Aerobic Exercise Inhibits CUMS-Depressed Mice Hippocampal Inflammatory Response via Activating Hippocampal miR-223/TLR4/MyD88-NF-κB Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Instruments and Reagents
2.2. Animal Processing and Grouping
2.3. CUMS Depression Mouse Model
2.4. Aerobic Exercise Protocol
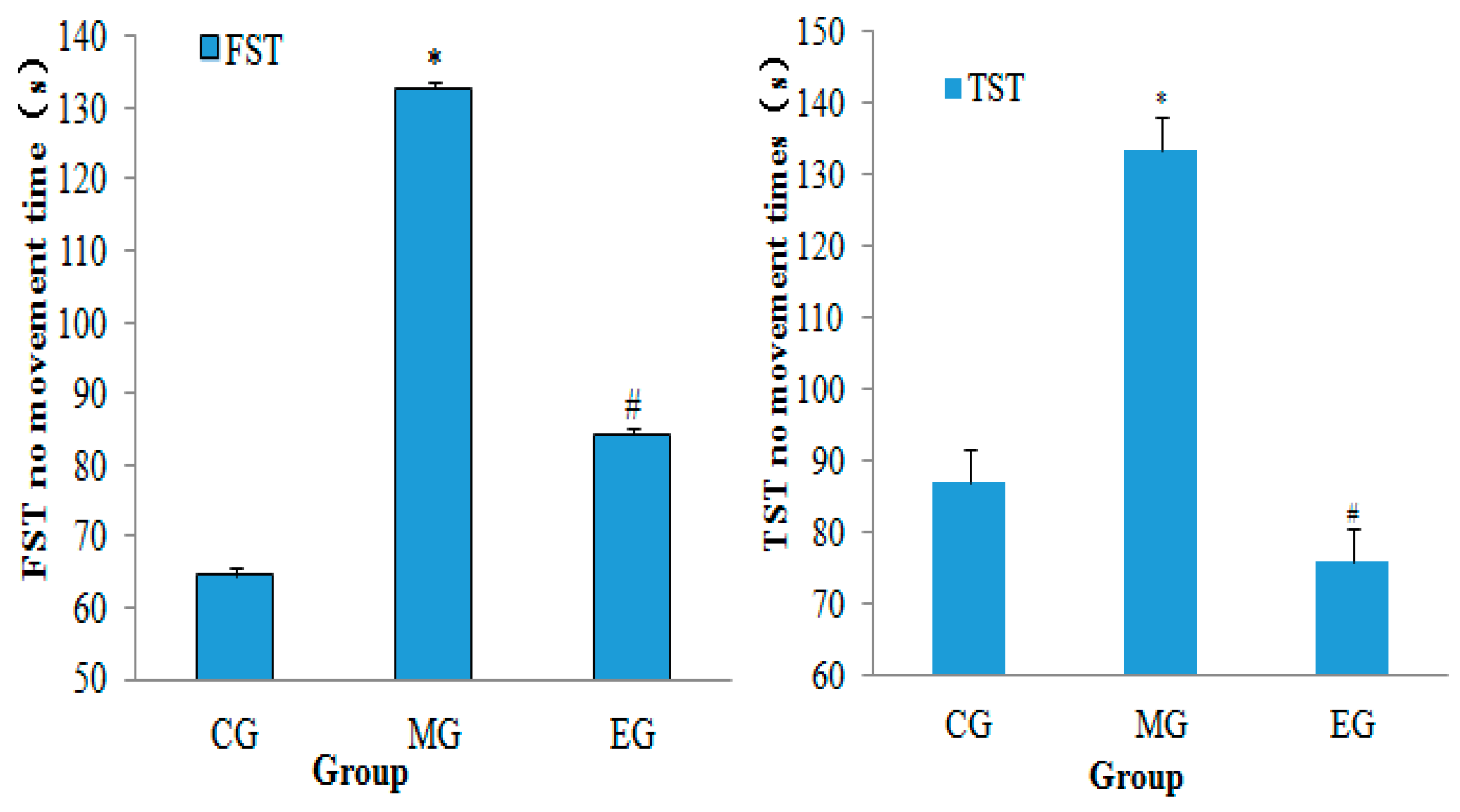
2.5. Neurobehavioral Assessment
2.6. Sample Processing
2.7. Enzyme-linked Immunosorbent Assay (ELISA)
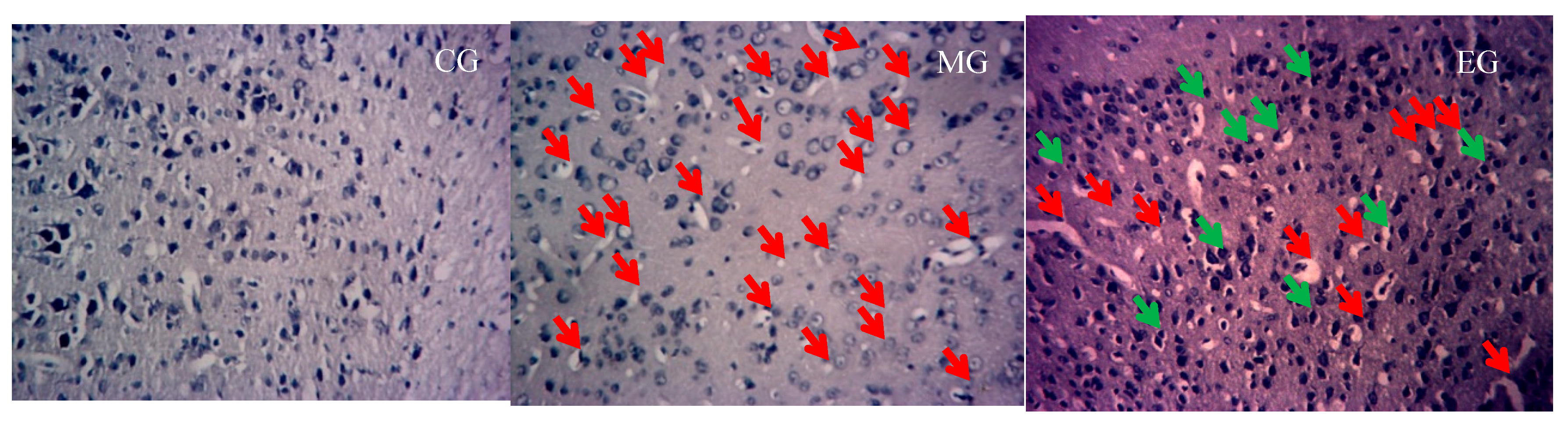
2.8. Nissl Staining
2.9. Immunohistochemistry Staining
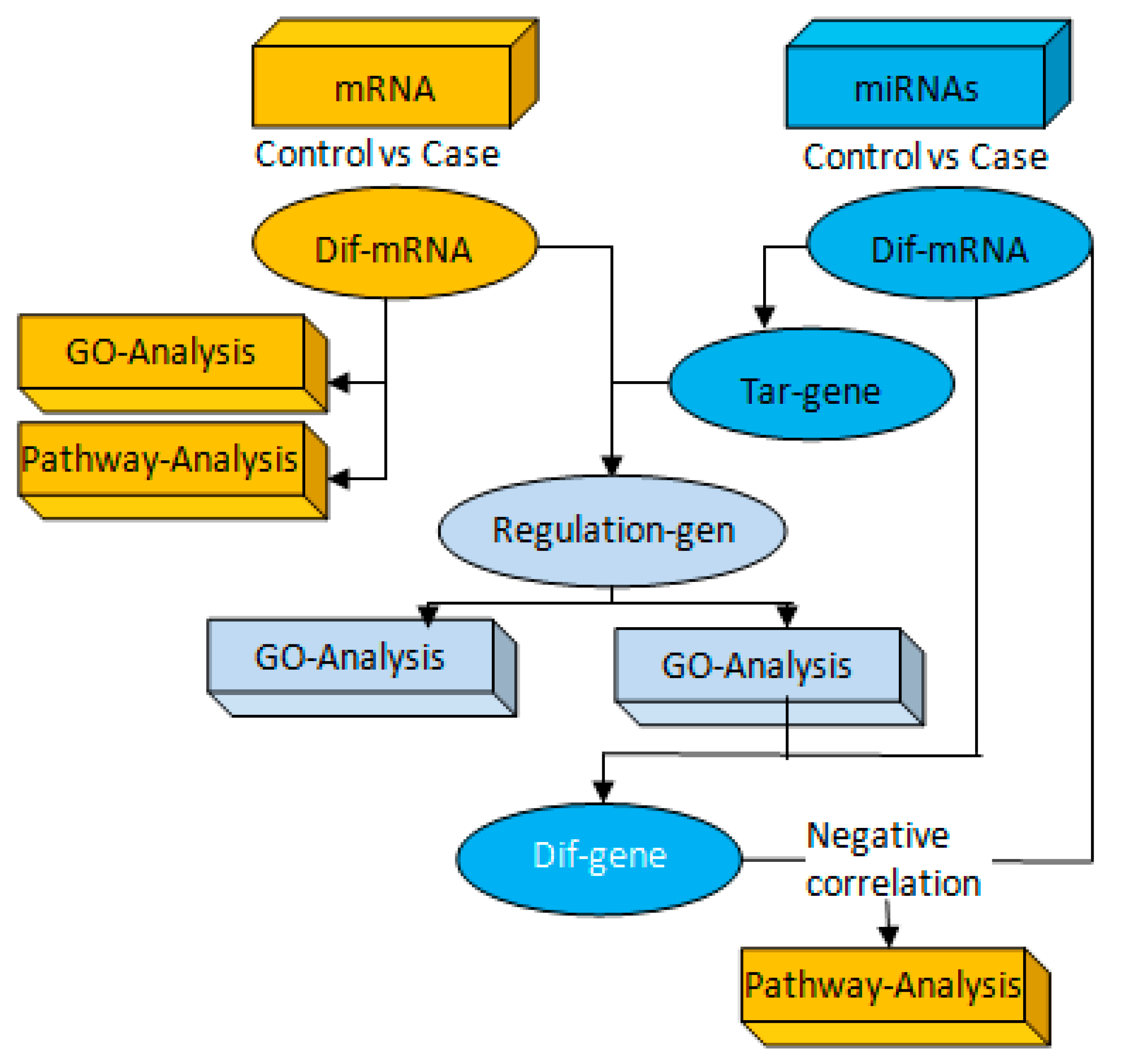
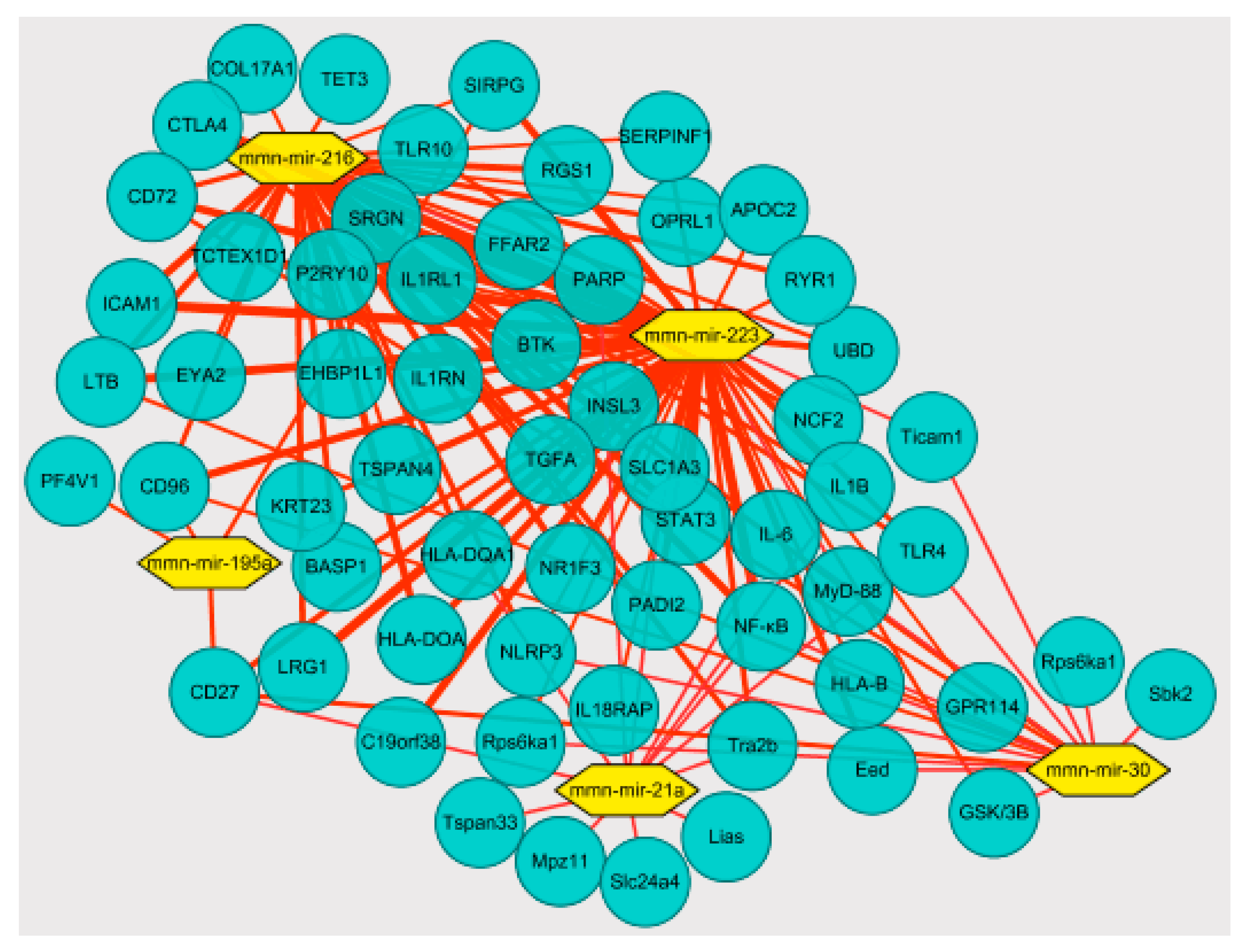
2.10. Association Analysis of miRNAs and mRNA Targeting Regulation
2.11. RT–PCR
2.12. Western Blot Analysis
2.13. Statistics
3. Results
3.1. Neurobehavioral Assessment
3.2. The Changes of Nissle Body in the Hippocampus
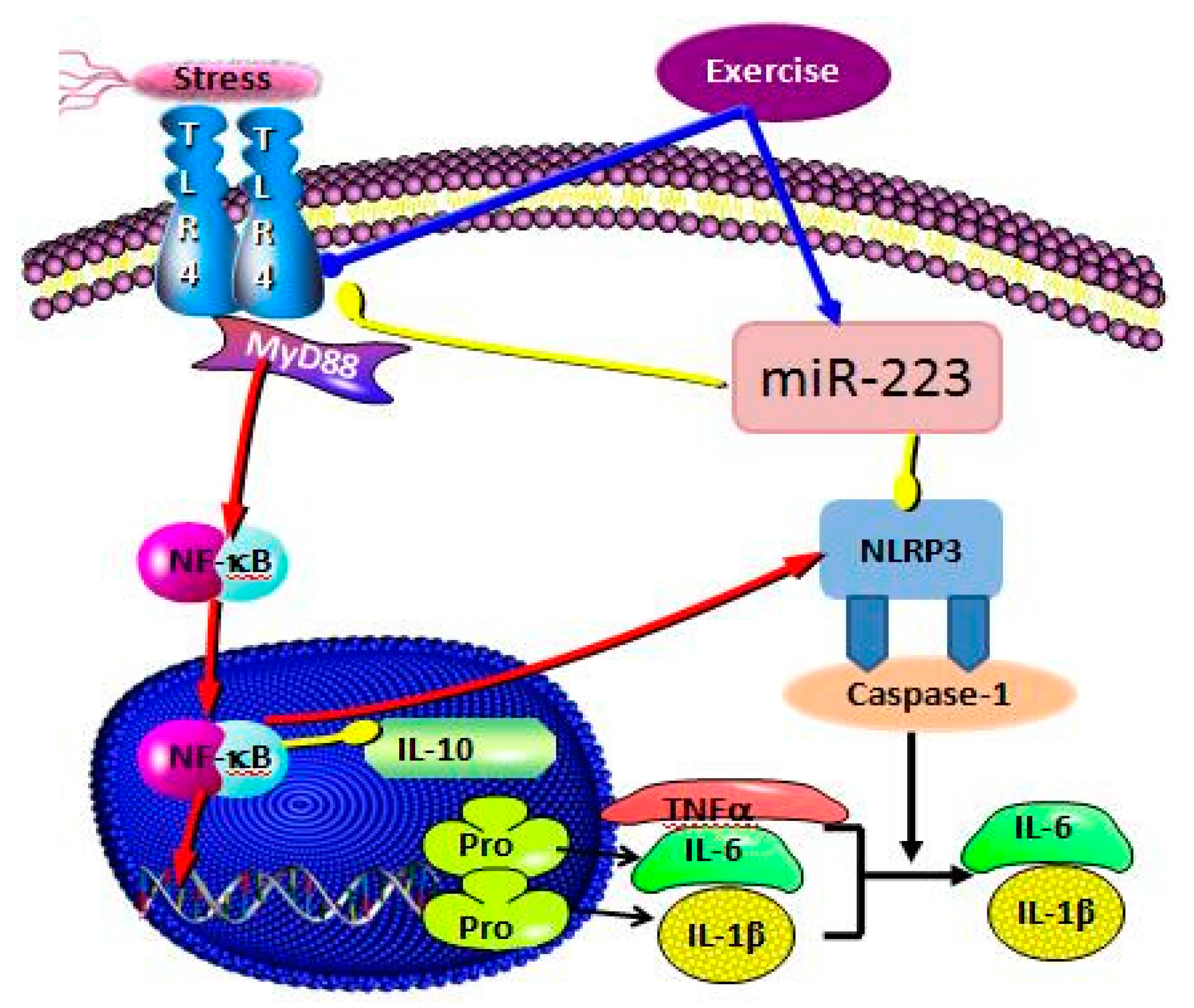
3.3. Correlation Analysis of Targeting the TLR4/MyD88-NF-κB Pathway by miR-223 Activation Based on Aerobic Exercise
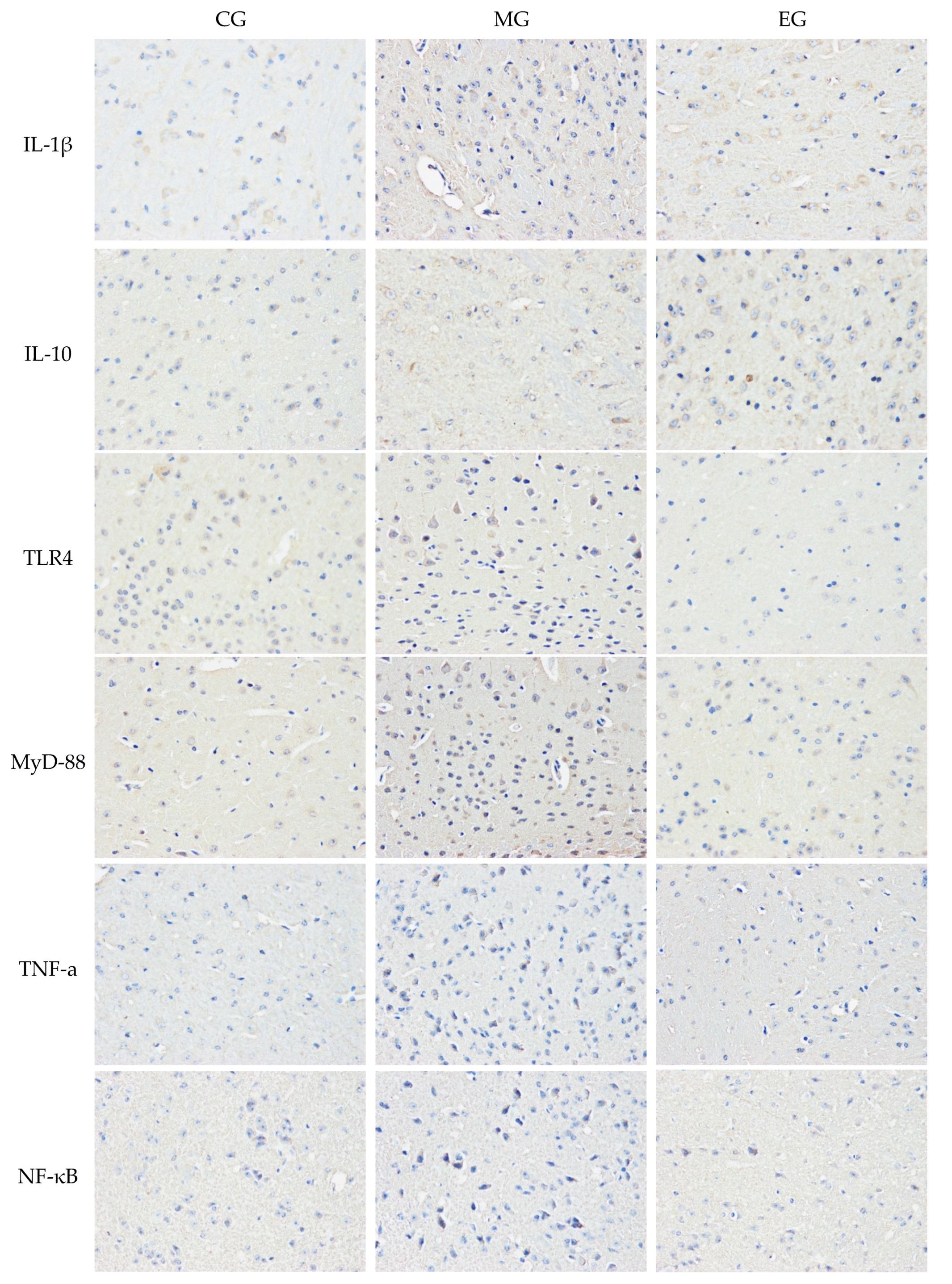
3.4. The Immunohistochemistry
3.5. ELISA
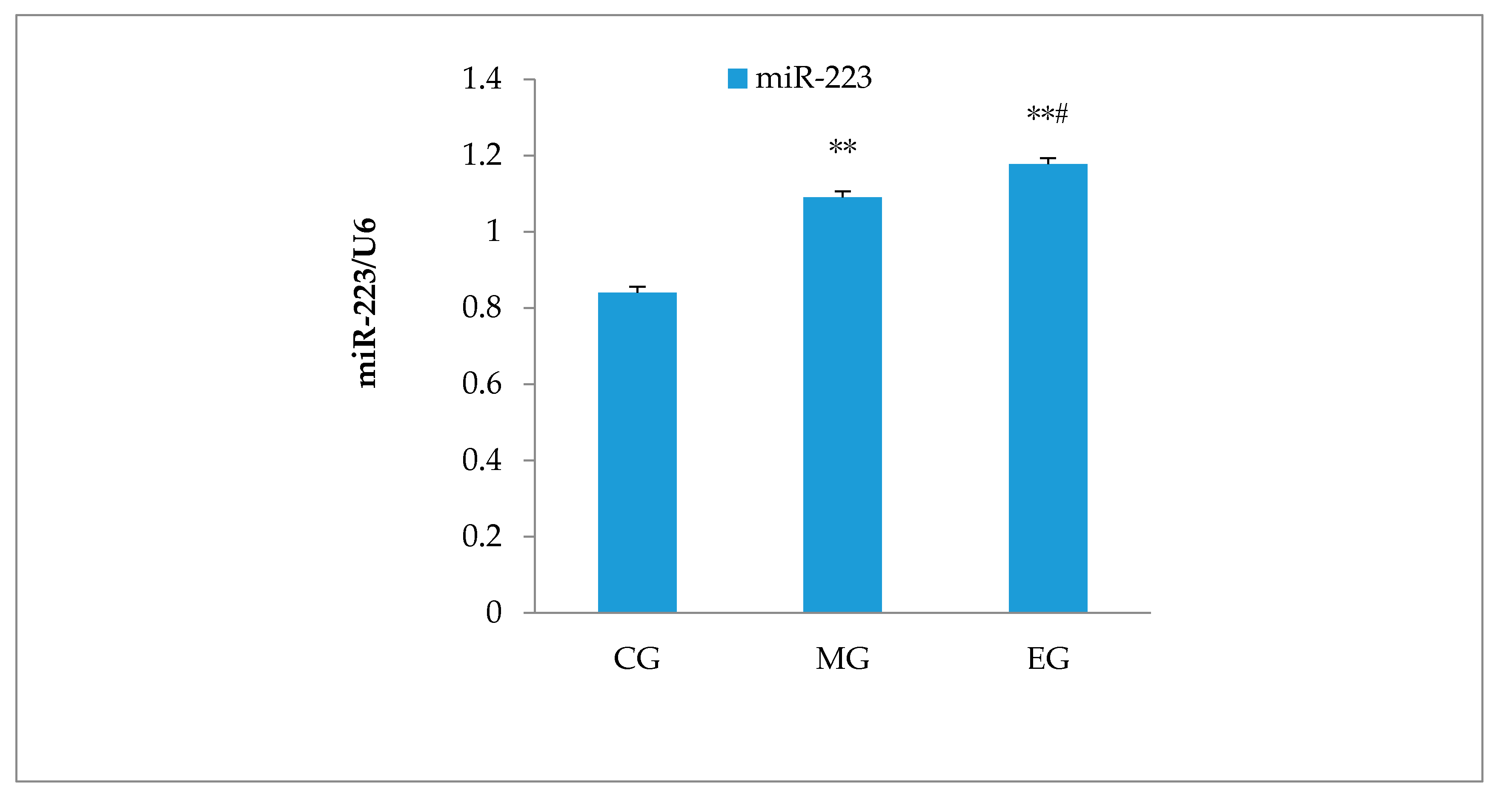
3.6. Hippocampal miR-223 Expression
3.7. RT–PCR
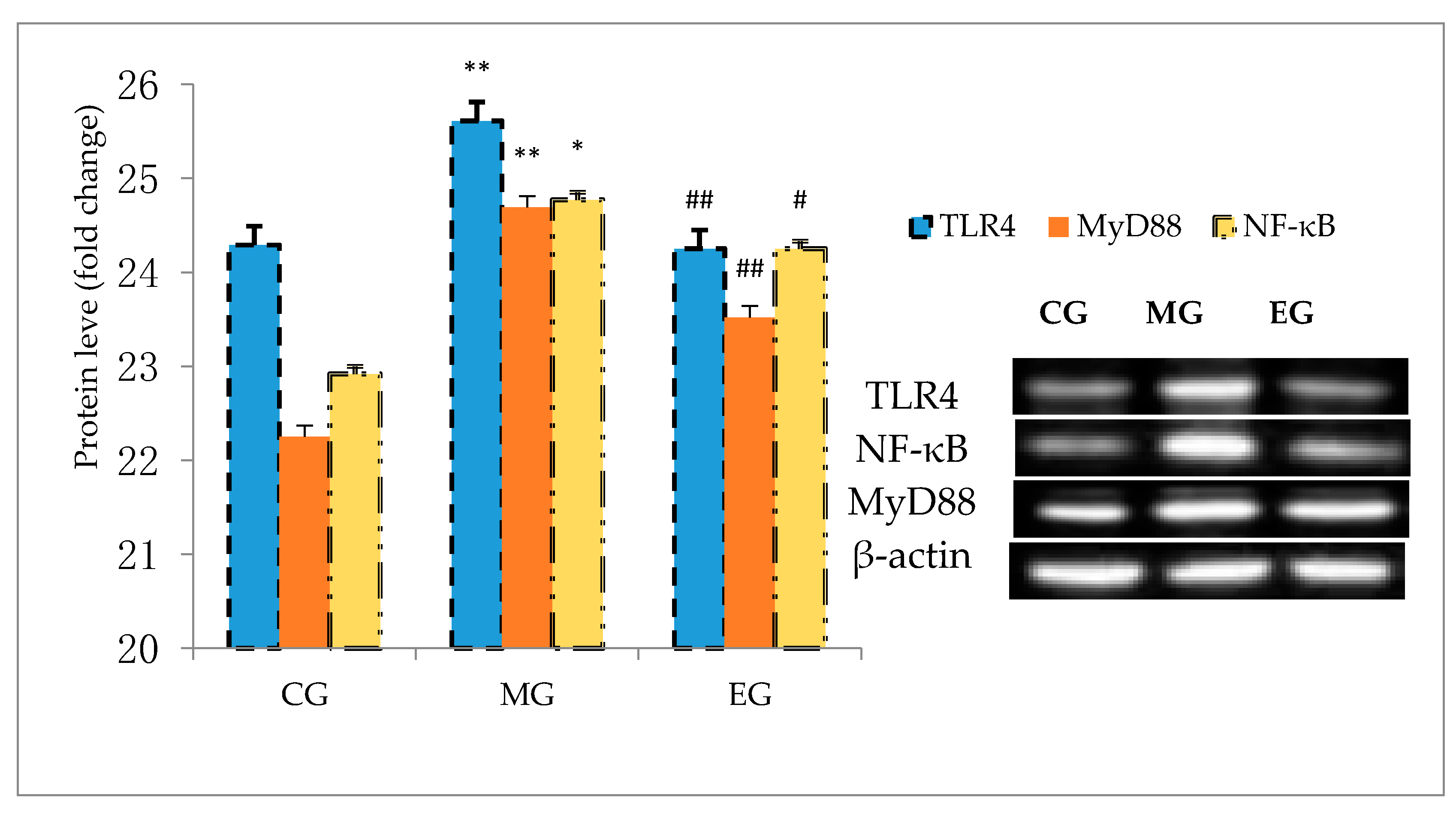
3.8. Western Blot
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lurie, D.I. An integrative approach to neuroinflammation in psychiatric disorders and neuropathic pain. J. Exp. Neurosci. 2018, 12, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Dey, A.; Hankey Giblin, P.A. Insights into Macrophage Heterogeneity and Cytokine-Induced Neuroinflammation in Major Depressive Disorder. Pharmaceuticals 2018, 11, 64. [Google Scholar] [CrossRef] [PubMed]
- Carboni, L.; McCarthy, D.J.; Delafont, B.; Filosi, M.; Ivanchenko, E.; Ratti, E.; Learned, S.M.; Alexander, R.; Domeniciet, E. Biomarkers for response in major depression: Comparing paroxetine and venlafaxine from two randomised placebo-controlled clinical studies. Transl. Psychiatry 2019, 9, 182. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Zhou, F.; Yuan, X.; Yang, T.; Liang, X.; Wang, Y.; Tu, H.; Chang, J.; Nan, K.; Wei, Y. Reactive Oxygen Species Are Involved in the Development of Gastric Cancer and Gastric Cancer-Related Depression through ABL1-Mediated Inflammation Signaling Pathway. Oxidative Med. Cell. Longev. 2019, 15, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Mattina, G.F.; Van Lieshout, R.J.; Steiner, M. Inflammation, depression and cardiovascular disease in women: The role of the immune system across critical reproductive events. Ther. Adv. Cardiovasc. Dis. 2019, 13, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Itagaki, K.; Takebayashi, M.; Abe, H.; Shibasaki, C.; Kajitani, N.; Okada-Tsuchioka, M.; Hattori, K.; Yoshida, S.; Kunugi, H.; Yamawaki, S. Reduced Serum and Cerebrospinal Fluid Levels of Autotaxin in Major Depressive Disorder. Int. J. Neuropshchopharmacology 2019, 22, 262–269. [Google Scholar] [CrossRef]
- Zeraati, M.; Enayati, M.; Kafami, L.; Shahidi, S.H.; Salari, A.A. Gut microbiota depletion from early adolescence alters adult immunological and neurobehavioral responses in a mouse model of multiple sclerosis. Neuropharmacology 2019, 25, 107685. [Google Scholar] [CrossRef]
- Rebane, A.; Akdis, C.A. MicroRNAs: Essential players in the regulation of inflammation. J. Allergy Clin. Immunol. 2013, 132, 15–26. [Google Scholar] [CrossRef]
- Tezcan, G.; Martynova, E.; Gilazieva, Z.E.; Rizvanov, A.; Khaiboullina, S. MicroRNA Post-transcriptional Regulation of the NLRP3 Inflammasome in Immunopathologies. Front. Pharmacol. 2019, 10, 451. [Google Scholar] [CrossRef]
- Geng, Y.W.; Lin, Q.Q.; Ma, H.M.; Tian, Z.J. Aerobic Interval Training Activates the Renal miR-21/TLR4/NF-κB Signaling Pathway and Inhibits Renal Inflammation in Rats with Myocardial Infarction. J. Beijing Sport Univ. 2018, 41, 70–74. [Google Scholar]
- Tokarz, P.; Blasiak, J. The role of miRNA in metastatic colorectal cancer and its significance in cancer prognosis and treatment. Acta Biochim. Pol. 2012, 59, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Su, L.; Chen, R.Q.; Lan, R.L.; Fu, L.X. miR-223 protects mice from acute radiation-induced lung injury by inhibiting NLRP3. Chin. J. Radiol. Med. Prot. 2019, 39, 166–171. [Google Scholar]
- Neudecker, V.; Haneklaus, M.; Jensen, O.; Khailova, L.; Masterson, J.C.; Tye, H.; Biette, K.; Jedlicka, P.; Brodsky, K.S.; Gerich, M.E.; et al. Myeloid-derived miR-223 regulates intestinal inflammation via repression of the NLRP3 inflammasome. J. Exp. Med. 2017, 214, 1737–1752. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Fu, L.; Bu, Y.; Yao, Y.; Wang, Y. Downregulated expression of miR-223 promotes Toll-like receptor-activated inflammatory responses in macrophages by targeting RhoB. Mol. Immunol. 2017, 91, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Colbert, J.F.; Ford, J.A.; Haeger, S.M.; Yang, Y.; Dailey, K.L.; Allison, K.C.; Neudecker, V.; Evans, C.M.; Richardson, V.L.; Brodsky, K.S.; et al. A model-specific role of microRNA-223 as a mediator of kidney injury during experimental sepsis. Am. J. Physiol. Ren. Physiol. 2017, 313, 553–559. [Google Scholar] [CrossRef]
- Nielsen, S.; Åkerström, T.; Rinnov, A.; Yfanti, C.; Scheele, C.; Pedersen, B.K.; Laye, M.J. The miRNA Plasma Signature in Response to Acute Aerobic Exercise and Endurance Training. PLoS ONE 2014, 9, e87308. [Google Scholar] [CrossRef]
- De Gonzalo-Calvo, D.; Dávalos, A.; Montero, A.; García-González, Á.; Tyshkovska, I.; González-Medina, A.; Soares, S.M.; Martínez-Camblor, P.; Casas-Agustench, P.; Rabadán, M.; et al. Circulating inflammatory miRNA signature in response to different doses of aerobic exercise. J. Appl. Physiol. 2015, 119, 124–134. [Google Scholar] [CrossRef]
- Taghizadeh, M.; Ahmadizad, S.; Naderi, M. Effects of endurance training on hsa-miR-223, P2RY12 receptor expression and platelet function in type 2 diabetic patients. Clin. Hemorheol. Microcirc. 2018, 68, 391–399. [Google Scholar] [CrossRef]
- Wang, L.; Chen, J.D. Progress in studies on TLR4 signaling pathway and major depressive disorder. J. Cent. South Univ. Med Sci. 2017, 42, 725–729. [Google Scholar]
- Li, N.; Gu, H.F.; Xu, Z.Q.; Hu, L.; Li, H.; Zhang, R.J.; Chen, R.M.; Tang, Y.L.; Liao, D.F. Chronic Unpredictable Mild Stress Promotes Atherosclerosis via HMGB1/TLR4-Mediated Downregulation of PPARγ/LXRα/ABCA1 in ApoE-/- Mice. Front. Physiol. 2019, 10, 1–13. [Google Scholar]
- Lin, X.Y.; Liu, J.M. TLR4/MyD88/NF-κB Signaling Pathway and Ulcerative Colitis. Chin. J. Gastroenterol. 2013, 18, 244–246. [Google Scholar]
- Liu, H.Y.; Chen, K.; Feng, W.Q.; Wu, X.; Li, H. TLR4-MyD88/Mal-NF-κB Axis Is Involved in Infection of HSV-2 in Human Cervical Epithelial Cells. PLoS ONE 2013, 8, e80327. [Google Scholar]
- Li, Z.; Tang, X.; Bao, H. MP059C-REACTIVE PROTEIN INDUCES RENAL INFLAMMATION VIA A CD32A-NF-ΚB-TLR4/MYD88 CIRCUIT. Nephrol. Dial. Transplant. 2017, 32, 447. [Google Scholar] [CrossRef]
- Tie, H.T.; Wang, J.Y. Negative regulation of TLR signaling by microRNA. Chem. Life 2012, 32, 347–352. [Google Scholar]
- O’neill, L.A.; Sheedy, F.J.; McCoy, C.E. microRNAs: The fine-tuners of Toll-like receptor signaling. Nat. Rev. Immunol. 2011, 11, 163–175. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, H.; Liu, Y.; Song, Y.; Lai, L.; Han, Q.; Cao, X.; Wang, Q. Inducible microRNA-223 down-regulation promotes TLR-triggered IL-6 and IL-1β production in macrophages by targeting STAT3. PLoS ONE 2012, 7, e42971. [Google Scholar] [CrossRef]
- Su, W.H.; Zhang, Y.; Chen, Y.; Gong, H.; Lian, Y.J.; Peng, W.; Liu, Y.Z.; Wang, Y.X.; You, Z.L.; Feng, S.J.; et al. NLRP3 gene knockout blocks NF-kB and MAPK signaling pathway in CUMS-induced depression mouse model. Behav. Brain Res. 2017, 322, 1–8. [Google Scholar] [CrossRef]
- Bedford, T.G.; Tipton, C.M.; Wilson, N.C.; Oppliger, R.A.; Gisolfi, C.V. Maximum oxygen consumption of rats and its changes with various ex-perimental procedures. J. Appl. Physiol. 1979, 47, 1278–1283. [Google Scholar] [CrossRef]
- Mao, H.F.; Xie, J.; Chen, J.Q.; Tang, C.F.; Chen, W.; Zhou, B.C.; Chen, R.; Qu, H.L.; Wu, C.Z. Aerobic exercise combined with huwentoxin-I mitigates chronic cerebral ischemia injury. Neural Regen. Res. 2017, 12, 596–602. [Google Scholar]
- Chen, W.; Chen, J.Q.; Mao, H.F.; Zhou, B.C.; Qu, H.L.; Li, D.; Zhang, Z.Y.; Peng, Q. The effect of aerobic exercise and Lycium ruthenicum murr polysaccharide on chronic cerebral ischemia in mice and differential expression of Notch pathway tissue. Chin. J. Arterioscler. 2017, 25, 783–790. [Google Scholar]
- Kohut, M.L.; McCann, D.A.; Russell, D.W.; Konopka, D.N.; Cunnick, J.E.; Franke, W.D.; Castillo, M.C.; Reighard, A.E.; Vanderah, E. Aerobic exercise, but not flexibility/resistance exercise, reduces serum IL-18, CRP, and IL-6 independent of beta-bloc-kers, BMI, and psychosocial factors in older adults. Brain Behav. Immun. 2006, 20, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.J.; Lv, Y.H. Aerobic exercise regulate the capability of learning and memory via IGF1 signaling pathway of depression rat. J. Guangzhou Sport Univ. 2014, 34, 101–106. [Google Scholar]
- Jyonouchi, H.; Geng, L.; Streck, D.L.; Dermody, J.J.; Toruner, G.A. MicroRNA expression changes in association with changes in interleukin-1ß/interleukin10 ratios produced by monocytes in autism spectrum disorders: Their association with neuropsychiatric symptoms and comorbid conditions (observational study). J. Neuroinflammation 2017, 14, 229–242. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Bai, X.J.; Song, Q.; Fan, F.L.; Hu, Z.; Cheng, G.S.; Zhang, Y.S. miR-223 Inhibits Lipid Deposition and Inflammation by Suppressing Toll-Like Receptor 4 Signaling in Macrophages. Int. J. Mol. Sci. 2015, 16, 24965–24982. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.B.; Wang, R.; Wu, R.D.; Chen, S.; Lin, M.Y.; Wang, S.M.; Yao, C. MiR-223 inhibits HMGB1 secretion from human arterial smooth muscle cells by down-regulating NLRP3. J. Third Mil. Med Univ. 2018, 40, 1399–1406. [Google Scholar]
- Zhou, W.; Pal, A.S.; Hsu, A.Y.; Gurol, T.; Zhu, X.; Wirbisky-Hershberger, S.E.; Freeman, J.L.; Kasinski, A.L.; Deng, Q. MicroRNA-223 Suppresses the Canonical NF-κB Pathway in Basal Keratinocytes to Dampen Neutrophilic Inflammation. Cell Rep. 2018, 22, 1810–1823. [Google Scholar] [CrossRef]
- Yang, F.; Xu, Y.; Liu, C.; Ma, C.; Zou, S.; Xu, X.; Jia, J.; Liu, Z. NF-κB/miR-223-3p/ARID1A axis is involved in Helicobacter pylori CagA-induced gastric carcinogenesis and progression. Cell Death Dis. 2018, 9, 12–25. [Google Scholar] [CrossRef]
- He, Y.; Feng, D.; Li, M.; Gao, Y.; Ramirez, T.; Cao, H.; Kim, S.J.; Yang, Y.; Cai, Y.; Ju, C.; et al. Hepatic mitochondrial DNA/Toll-like receptor 9/MicroRNA-223 forms a negative feedback loop to limit neutrophil overactivation and acetaminophen hepatotoxicity in mice. Hepatology 2017, 66, 220–234. [Google Scholar] [CrossRef]
- Wu, J.; Niu, P.; Zhao, Y.; Cheng, Y.; Chen, W.; Lin, L.; Lu, J.; Cheng, X.; Xu, Z. Impact of miR-223-3p and miR-2909 on inflammatory factors IL-6, IL-1ß, and TNF-α, and the TLR4/TLR2/NF-κB/STAT3 signaling pathway induced by lipopolysaccharide in human adipose stem cells. PLoS ONE 2019, 14, e0212063. [Google Scholar] [CrossRef]
- Izumi, B.; Nakasa, T.; Tanaka, N.; Nakanishi, K.; Kamei, N.; Yamamoto, R.; Nakamae, T.; Ohta, R.; Fujioka, Y.; Yamasaki, K.; et al. MicroRNA-223 expression in neutrophils in the early phase of secondary damage after spinal cord injury. Neurosci. Lett. 2011, 492, 114–118. [Google Scholar] [CrossRef]
- Wang, W.X.; Visavadiya, N.P.; Pandya, J.D.; Nelson, P.T.; Sullivan, P.G.; Springer, J.E. Mitochondria-associated microRNAs in rat hippocampus following traumatic brain injury. Exp. Neurol. 2015, 265, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Harrison, E.B.; Emanuel, K.; Lamberty, B.G.; Morsey, B.M.; Li, M.; Kelso, M.L.; Yelamanchili, S.V.; Fox, H.S. Induction of miR-155 after Brain Injury Promotes Type 1 Interferon and has a Neuroprotective Effect. Front. Mol. Neurosci. 2017, 28, 10. [Google Scholar] [CrossRef] [PubMed]
- Harraz, M.M.; Eacker, S.M.; Wang, X.; Dawson, T.M.; Dawson, V.L. MicroRNA-223 is neuroprotective by targeting glutamate receptors. Proc. Natl. Acad. Sci. USA 2012, 109, 18962–18967. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Tan, M.; Xiang, Q.; Zhou, Z.; Yan, H. Thrombin-activated platelet-derived exosomes regulate endothelial cell expression of ICAM-1 via microRNA-223 during the thrombosis-inflammation response. Thromb. Res. 2017, 154, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; He, Y.; Zhou, Z.; Ramirez, T.; Gao, Y.; Gao, Y.; Ross, R.A.; Cao, H.; Cai, Y.; Xu, M.; et al. MicroRNA-223 ameliorates alcoholic liver injury by inhibiting the IL-6-p47phox-oxidative stress pathway in neutrophils. Get 2017, 66, 705–715. [Google Scholar] [CrossRef]
- Qu, H.L.; Xie, J.; Chen, J.Q.; Liu, R.L.; Peng, Q.; Li, D.; Chen, W.; Chen, Y.L.; Chen, R.; Shao, C.F.; et al. Aerobic Exercise Activates the Hippocampus BDNF/miR-195/Bcl-2 Signaling Pathway Axis and Inhibits Hip-pocampus Neuronal Apoptosis in Mice Model with CUMS Depression. J. Tianjin Univ. Sport 2018, 33, 148–155. [Google Scholar]
- Deiuliis, J.A.; Syed, R.; Duggineni, D.; Rutsky, J.; Rengasamy, P.; Zhang, J.; Huang, K.; Needleman, B.; Mikami, D.; Perry, K.; et al. Visceral Adipose MicroRNA 223 Is Upregulated in Human and Murine Obesity and Modulates the Inflammatory Phenotype of Macrophages. PLoS ONE 2016, 11, e0165962. [Google Scholar] [CrossRef]
- Sari, A.N.; Korkmaz, B.; Serin, M.S.; Kacan, M.; Unsal, D.; Buharalioglu, C.K.; Firat, S.S.; Manthati, V.L.; Falck, J.R.; Malik, K.U.; et al. Effects of 5,14-HEDGE, a 20-HETE mimetic, on lipopolysaccharide-induced changes in MyD88/TAK1/IKKβ/IκB-α/NF-κB pathway and circulating miR-150, miR-223, and miR-297 levels in a rat model of septic shock. Inflamm. Res. 2014, 63, 741–756. [Google Scholar] [CrossRef]
- Huang, L.; Li, F.; Deng, P.; Hu, C. MicroRNA-223 Promotes Tumor Progression in Lung Cancer A549 Cells via Activation of the NF-κB Signaling Pathway. Oncol. Res. 2016, 24, 405–413. [Google Scholar] [CrossRef]
- Chivero, E.T.; Guo, M.L.; Periyasamy, P.; Liao, K.; Callen, S.E.; Buch, S. HIV-1 Tat Primes and Activates Microglial NLRP3 Inflammasome-Mediated Neuroinflammation. J. Neurosci. Off. J. Soc. Neurosci. 2017, 37, 3599–3609. [Google Scholar] [CrossRef]
- Liu, M.; Li, C.; Zhao, G.Q.; Lin, J.; Che, C.Y.; Xu, Q.; Wang, Q.; Xu, R.; Niu, Y.W. Boxb mediate BALB/c mice corneal inflammation through a TLR4/MyD88-dependent signaling pathway in Aspergillus fumigatus keratitis. Int. J. Ophthalmol. 2018, 11, 548–552. [Google Scholar] [PubMed]
- Yan, Y.R.; Lu, K.X.; Ye, T.; Zhang, Z. MicroRNA-223 attenuates LPS-induced inflammation in an acute lung injury model via the NLRP3 inflammasome and TLR4/NF-κB signaling pathway via RHOB. Int. J. Mol. Med. 2019, 43, 1467–1477. [Google Scholar] [CrossRef] [PubMed]
- Qu, H.L.; Xie, J.; Chen, J.Q.; Liu, R.L.; Tang, C.F.; Chen, Y.L.; Chen, W.; Li, D.; Peng, Q.; Chen, R. Aerobic Training Inhibits Hippocampal Inflammation by Activating the Hippocampus TLR4/miR223/NLRP3 Signaling Pathway Axis in Mice with CUMS Depression. China Sport Sci. 2019, 39, 39–50. [Google Scholar]
- Moriya, N.; Shibasaki, S.; Karasaki, M.; Iwasaki, T. The Impact of MicroRNA-223-3p on IL-17 Receptor D Expression in Synovial Cells. PLoS ONE 2017, 12, e0169702. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, J.; Cheng, Y.; Jiang, Y.; Li, G. Over-expression of microRNA-223 inhibited the proinflammatory responses in Helicobacter pylori-infection macrophages by down-regulating IRAK-1. Am. J. Transl. Res. 2016, 8, 615–622. [Google Scholar] [PubMed]
| Gene | Upstream Primer | Downstream Primer |
|---|---|---|
| TLR4 | 5′-ACAAACGCCGGAACTTTTCG-3′ | 5′- GTCGGACACACACAACTTAAGC-3′ |
| MyD88 | 5′-CGCCGCCTATCGCTGTTCTTG-3′ | 5′- TTCTCGGACTCCTGGTTCTGCTG-3′ |
| NF-κB | 5′-ATCATCGAACAGCCGAAGCA-3′ | 5′-TGATGGTGGGGTGTGTCTTG-3′ |
| GAPDH | 5′- CATGGCCTTCCGTGTTCCTA-3′ | 5′- CCTGCTTCACCACCTTCTTGAT-3′ |
| Group | CA1 (Number) | CA3 (Number) | Nissl Bodies (Number) |
|---|---|---|---|
| CG | 56.8 ± 6.6 | 31.3 ± 8.6 | 81.3 ± 15.6 |
| MG | 55.5 ± 4.9 | 22.7 ± 9.3 ** | 51.0 ± 17.9 ** |
| EG | 55.2 ± 5.8 | 29.6 ± 7.0 ## | 72.6 ± 14.5 # |
| Group | CG-CA1 | CG-CA3 | MG-CA1 | MG-CA3 | EG-CA1 | CG-CA3 |
|---|---|---|---|---|---|---|
| IL-1β | 31.64 ± 5.79 | 25.13 ± 2.87 | 51.36 ± 7.12 ** | 52.04 ± 5.99 ** | 30.07 ± 3.55 ## | 32.17 ± 2.30 ## |
| IL-10 | 17.37 ± 2.45 | 18.81 ± 2.76 | 20.62 ± 3.75 | 22.19 ± 2.75 * | 38.67 ± 5.31 ## | 41.11 ± 5.56 ## |
| TLR4 | 27.13 ± 4.55 | 26.37 ± 6.19 | 45.92 ± 6.24 ** | 47.90 ± 7.33 ** | 38.29 ± 7.54 # | 33.03 ± 6.71 ## |
| MyD88 | 22.81 ± 4.77 | 28.39 ± 6.30 | 39.12 ± 7.76 ** | 42.19 ± 7.23 ** | 35.67 ± 5.31 # | 32.69 ± 7.58 ## |
| TNF-α | 15.49 ± 3.25 | 14.04 ± 1.37 | 31.99 ± 3.06 ** | 36.55 ± 2.79 ** | 24.52 ± 3.37 # | 21.93 ± 3.40 ## |
| NF-κB | 22.71 ± 4.54 | 20.33 ± 3.06 | 47.07 ± 6.39 ** | 50.92 ± 4.98 ** | 33.84 ± 6.71 ## | 26.40 ± 3.91 ## |
| Group | IL-1β (pg/mL) | IL-10 (pg/mL) | TLR4 (ng/mL) | NF-κB (pg/mL) | TNF-α (pg/mL) |
|---|---|---|---|---|---|
| CG | 1.71 ± 0.90 | 5.39 ± 2.67 | 24,140.00 ± 1560.39 | 19.99 ± 1.29 | 4.90 ± 0.51 |
| MG | 4.55 ± 1.37 * | 7.36 ± 2.93 ** | 34,720.00 ± 1436.20 ** | 29.98 ± 1.42 ** | 8.78 ± 0.46 ** |
| EG | 2.71 ± 1.69 # | 13.68 ± 5.74 ## | 24,845.00 ± 1234.14 ## | 13.98 ± 1.34 *## | 5.68 ± 0.63 # |
| Group | IL-1β | IL-10 | TLR4 | MyD88 | NF-κB |
|---|---|---|---|---|---|
| CG | 1.24 ± 0.042 | 1.28 ± 0.040 | 1.14 ± 0.033 | 1.16 ± 0.062 | 1.11 ± 0.041 |
| MG | 1.29 ± 0.033 * | 1.33 ± 0.071 | 1.19 ± 0.028 * | 1.61 ± 0.096 ** | 1.20 ± 0.034 ** |
| EG | 1.26 ± 0.058 | 1.42 ± 0.004 **# | 1.16 ± 0.035 ## | 1.39 ± 0.033 ## | 1.12 ± 0.041 # |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qu, H.; Liu, R.; Chen, J.; Zheng, L.; Chen, R. Aerobic Exercise Inhibits CUMS-Depressed Mice Hippocampal Inflammatory Response via Activating Hippocampal miR-223/TLR4/MyD88-NF-κB Pathway. Int. J. Environ. Res. Public Health 2020, 17, 2676. https://doi.org/10.3390/ijerph17082676
Qu H, Liu R, Chen J, Zheng L, Chen R. Aerobic Exercise Inhibits CUMS-Depressed Mice Hippocampal Inflammatory Response via Activating Hippocampal miR-223/TLR4/MyD88-NF-κB Pathway. International Journal of Environmental Research and Public Health. 2020; 17(8):2676. https://doi.org/10.3390/ijerph17082676
Chicago/Turabian StyleQu, Honglin, Ruilian Liu, Jiaqin Chen, Lan Zheng, and Rui Chen. 2020. "Aerobic Exercise Inhibits CUMS-Depressed Mice Hippocampal Inflammatory Response via Activating Hippocampal miR-223/TLR4/MyD88-NF-κB Pathway" International Journal of Environmental Research and Public Health 17, no. 8: 2676. https://doi.org/10.3390/ijerph17082676
APA StyleQu, H., Liu, R., Chen, J., Zheng, L., & Chen, R. (2020). Aerobic Exercise Inhibits CUMS-Depressed Mice Hippocampal Inflammatory Response via Activating Hippocampal miR-223/TLR4/MyD88-NF-κB Pathway. International Journal of Environmental Research and Public Health, 17(8), 2676. https://doi.org/10.3390/ijerph17082676





