Full-Digital Workflow for Fabricating a Custom-Made Direct Metal Laser Sintering (DMLS) Mandibular Implant: A Case Report
Abstract
1. Background
2. Case Presentation
2.1. Data Acquisition
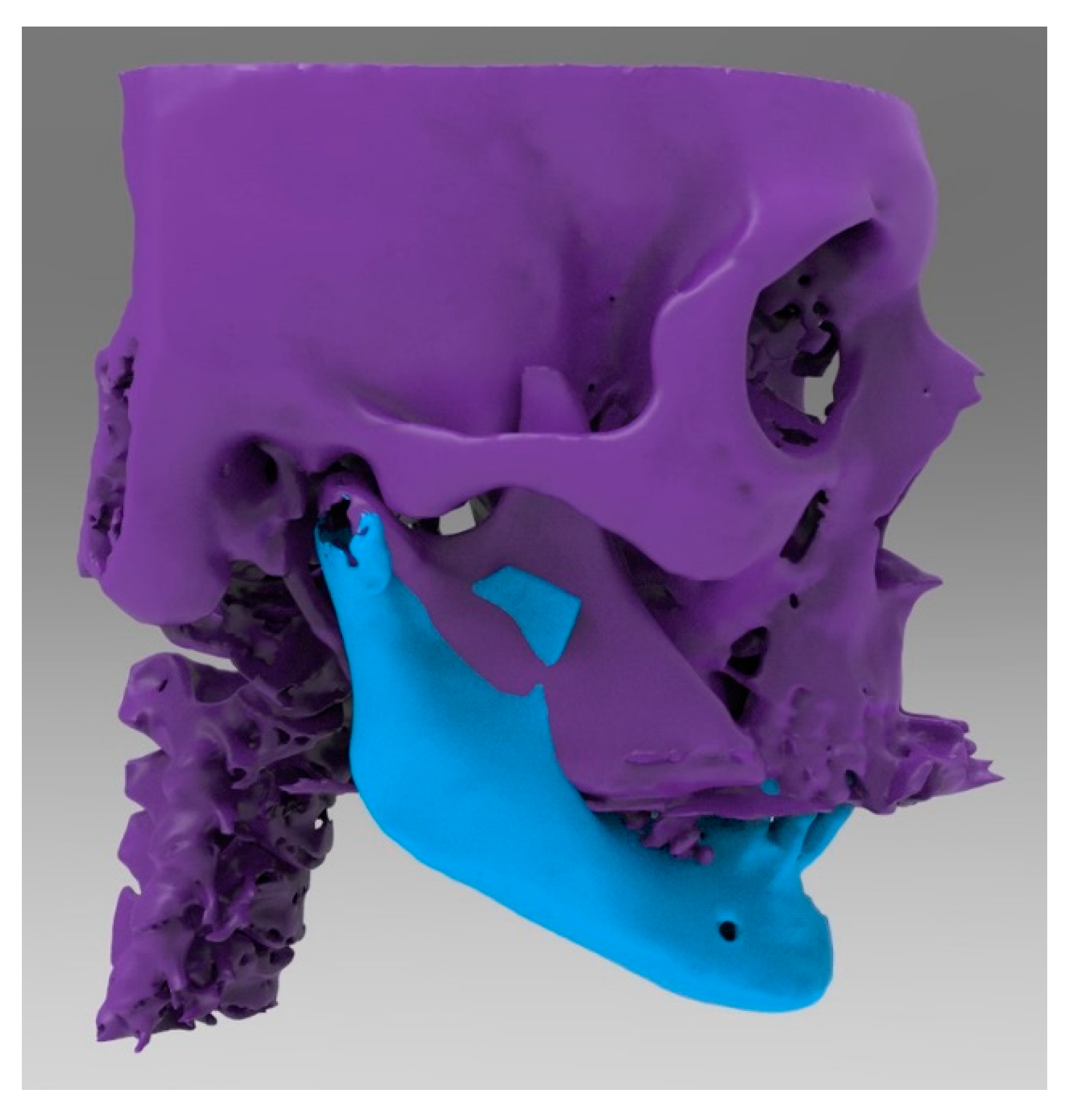
2.2. CAD 3D Modelling
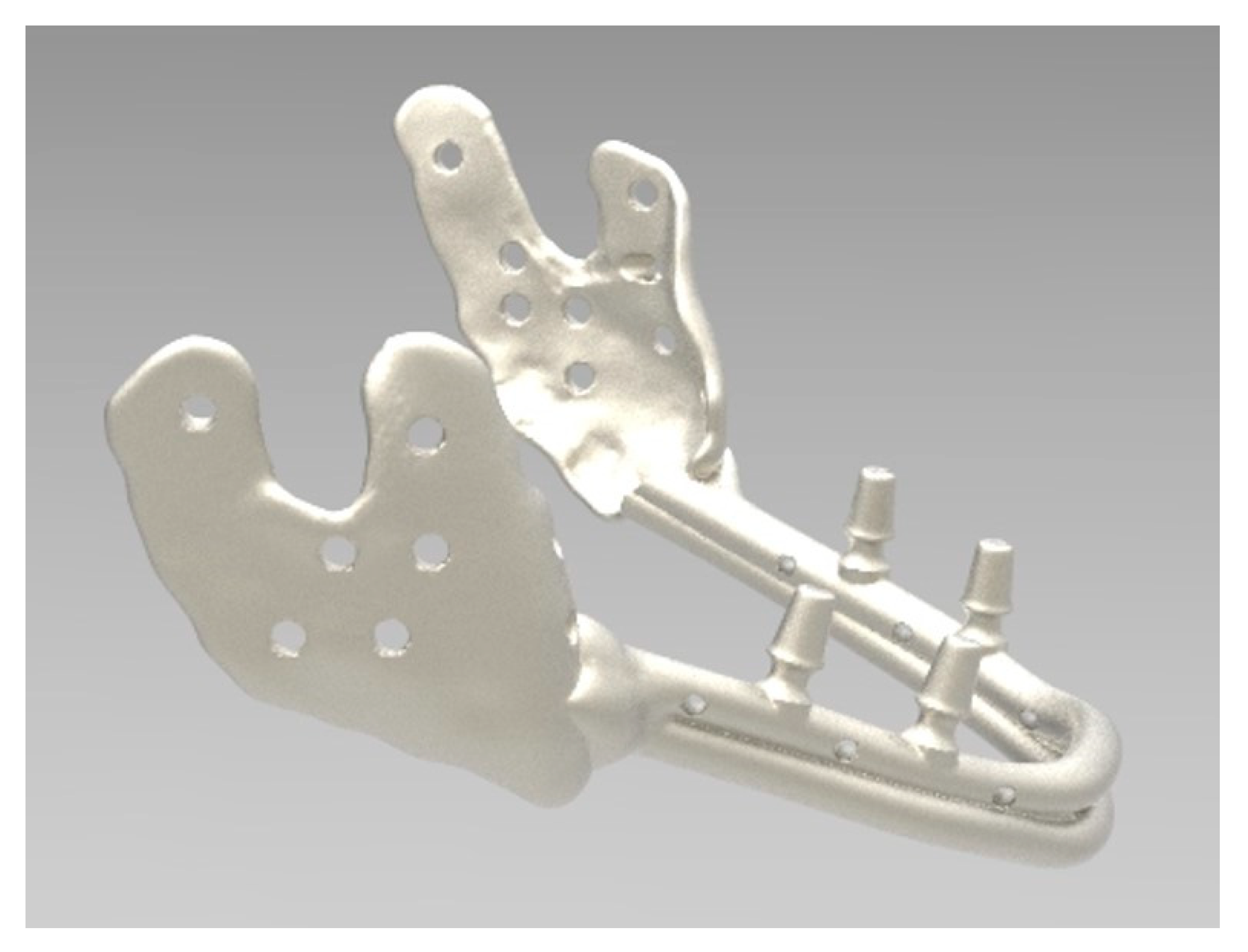
2.3. Direct Metal Laser Sintering (DMLS)
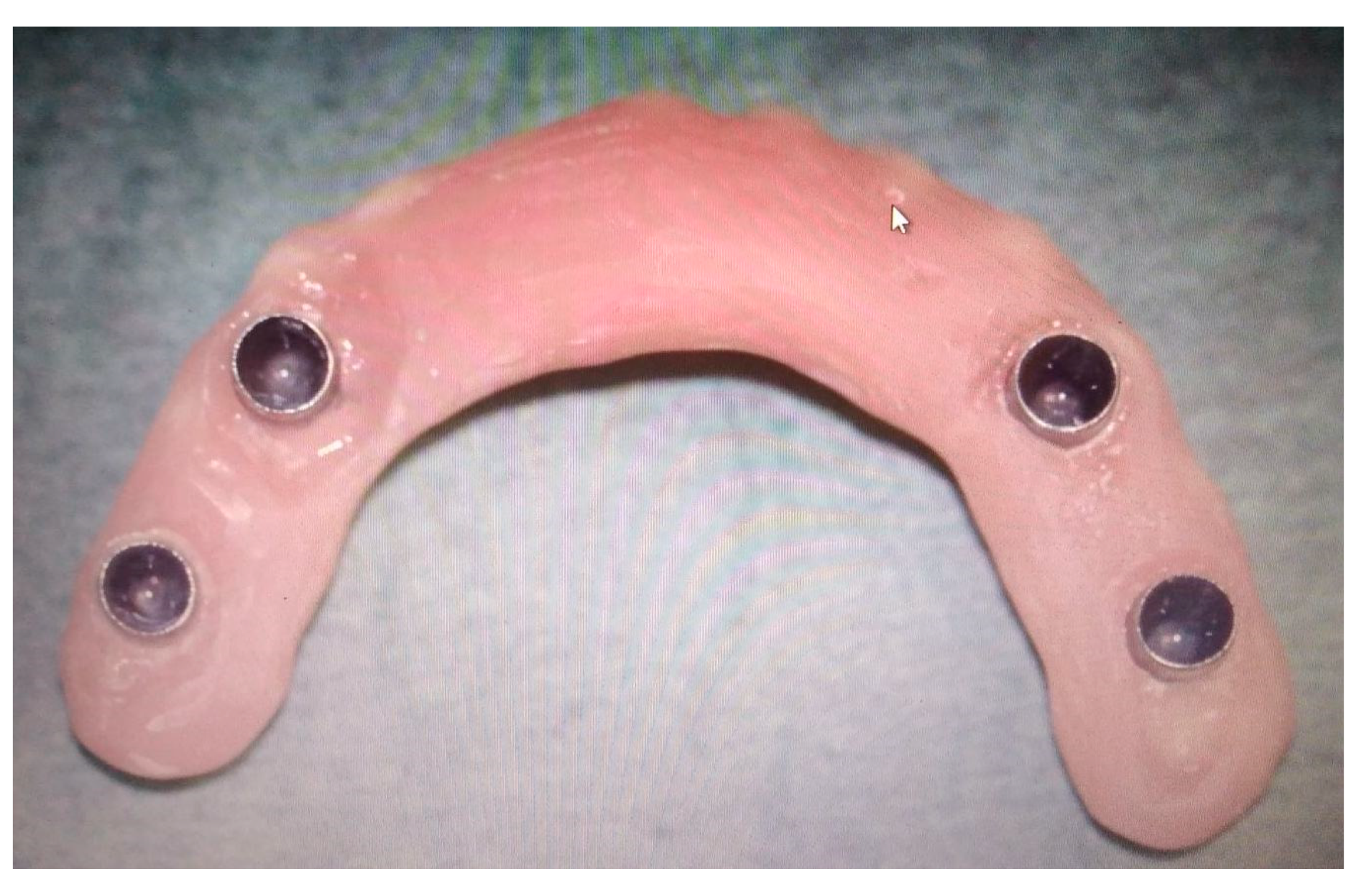
2.4. Post-Production
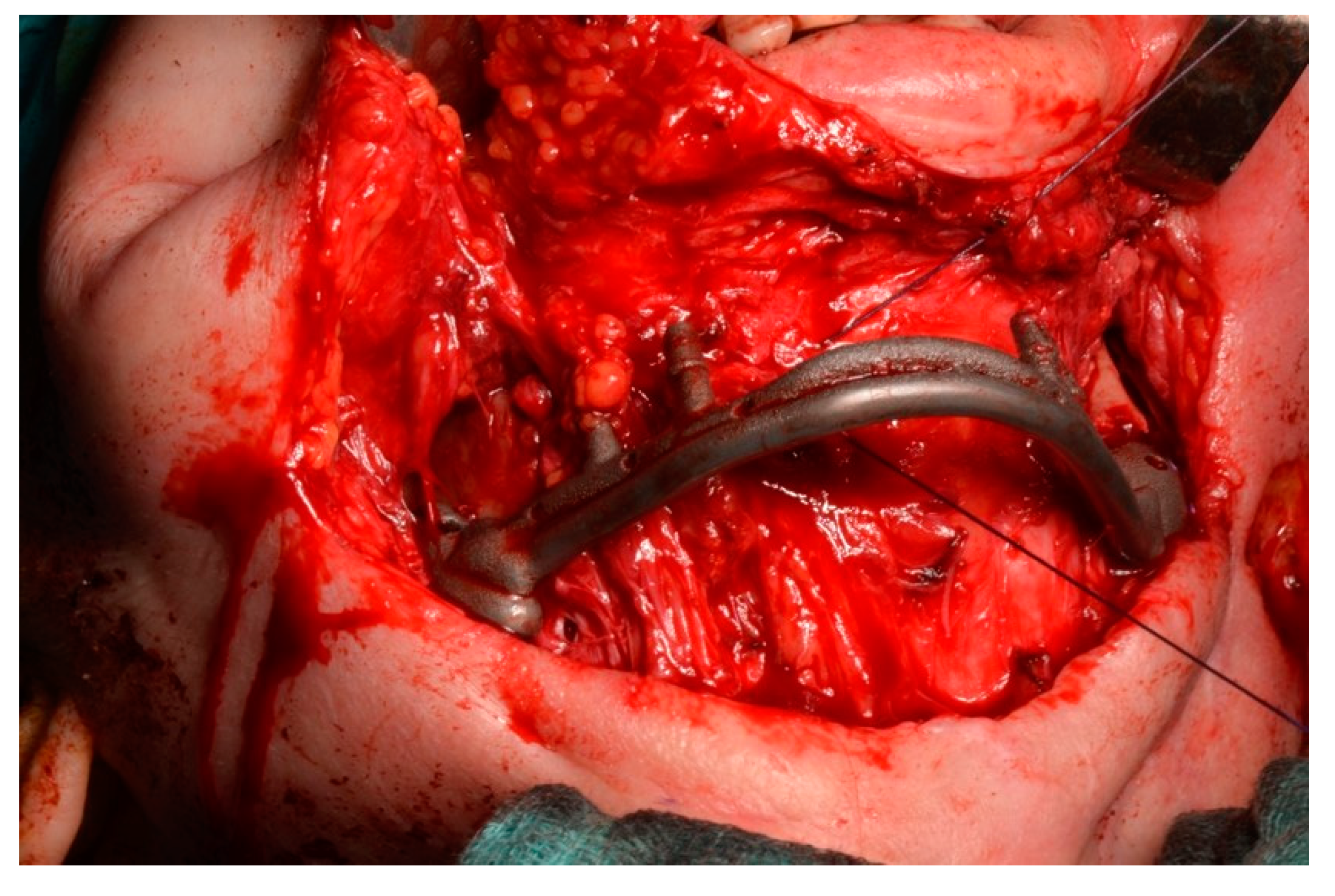
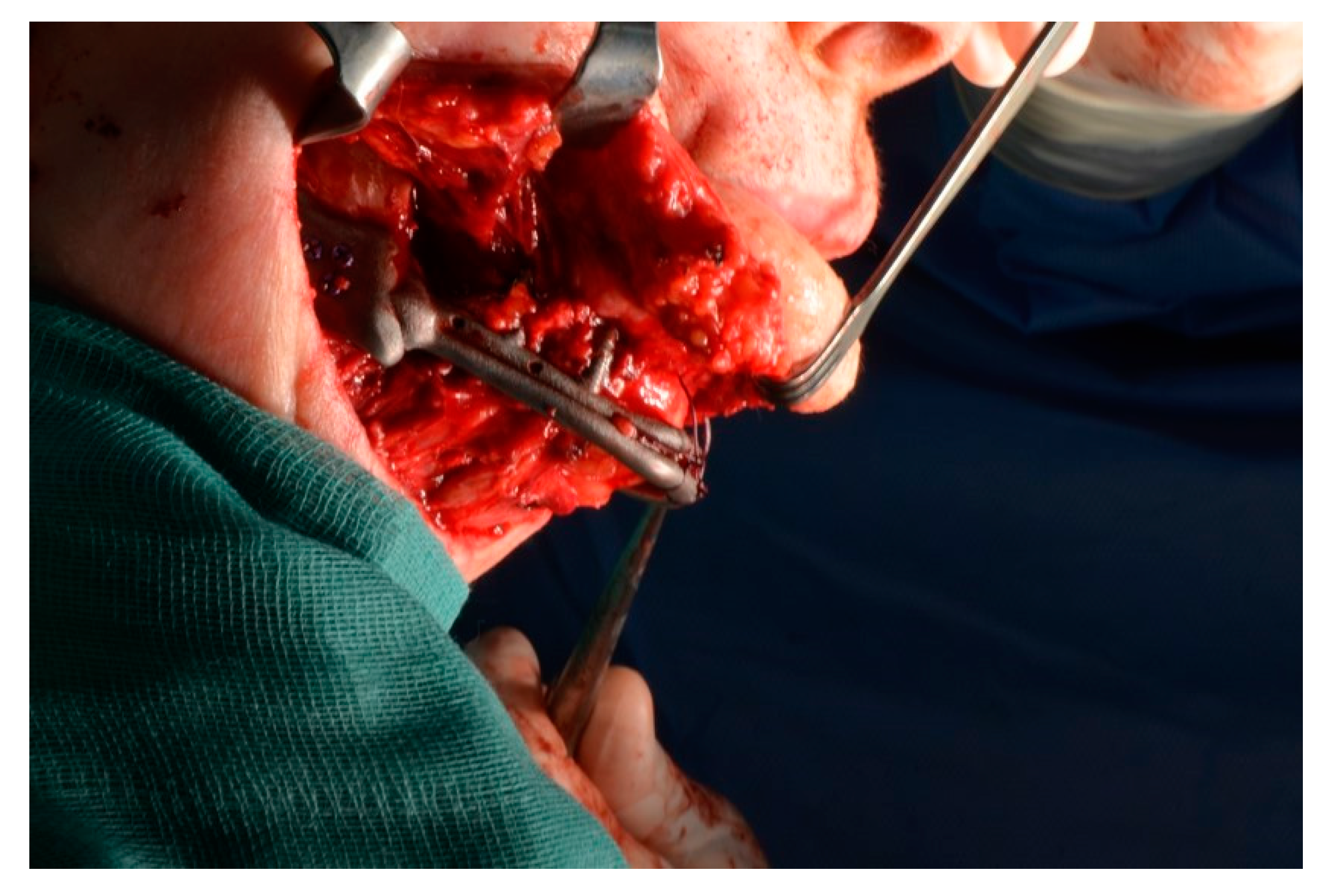
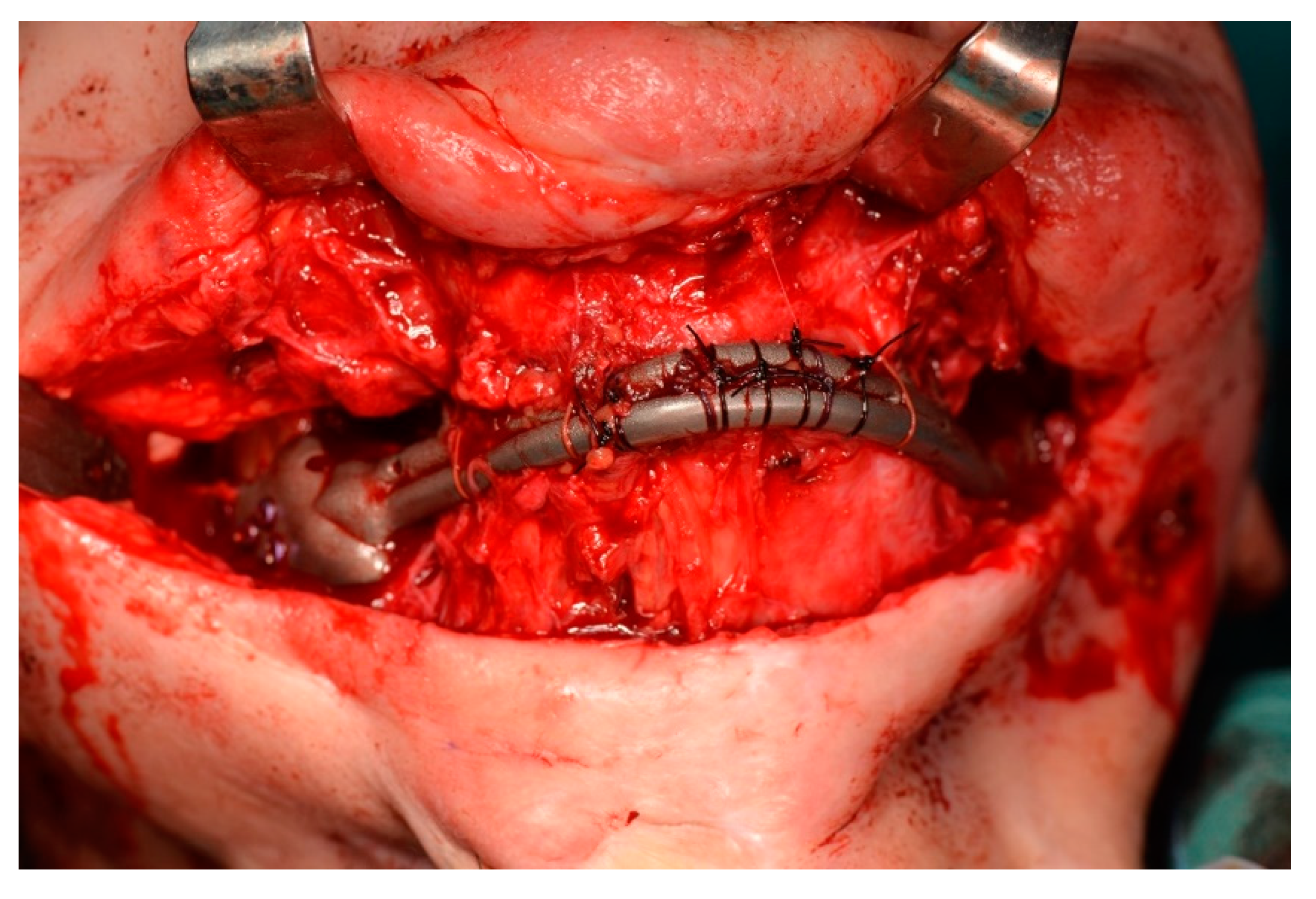
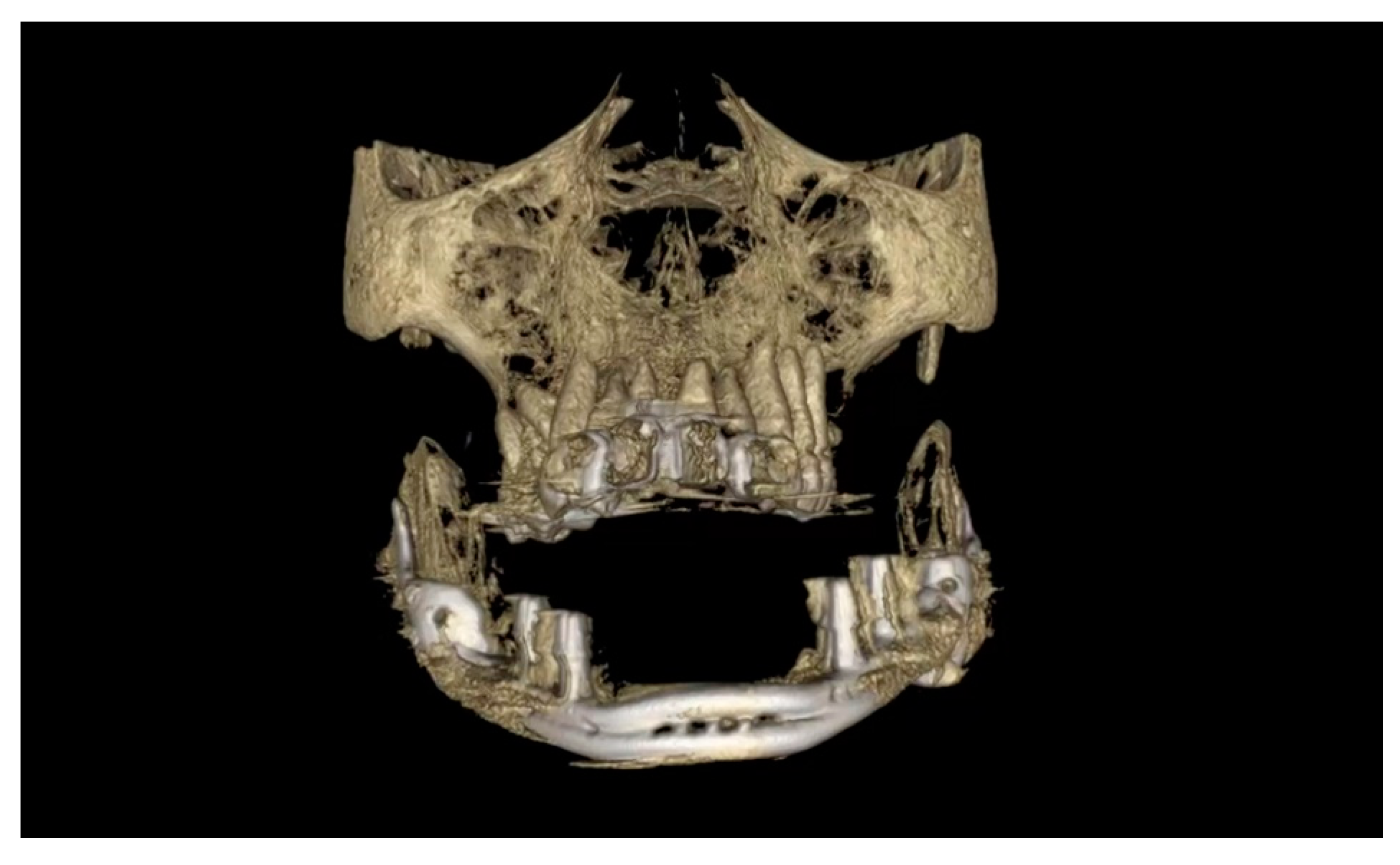
2.5. Surgery
- The prosthesis reflected the original anatomy of the jaw.
- The prosthesis made it possible to suspend soft tissue by holes in the anterior portion.
- The prosthesis had the possibility of osteosynthesis by screws in the posterior portions.
- A temporary tracheostomy to protect the airway was performed.
- A wide cutaneous neck incision was made to achieve the residual portions of the mandible and prepare the surgical field to receive the prosthesis.
- Bilateral coronoidotomy was performed to release the ramus. The structures were rotated and repositioned.
- The jaw prosthesis placement and fixation was completed using screws (Tekka®, Pesaro, Italy) of 2 mm Ø and 7 to 9 mm length to attach them to the mandibular ramus.
- Soft tissue was suspended to the anterior portion of the prosthesis.
- An intraoral mucosa incision was made to expose the stumps for the following dental rehabilitation.
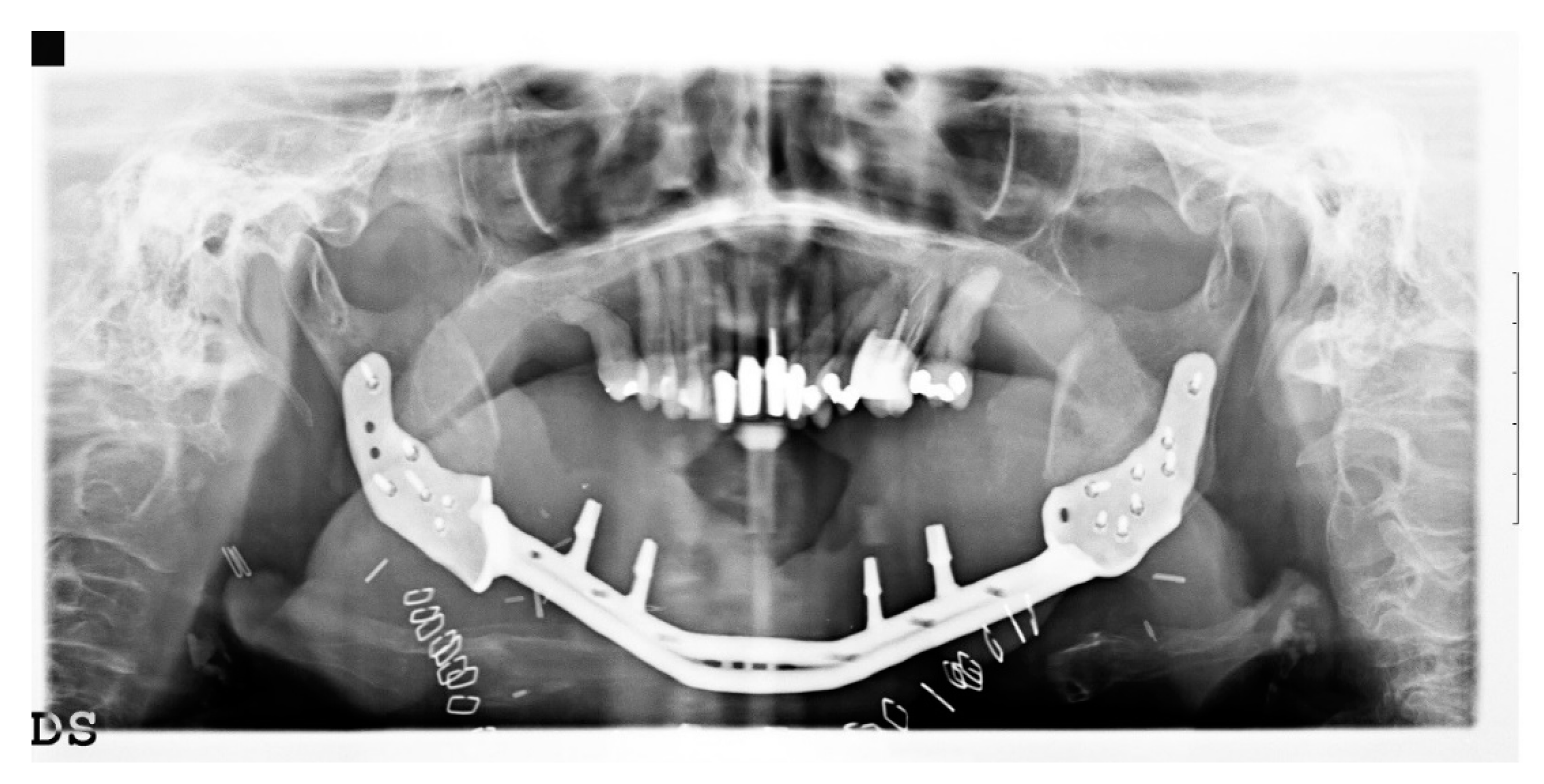
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| DMLS | Direct Metal Laser Sintering |
| AM | Additive Manufacturing |
| CAD/CAM | Computer-aided design/Computer-aided technology |
| CBCT | Cone-beam computed tomography |
| CT | Computerized tomography |
| PCBM | Particulate cancellous bone and marrow |
References
- Yu, Y.; Zhang, W.B.; Liu, X.J.; Guo, C.B.; Yu, G.Y.; Peng, X. Double-Barrel Fibula Flap Versus Vascularized Iliac Crest Flap for Mandibular Reconstruction. J. Oral Maxillofac. Surg. 2020. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.C.; Yoon, J.H.; Choi, B.; Lee, J. Mandibular reconstruction with autologous human bone marrow stem cells and autogenous bone graft in a patient with plexiform ameloblastoma. J. Craniofac. Surg. 2013, 24, e409–e411. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.S.; Lowe, D.; Kanatas, A.; Schache, A. Mandibular reconstruction with vascularised bone flaps: A systematic review over 25 years. Br. J. Oral Maxillofac. Surg. 2017, 55, 113–126. [Google Scholar] [CrossRef]
- Farronato, M.; Maspero, C.; Lanteri, V.; Fama, A.; Ferrati, F.; Pettenuzzo, A.; Farronato, D. Current state of the art in the use of augmented reality in dentistry: A systematic review of the literature. BMC Oral Health 2019, 19, 135. [Google Scholar] [CrossRef] [PubMed]
- Jardini, A.L.; Larosa, M.A.; Maciel Filho, R.; Zavaglia, C.A.; Bernardes, L.F.; Lambert, C.S.; Calderoni, D.R.; Kharmandayan, P. Cranial reconstruction: 3D biomodel and custom-built implant created using additive manufacturing. J. Cranio-Maxillofac. Surg. 2014, 42, 1877–1884. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, A.; Badiali, G.; Piersanti, L.; Marchetti, C. Computer-assisted piezoelectric surgery: A navigated approach toward performance of craniomaxillofacial osteotomies. J. Craniofac. Surg. 2015, 26, 867–872. [Google Scholar] [CrossRef]
- Mangano, A.; Beretta, M.; Luongo, G.; Mangano, C.; Mangano, F. Conventional Vs Digital Impressions: Acceptability, Treatment Comfort and Stress among Young Orthodontic Patients. Open Dent. J. 2018, 12, 118–124. [Google Scholar] [CrossRef]
- Traini, T.; Mangano, C.; Sammons, R.L.; Mangano, F.; Macchi, A.; Piattelli, A. Direct laser metal sintering as a new approach to fabrication of an isoelastic functionally graded material for manufacture of porous titanium dental implants. Dent. Mater. 2008, 24, 1525–1533. [Google Scholar] [CrossRef]
- Mangano, F.; Chambrone, L.; van Noort, R.; Miller, C.; Hatton, P.; Mangano, C. Direct metal laser sintering titanium dental implants: A review of the current literature. Int. J. Biomater. 2014, 2014, 461534. [Google Scholar] [CrossRef]
- Ikawa, T.; Shigeta, Y.; Hirabayashi, R.; Hirai, S.; Hirai, K.; Harada, N.; Kawamura, N.; Ogawa, T. Computer assisted mandibular reconstruction using a custom-made titan mesh tray and removable denture based on the top-down treatment technique. J. Prosthodont. Res. 2016, 60, 321–331. [Google Scholar] [CrossRef]
- Roos, M.E.; Claassen, J.; Booysen, G.; van den Heever, J.; Seedat, R.Y. 3D printed titanium prosthesis reconstruction following subtotal maxillectomy for myoepithelial carcinoma—A case report. J. Stomatol. Oral Maxillofac. Surg. 2019. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, S.; Ricotta, F.; Maiolo, V.; Savastio, G.; Contedini, F.; Cipriani, R.; Bortolani, B.; Cercenelli, L.; Marcelli, E.; Marchetti, C.; et al. Computer-assisted surgery for reconstruction of complex mandibular defects using osteomyocutaneous microvascular fibular free flaps: Use of a skin paddle-outlining guide for soft-tissue reconstruction. A technical report. J. Cranio-Maxillofac. Surg. 2019, 47, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Batstone, M.D. Reconstruction of major defects of the jaws. Aust. Dent. J. 2018, 63 (Suppl. 1), S108–S113. [Google Scholar] [CrossRef] [PubMed]
- Fawzy, H.H.; Choi, J.W. Evaluation of virtual surgical plan applicability in 3D simulation-guided two-jaw surgery. J. Cranio-Maxillofac. Surg. 2019, 47, 860–866. [Google Scholar] [CrossRef] [PubMed]
- Rana, M.; Chin, S.J.; Muecke, T.; Kestin, M.; Groebe, A.; Riecke, B.; Heiland, M.; Gellrich, N.C. Increasing the accuracy of mandibular reconstruction with free fibula flaps using functionalized selective laser-melted patient-specific implants: A retrospective multicenter analysis. J. Cranio-Maxillofac. Surg. 2017, 45, 1212–1219. [Google Scholar] [CrossRef]
- Dolgolev, A.; Reshetov, I.; Svyatoslavov, D.; Sinelnikov, M.; Kudrin, K.; Dub, V.; Anikin, V. Experimental Biointegration of a Titanium Implant in Delayed Mandibular Reconstruction. J. Pers. Med. 2020, 10, 6. [Google Scholar] [CrossRef]
- Luu, K.; Pakdel, A.; Wang, E.; Prisman, E. In house virtual surgery and 3D complex head and neck reconstruction. J. Otolaryngol.-Head Neck Surg. 2018, 47, 75. [Google Scholar] [CrossRef]
- Abbate, V.; Orabona, G.D.A.; Solari, D.; Bonavolontà, P.; Iaconetta, G.; Califano, L. Mandibular Surgical Navigation: An Innovative Guiding Method. J. Craniofac. Surg. 2017, 28, 2122–2126. [Google Scholar] [CrossRef]
- Patini, R.; Mangino, G.; Martellacci, L.; Quaranta, G.; Masucci, L.; Gallenzi, P. The Effect of Different Antibiotic Regimens on Bacterial Resistance: A Systematic Review. Antibiotics (Basel) 2020, 9, 22. [Google Scholar] [CrossRef]
- Martellacci, L.; Quaranta, G.; Patini, R.; Isola, G.; Gallenzi, P.; Masucci, L. A Literature Review of Metagenomics and Culturomics of the Peri-implant Microbiome: Current Evidence and Future Perspectives. Materials (Basel) 2019, 12, 3010. [Google Scholar] [CrossRef]
- Tarsitano, A.; Battaglia, S.; Ricotta, F.; Bortolani, B.; Cercenelli, L.; Marcelli, E.; Cipriani, R.; Marchetti, C. Accuracy of CAD/CAM mandibular reconstruction: A three-dimensional, fully virtual outcome evaluation method. J. Cranio-Maxillofac. Surg. 2018, 46, 1121–1125. [Google Scholar] [CrossRef] [PubMed]
- Huo, J.; Dérand, P.; Rännar, L.E.; Hirsch, J.M.; Gamstedt, E.K. Failure location prediction by finite element analysis for an additive manufactured mandible implant. Med. Eng. Phys. 2015, 37, 862–869. [Google Scholar] [CrossRef] [PubMed]








© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grecchi, F.; Zecca, P.A.; Macchi, A.; Mangano, A.; Riva, F.; Grecchi, E.; Mangano, C. Full-Digital Workflow for Fabricating a Custom-Made Direct Metal Laser Sintering (DMLS) Mandibular Implant: A Case Report. Int. J. Environ. Res. Public Health 2020, 17, 2693. https://doi.org/10.3390/ijerph17082693
Grecchi F, Zecca PA, Macchi A, Mangano A, Riva F, Grecchi E, Mangano C. Full-Digital Workflow for Fabricating a Custom-Made Direct Metal Laser Sintering (DMLS) Mandibular Implant: A Case Report. International Journal of Environmental Research and Public Health. 2020; 17(8):2693. https://doi.org/10.3390/ijerph17082693
Chicago/Turabian StyleGrecchi, Francesco, Piero Antonio Zecca, Aldo Macchi, Alessandro Mangano, Federica Riva, Emma Grecchi, and Carlo Mangano. 2020. "Full-Digital Workflow for Fabricating a Custom-Made Direct Metal Laser Sintering (DMLS) Mandibular Implant: A Case Report" International Journal of Environmental Research and Public Health 17, no. 8: 2693. https://doi.org/10.3390/ijerph17082693
APA StyleGrecchi, F., Zecca, P. A., Macchi, A., Mangano, A., Riva, F., Grecchi, E., & Mangano, C. (2020). Full-Digital Workflow for Fabricating a Custom-Made Direct Metal Laser Sintering (DMLS) Mandibular Implant: A Case Report. International Journal of Environmental Research and Public Health, 17(8), 2693. https://doi.org/10.3390/ijerph17082693







