A. Clonal integration and effects of simulated herbivory in old-field perennials. Oecologia 1988, 75, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.W.; Li, L.G.; Han, X.G.; Dong, M. Do rhizome severing and shoot defoliation affect clonal growth of Leymus chinensis at ramet population level? Acta Oecol. 2004, 26, 255–260. [Google Scholar] [CrossRef]
- Guo, J.; Li, H.; Yang, Y. Phenotypic plasticity in sexual reproduction based on nutrients supplied from vegetative ramets in a Leymus chinensis population. Front Plant Sci. 2019, 10, 1681. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.C. Biological Ecology of Leymus Chinensis; Jilin Science & Technology: Changchun, China, 2004. [Google Scholar]
- Wang, D.L.; Ba, L. Ecology of meadow steppe in northeast China. Rangeland J. 2008, 30, 247–254. [Google Scholar] [CrossRef]
- Zhang, Y.C.; Zhang, Q.Y. Clonal integration of Fragaria orientalis in reciprocal and coincident patchiness resources: Cost-benefit analysis. PLoS ONE 2013, 8, e80623. [Google Scholar] [CrossRef]
- Slade, A.J.; Hutchings, M.J. An analysis of the costs and benefits of physiological integration between ramets in the clonal perennial herb Glechoma hederacea. Oecologia 1987, 73, 425–431. [Google Scholar] [CrossRef]
- Bullock, J.M.; Mortimer, A.M.; Begon, M. Physiological integration among tillers of Holcus lanatus: Age-dependence and responses to clipping and competition. New Phytol. 1994, 128, 737–747. [Google Scholar] [CrossRef]
- Březina, S.; Koubek, T.; Münzbergová, Z.; Herben, T. Ecological benefits of integration of Calamagrostis epigejos ramets under field conditions. Flora 2006, 201, 461–467. [Google Scholar] [CrossRef]
- Pitelka, L.F.; Ashmun, J.W. Physiology and Integration of Ramets in Clonal plants. In Population Biology and Evolution of Clonal Organisms; Yale University Press: New Haven, CT, USA, 1985; pp. 399–436. [Google Scholar]
- Olson, B.E.; Wallander, R.T. Carbon allocation in Euphorbia esula and neighbours after defoliation. Can. J. Bot. 2000, 77, 1641–1647. [Google Scholar] [CrossRef]
- Hellström, K.; Kytöviita, M.M.; Tuomi, J.; Rautio, P. Plasticity of clonal integration in the perennial herb Linaria vulgaris after damage. Funct. Ecol. 2006, 20, 413–420. [Google Scholar] [CrossRef]
- Wang, P.; Alpert, P.; Yu, F.H. Clonal integration affects allocation in the perennial herb Alternanthera philoxeroides in N-limited, homogeneous environments. Folia Geobot. 2017, 52, 303–315. [Google Scholar] [CrossRef]
- Roiloa, S.R.; Antelo, B.; Retuerto, R. Physiological integration modifies 15N in the clonal plant Fragaria vesca, suggesting preferential transport of nitrogen to water-stressed offspring. Ann. Bot. 2014, 114, 399–411. [Google Scholar] [CrossRef]
- Stuefer, J.F.; During, H.J.; De Kroon, H. High benefits of clonal integration in two stoloniferous species, in response to heterogeneous light environments. J. Ecol. 1994, 82, 511–518. [Google Scholar] [CrossRef]
- Janeček, S.; Kantorová, J.; Bartos, M.; Klimešová, J. Integration in the clonal plant Eriophorum angustifolium: An experiment with a three-member-clonal system in a patchy environment. Evol. Ecol. 2008, 22, 325–336. [Google Scholar] [CrossRef]
- You, W.H.; Fan, S.F.; Yu, D.; Xie, D.; Liu, C.H. An invasive clonal plant benefits from clonal integration more than a co-occurring native plant in nutrient-patchy and competitive environments. PLoS ONE 2014, 9, e97246. [Google Scholar] [CrossRef]
- Olejniczak, P. Optimal allocation to vegetative and sexual reproduction in plants: The effect of ramet density. Evol. Ecol. 2003, 17, 265–275. [Google Scholar] [CrossRef]
- Obeso, J.R. The cost of reproduction in plants. New Phytol. 2002, 155, 321–348. [Google Scholar] [CrossRef]
- Loehle, C. Partitioning of reproductive effort in clonal plants: A benefit-cost model. Oikos 1987, 49, 199–208. [Google Scholar] [CrossRef]
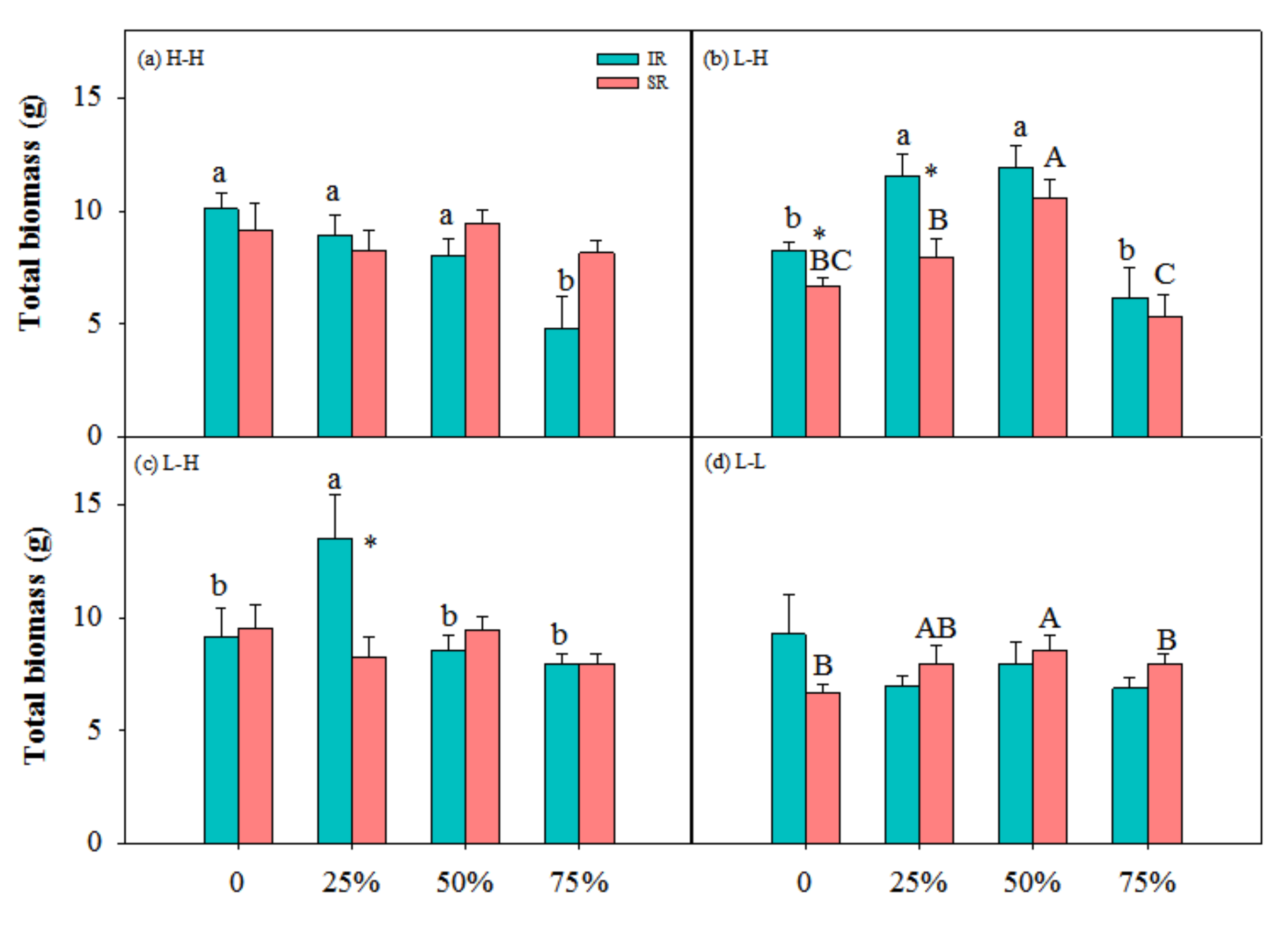
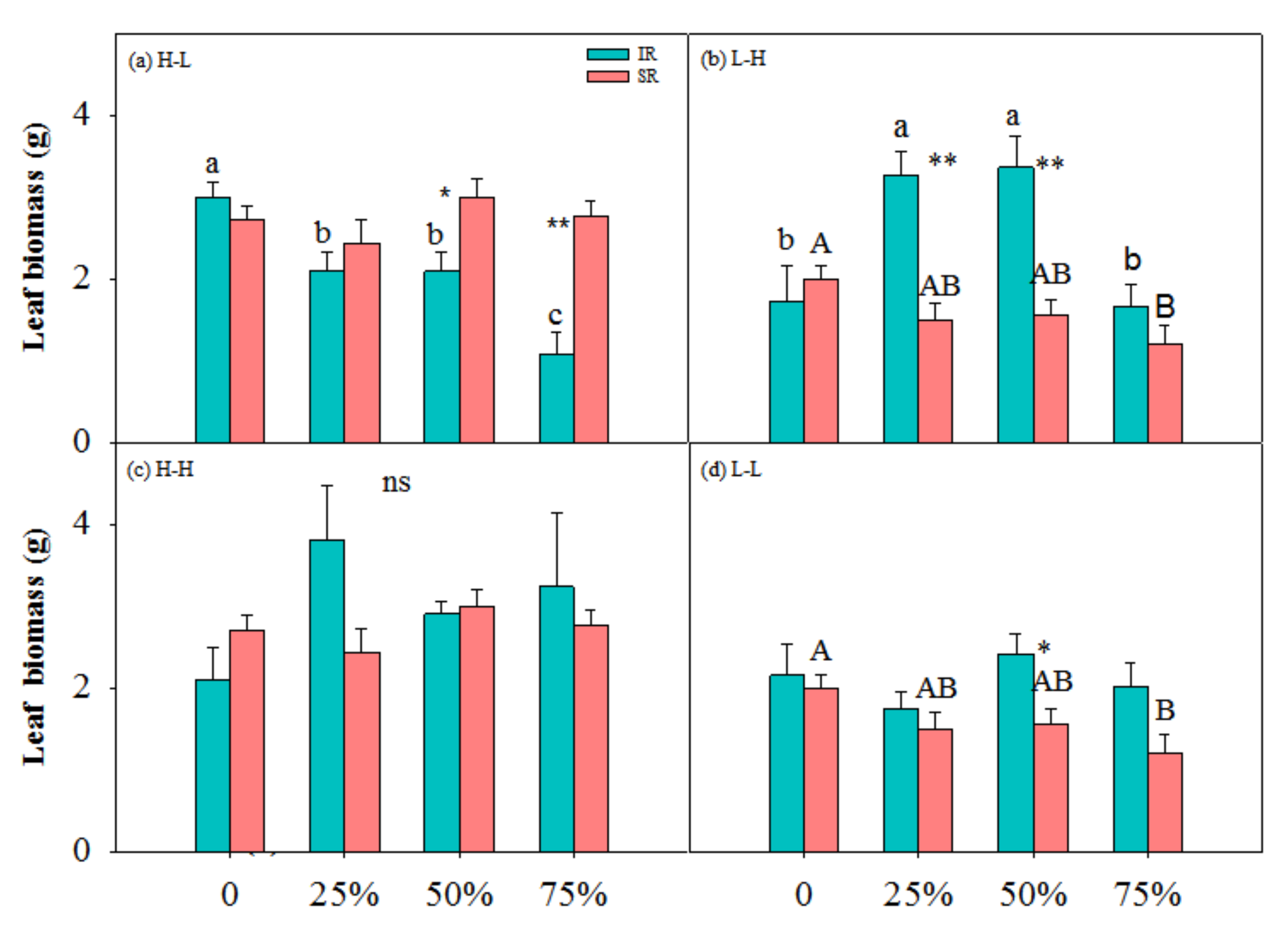

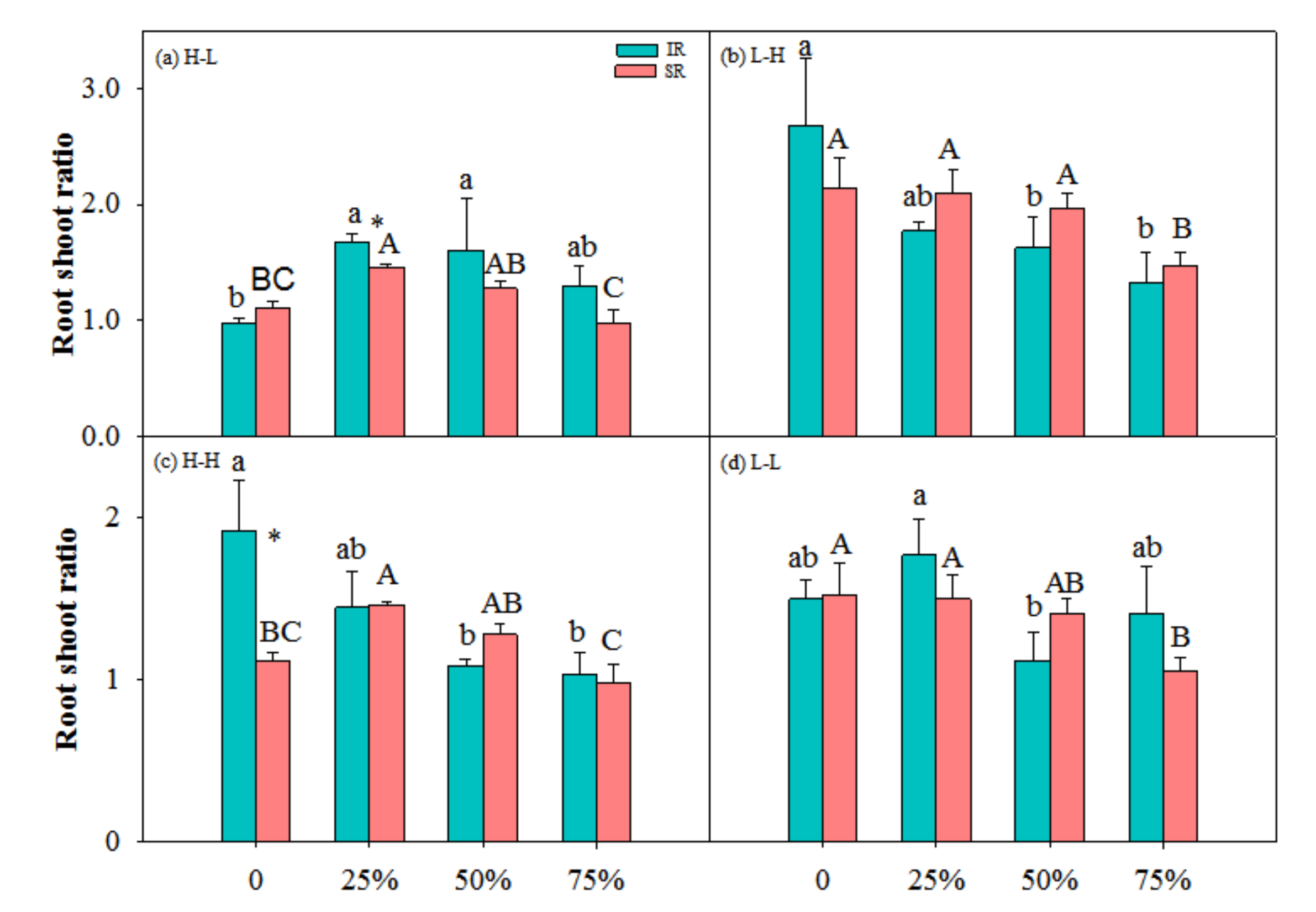
| Treatment | TB (g) | LB (g) | SB (g) | R/S | |||||
|---|---|---|---|---|---|---|---|---|---|
| df | F | p | F | p | F | p | F | p | |
| Integration (I) | 1 | 8.709 | 0.004 ** | 3.331 | 0.070 | 4.020 | 0.047 * | 1.941 | 0.166 |
| Clipping (C) | 3 | 5.725 | 0.001 ** | 2.486 | 0.064 | 2.772 | 0.044 * | 2.278 | 0.083 |
| Nutrient (N) | 3 | 1.495 | 0.219 | 2.033 | 0.113 | 2.614 | 0.054 | 1.074 | 0.363 |
| I × C | 3 | 1.290 | 0.210 | 3.133 | 0.028 * | 0.834 | 0.477 | 3.339 | 0.022 * |
| I × N | 3 | 4.314 | 0.006 ** | 4.005 | 0.009 ** | 3.107 | 0.029 * | 1.891 | 0.134 |
| C × N | 9 | 3.903 | 0.000 ** | 4.554 | 0.000 ** | 5.069 | 0.000 *** | 1.700 | 0.096 |
| I × C × N | 9 | 0.462 | 0.898 | 1.401 | 0.194 | 1.282 | 0.253 | 2.752 | 0.006 ** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Chen, C.; Pan, Y.; Zhang, Y.; Gao, Y. The Intensity of Simulated Grazing Modifies Costs and Benefits of Physiological Integration in a Rhizomatous Clonal Plant. Int. J. Environ. Res. Public Health 2020, 17, 2724. https://doi.org/10.3390/ijerph17082724
Liu J, Chen C, Pan Y, Zhang Y, Gao Y. The Intensity of Simulated Grazing Modifies Costs and Benefits of Physiological Integration in a Rhizomatous Clonal Plant. International Journal of Environmental Research and Public Health. 2020; 17(8):2724. https://doi.org/10.3390/ijerph17082724
Chicago/Turabian StyleLiu, Jushan, Chen Chen, Yao Pan, Yang Zhang, and Ying Gao. 2020. "The Intensity of Simulated Grazing Modifies Costs and Benefits of Physiological Integration in a Rhizomatous Clonal Plant" International Journal of Environmental Research and Public Health 17, no. 8: 2724. https://doi.org/10.3390/ijerph17082724
APA StyleLiu, J., Chen, C., Pan, Y., Zhang, Y., & Gao, Y. (2020). The Intensity of Simulated Grazing Modifies Costs and Benefits of Physiological Integration in a Rhizomatous Clonal Plant. International Journal of Environmental Research and Public Health, 17(8), 2724. https://doi.org/10.3390/ijerph17082724





