The Potential Role of Migratory Birds in the Rapid Spread of Ticks and Tick-Borne Pathogens in the Changing Climatic and Environmental Conditions in Europe
Abstract
1. Introduction
2. Tick Species Most Frequently Infesting Migratory Birds in Europe
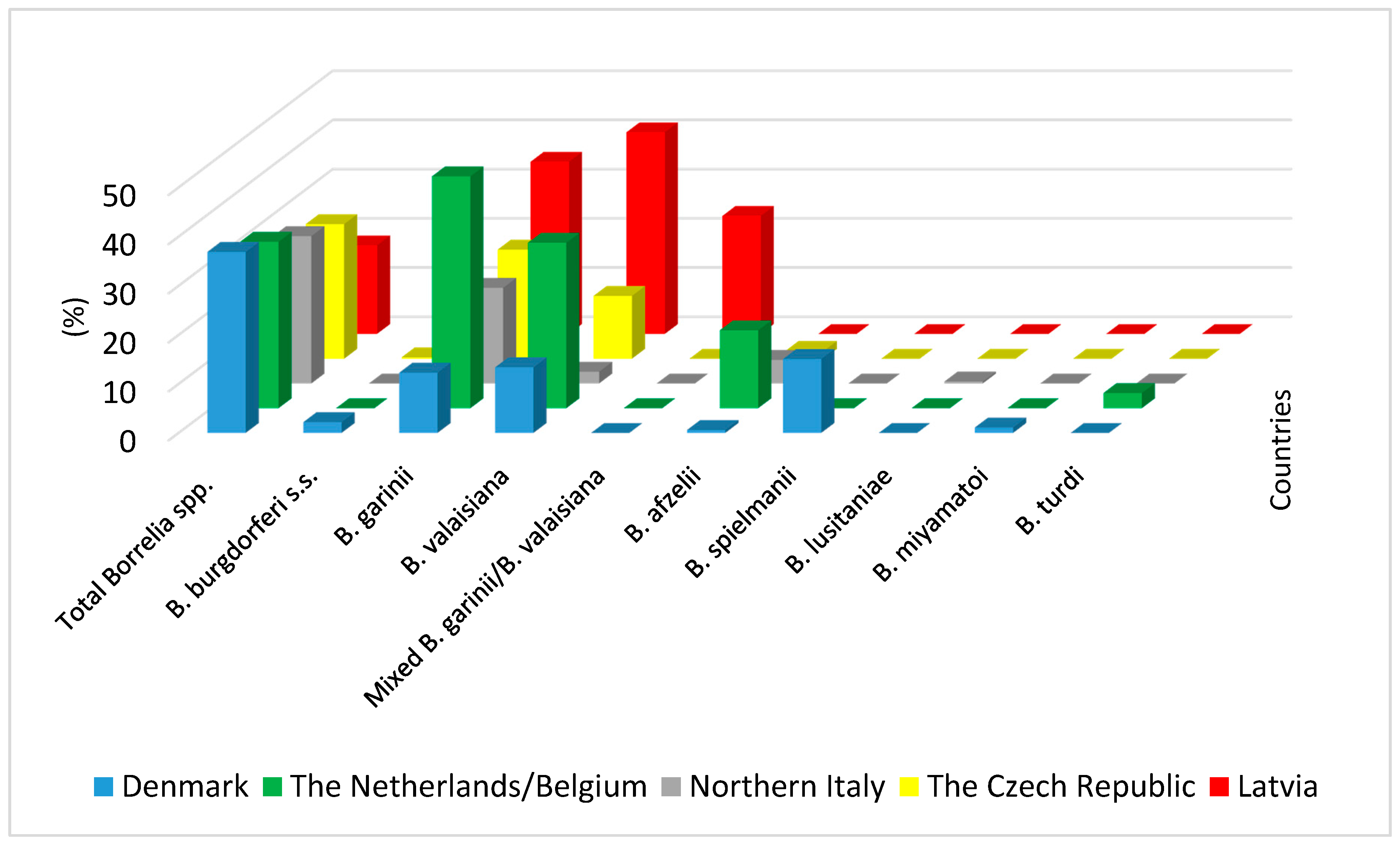
3. Occurrence of Tick-Borne Pathogens in Ticks Infesting Birds in Europe
4. Impact of Climatic and Environmental Factors on the Behaviour of Migratory Birds
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Nowak-Chmura, M. Tick fauna (Ixodida) of Central Europe; Wyd Nauk Uniw Ped: Kraków, Poland, 2013; pp. 88–211. [Google Scholar]
- Jaenson, T.G.T.; Jaenson, D.G.E.; Eisen, L.; Petersson, E.; Lindgren, E. Changes in the geographical distribution and abundance of the tick Ixodes ricinus during the past 30 years in Sweden. Parasit. Vectors 2012, 5, 8–22. [Google Scholar] [CrossRef]
- Medlock, J.M.; Hansford, K.M.; Bormane, A.; Derdakova, M.; Estrada-Peña, A.; George, J.-C.; Golovljova, I.; Jaenson, T.G.; Jensen, J.K.; Jensen, P.M.; et al. Driving forces for changes in geographical distribution of Ixodes ricinus ticks in Europe. Parasit. Vectors 2013, 6, 1. [Google Scholar] [CrossRef] [PubMed]
- Jore, S.; Vanwambeke, S.O.; Viljugrein, H.; Isaksen, K.; Kristoffersen, A.B.; Woldehiwet, Z.; Johansen, B.; Brun, E.; Brun-Hansen, H.; Westermann, S.; et al. Climate and environmental change drives Ixodes ricinus geographical expansion at the northern range margin. Parasit. Vectors 2014, 7, 11. [Google Scholar] [CrossRef] [PubMed]
- Chitimia-Dobler, L. Spatial distribution of Dermacentor reticulatus in Romania. Vet. Parasitol. 2015, 214, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Földvári, G.; Široký, P.; Szekeres, S.; Majoros, G.; Sprong, H. Dermacentor reticulatus: A vector on the rise. Parasit. Vectors 2016, 9, 314. [Google Scholar] [CrossRef]
- Rubel, F.; Brugger, K.; Pfeffer, M.; Chitimia-Dobler, L.; Didyk, Y.M.; Leverenz, S.; Dautel, H.; Kahl, O. Geographical distribution of Dermacentor marginatus and Dermacentor reticulatus in Europe. Ticks Tick Borne Dis. 2016, 7, 224–233. [Google Scholar] [CrossRef]
- Rubel, F.; Brugger, K.; Walter, M.; Vogelgesang, J.R.; Didyk, Y.M.; Fu, S.; Kahl, O. Geographical distribution, climate adaptation and vector competence of the Eurasian hard tick Haemaphysalis concinna. Ticks Tick Borne Dis. 2018, 9, 1080–1089. [Google Scholar] [CrossRef]
- Estrada-Peña, A.; Jameson, L.; Medlock, J.; Vatansever, Z.; Tishkova, F. Unraveling the ecological complexities of tick-associated Crimean-Congo hemorrhagic fever virus transmission: A gap analysis for the western Palearctic. Vector Borne Zoonotic Dis. 2012, 12, 743–752. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control and European Food Safety Authority. Tick Maps; ECDC: Stockholm, Sweden, 2019; Available online: https://ecdc.europa.eu/en/disease-vectors/surveillance-and-disease-data/tick-maps (accessed on 15 December 2019).
- Gray, J.; Dantas-Torres, F.; Estrada-Peña, A.; Levin, M. Systematics and ecology of the brown dog tick, Rhipicephalus sanguineus. Ticks Tick Borne Dis. 2013, 4, 171–180. [Google Scholar] [CrossRef]
- Hasle, G. Transport of ixodid ticks and tick-borne pathogens by migratory birds. Front. Cell. Infect. Microbiol. 2013, 3, 48. [Google Scholar] [CrossRef]
- Wallménius, K.; Barboutis, C.; Fransson, T.; Jaenson, T.G.; Lindgren, P.E.; Nyström, F.; Olsen, B.; Salaneck, E.; Nilsson, K. Spotted fever Rickettsia species in Hyalomma and Ixodes ticks infesting migratory birds in the European Mediterranean area. Parasit. Vectors 2014, 7, 318. [Google Scholar] [CrossRef] [PubMed]
- Ciebiera, O.; Jerzak, L.; Nowak-Chmura, M.; Bocheński, M. Ticks (Acari: Ixodida) on birds (Aves) migrating through the Polish Baltic coast. Exp. Appl. Acarol. 2019, 77, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Hasle, G. Dispersal of Ticks and Tick-Borne Pathogens by Birds. Dynamics of Birds’ Transport of Ticks to Norway. Ph.D. Thesis, Department of Biology, University of Oslo, Institute for Health and Society, Faculty of Medicine, University of Oslo, Oslo, Norway, 2010. [Google Scholar]
- Buczek, A.; Bartosik, K.; Buczek, A.M.; Buczek, W.; Stanko, M. Conspecific hyperparasitism in the Hyalomma excavatum tick and considerations on the biological and epidemiological implications of this phenomenon. Ann. Agric. Environ. Med. 2019, 26, 548–554. [Google Scholar] [CrossRef] [PubMed]
- Buczek, A.; Bartosik, K.; Buczek, W.; Buczek, A.M.; Kulina, D.; Kulisz, J.; Tomasiewicz, K. A unique phenomenon of oral-anal contact between ticks observed in two tick species Ixodes ricinus and Dermacentor reticulatus. Ann. Agric. Environ. Med. 2018, 25, 686–689. [Google Scholar] [CrossRef]
- Carroll, J.F.; Schmidtmann, E.T. Dispersal of blacklegged tick (Acari: Ixodidae) nymphs and adults at the woods-pasture interface. J. Med. Entomol. 1996, 33, 554–558. [Google Scholar] [CrossRef]
- Buczek, A.; Zając, Z.; Woźniak, A.; Kulina, D.; Bartosik, K. Locomotor activity of adult Dermacentor reticulatus ticks (Ixodida: Ixodidae) in natural conditions. Ann. Agric. Environ. Med. 2017, 24, 271–275. [Google Scholar] [CrossRef]
- Dutkiewicz, J.; Siuda, K. Rhipicephalus rossicus Jakimov et Kohl-Jakimova, 1911—A new tick genus and species (Acarina, Ixodidae) in the Polish fauna. Fragm. Faun. 1969, 15, 99–105. (In Polish) [Google Scholar] [CrossRef]
- Qviller, L.; Risnes-Olsen, N.; Bærum, K.M.; Meisingset, E.L.; Loe, L.E.; Ytrehus, B.; Viljugrein, H.; Mysterud, A. Landscape level variation in tick abundance relative to seasonal migration in red deer. PLoS ONE 2013, 8, e71299. [Google Scholar] [CrossRef]
- Mysterud, A.; Qviller, L.; Meisingset, E.L.; Viljugrein, H. Parasite load and seasonal migration in red deer. Oecologia 2016, 180, 401–407. [Google Scholar] [CrossRef]
- Vial, L.; Stachurski, F.; Leblond, A.; Huber, K.; Vourc’h, G.; René-Martellet, M.; Desjardins, I.; Balança, G.; Grosbois, V.; Pradier, S.; et al. Strong evidence for the presence of the tick Hyalomma marginatum Koch, 1844 in southern continental France. Ticks Tick Borne Dis. 2016, 7, 1162–1167. [Google Scholar] [CrossRef]
- Hoogstraal, H.; Kaiser, M.N.; Traylor, M.A.; Guindy, E.; Gaber, S. Ticks (Ixodidae) on birds migrating from Europe and Asia to Africa, 1959–1961. Bull. World Health Organ. 1963, 28, 235–262. [Google Scholar] [PubMed]
- Lee, K.; Medlock, J.; Heo, S. Severe fever with thrombocytopenia syndrome virus, Crimean-Congo haemorrhagic fever virus, and migratory birds. J. Bacteriol. Virol. 2013, 43, 235–243. [Google Scholar] [CrossRef][Green Version]
- Choi, C.Y.; Kang, C.W.; Kim, E.M.; Lee, S.; Moon, K.H.; Oh, M.R.; Yamauchi, T.; Yun, Y.M. Ticks collected from migratory birds, including a new record of Haemaphysalis formosensis, on Jeju Island, Korea. Exp. Appl. Acarol. 2014, 2, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Hornok, S.; Flaisz, B.; Takács, N.; Kontschán, J.; Csörgő, T.; Csipak, Á.; Jaksa, B.R.; Kováts, D. Bird ticks in Hungary reflect western, southern, eastern flyway connections and two genetic lineages of Ixodes frontalis and Haemaphysalis concinna. Parasit. Vectors 2016, 9, 101. [Google Scholar] [CrossRef] [PubMed]
- Sokolov, L.V.; Tropp, E.A.; Morozov, Y.; Efremov, V.D. Effect of the temperature factor on the long-term fluctuations of the timing of migration, breeding, and dispersal of passerine birds. Dokl. Biol. Sci. 2001, 379, 362–365. [Google Scholar] [CrossRef] [PubMed]
- Gray, J.S.; Dautel, H.; Estrada-Peña, A.; Kahl, O.; Lindgren, E. Effects of climate change on ticks and tick-borne diseases in Europe. Interdiscip. Perspect. Infect. Dis. 2009, 593232. [Google Scholar] [CrossRef] [PubMed]
- Lukan, M.; Bullova, E.; Petko, B. Climate warming and tick-borne encephalitis, Slovakia. Emerg. Infect. Dis. 2010, 16, 524–526. [Google Scholar] [CrossRef]
- Buczek, A.; Bartosik, K.A.; Wiśniowski, Ł.; Tomasiewicz, K. Changes in population abundance of adult Dermacentor reticulatus (Acari: Amblyommidae) in long-term investigations in eastern Poland. Ann. Agric. Environ. Med. 2013, 20, 269–272. [Google Scholar]
- Wilking, H.; Stark, K. Trends in surveillance data of human Lyme borreliosis from six federal states in eastern Germany, 2009–2012. Ticks Tick Borne Dis. 2014, 5, 219–224. [Google Scholar] [CrossRef]
- Estrada-Peña, A.; de la Fuente, J.; Latapia, T.; Ortega, C. The Impact of Climate Trends on a Tick Affecting Public Health: A Retrospective Modeling Approach for Hyalomma marginatum (Ixodidae). PLoS ONE 2015, 10, e0125760. [Google Scholar] [CrossRef]
- Emelianova, I.N. Ecology of the Tick Gnus Hyalomma Koch 1844 (Acarina: Ixodidae) in Central Caucasus and Adjacent Territories. Ph.D. Thesis, State University of Stavropol, Stavropol, Russia, 2006. [Google Scholar]
- Emelianova, I.N. Hyalomma Koch, 1844 (Acari: Ixodidae) Ticks of Central Precaucasia and Surrounding Territories. (Distribution, Ecology, Role in the Natural Foci of Crimean-Congo Heamorrhagic Fever). Master’s Thesis, State University of Stavropol, Stavropol, Russia, 2006. [Google Scholar]
- Kolonin, G.V. Birds as hosts of Ixodid ticks (Acari: Ixodidae). Entomol. Rev. 2008, 88, 1012–1015. [Google Scholar] [CrossRef]
- Movila, A.; Reye, A.L.; Dubinina, H.V.; Tolstenkov, O.O.; Toderas, I.; Hübschen, J.M.; Muller, C.P.; Alekseev, A.N. Detection of Babesia Sp. EU1 and members of spotted fever group rickettsiae in ticks collected from migratory birds at Curonian Spit, North-Western Russia. Vector Borne Zoonotic Dis. 2011, 11, 89–91. [Google Scholar] [CrossRef] [PubMed]
- Bona, M.; Stanko, M. First records of the tick Ixodes frontalis (Panzer, 1795) (Acari, Ixodidae) in Slovakia. Ticks Tick Borne Dis. 2013, 4, 478–481. [Google Scholar] [CrossRef] [PubMed]
- Biernat, B.; Stańczak, J.; Michalik, J.; Sikora, B.; Cieniuch, S. Rickettsia helvetica and R. monacensis infections in immature Ixodes ricinus ticks derived from sylvatic passerine birds in west-central Poland. Parasitol. Res. 2016, 115, 3469–3477. [Google Scholar] [CrossRef]
- Klaus, C.; Gethmann, J.; Hoffmann, B.; Ziegler, U.; Heller, M.; Beer, M. Tick infestation in birds and prevalence of pathogens in ticks collected from different places in Germany. Parasitol. Res. 2016, 115, 2729–2740. [Google Scholar] [CrossRef]
- Klitgaard, K.; Højgaard, J.; Isbrand, A.; Madsen, J.J.; Thorup, K.; Bødker, R. Screening for multiple tick-borne pathogens in Ixodes ricinus ticks from birds in Denmark during spring and autumn migration seasons. Ticks Tick Borne Dis. 2019, 10, 546–552. [Google Scholar] [CrossRef]
- Nowak-Chmura, M.; Siuda, K.; Wegner, Z.; Piksa, K. Species diversity of ticks (Acari: Ixodida) on migrating birds on the baltic sea coast of Poland. Zool. Stud. 2012, 51, 1411–1417. [Google Scholar]
- Hornok, S.; Kováts, D.; Csörgő, T.; Meli, M.L.; Gönczi, E.; Hadnagy, Z.; Takács, N.; Farkas, R.; Hofmann-Lehmann, R. Birds as potential reservoirs of tick-borne pathogens: First evidence of bacteraemia with Rickettsia helvetica. Parasit. Vectors 2014, 7, 128. [Google Scholar] [CrossRef]
- Lommano, E.; Dvořák, C.; Vallotton, L.; Jenni, L.; Gern, L. Tick-borne pathogens in ticks collected from breeding and migratory birds in Switzerland. Ticks Tick Borne Dis. 2014, 5, 871–882. [Google Scholar] [CrossRef]
- Sándor, A.D.; Mărcuţan, D.I.; D’Amico, G.; Gherman, C.M.; Dumitrache, M.O.; Mihalca, A.D. Do the ticks of birds at an important migratory hotspot reflect the seasonal dynamics of Ixodes ricinus at the migration initiation site? A case study in the Danube Delta. PLoS ONE 2014, 9, e89378. [Google Scholar] [CrossRef]
- Gryczyńska, A.; Welc-Falęciak, R. Long-term study of the prevalence of Borrelia burgdorferi s.l. infection in ticks (Ixodes ricinus) feeding on blackbirds (Turdus merula) in NE Poland. Exp. Appl. Acarol. 2016, 70, 381–394. [Google Scholar] [CrossRef] [PubMed]
- Hasle, G.; Bjune, G.A.; Midthjell, L.; Røed, K.H.; Leinaas, H.P. Transport of Ixodes ricinus infected with Borrelia species to Norway by northward-migrating passerine birds. Ticks Tick Borne Dis. 2011, 2, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Heylen, D.; De Coninck, E.; Jansen, F.; Madder, M. Differential diagnosis of three common Ixodes spp. ticks infesting songbirds of Western Europe: Ixodes arboricola, I. frontalis and I. ricinus. Ticks Tick Borne Dis. 2014, 5, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Norte, A.C.; da Silva, L.P.; Tenreiro, P.J.; Felgueiras, M.S.; Araújo, P.M.; Lopes, P.B.; Matos, C.; Rosa, A.; Ferreira, P.J.; Encarnação, P.; et al. Patterns of tick infestation and their Borrelia burgdorferi s.l. infection in wild birds in Portugal. Ticks Tick Borne Dis. 2015, 6, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Geller, J.; Nazarova, L.; Katargina, O.; Leivits, A.; Järvekülg, L.; Golovljova, I. Tick-borne pathogens in ticks feeding on migratory passerines in Western part of Estonia. Vector Borne Zoonotic Dis. 2013, 13, 443–448. [Google Scholar] [CrossRef]
- Berthová, L.; Slobodník, V.; Slobodník, R.; Olekšák, M.; Sekeyová, Z.; Svitálková, Z.; Kazimírová, M.; Špitalská, E. The natural infection of birds and ticks feeding on birds with Rickettsia spp. and Coxiella burnetti in Slovakia. Exp. Appl. Acarol. 2016, 68, 299–314. [Google Scholar] [CrossRef]
- Poupon, M.A.; Lommano, E.; Humair, P.F.; Douet, V.; Rais, O.; Schaad, M.; Jenni, L.; Gern, L. Prevalence of Borrelia burgdorferi Sensu Lato in Ticks Collected from Migratory Birds in Switzerland. Appl. Environ. Microbiol. 2006, 72, 976–979. [Google Scholar] [CrossRef]
- Heylen, D.; Fonville, M.; van Leeuwen, A.D.; Stroo, A.; Duisterwinkel, M.; van Wieren, S.; Diuk-Wasser, M.; de Bruin, A.; Sprong, H. Pathogen communities of songbird-derived ticks in Europe’s low countries. Parasit. Vectors 2017, 10, 497. [Google Scholar] [CrossRef]
- Pajoro, M.; Pistone, D.; Varotto Boccazzi, I.; Mereghetti, V.; Bandi, C.; Fabbi, M.; Scattorin, F.; Sassera, D.; Montagna, M. Molecular screening for bacterial pathogens in ticks (Ixodes ricinus) collected on migratory birds captured in northern Italy. Folia Parasitol (Praha) 2018, 65, 008. [Google Scholar] [CrossRef]
- Toma, L.; Mancini, F.; Di Luca, M.; Cecere, J.G.; Bianchi, R.; Khoury, C.; Quarchioni, E.; Manzia, F.; Rezza, G.; Ciervo, A. Detection of microbial agents in ticks collected from migratory birds in central Italy. Vector Borne Zoonotic Dis. 2014, 14, 199–205. [Google Scholar] [CrossRef]
- Diakou, A.; Norte, A.C.; de Carvalho, I.L.; Nuncio, S.; Novakova, M.; Kautman, M.; Alivizatos, H.; Kazantzidis, S.; Sychra, O.; Literak, I. Ticks and tick-borne pathogens in wild birds in Greece. Parasitol. Res. 2016, 115, 2011–2016. [Google Scholar] [CrossRef] [PubMed]
- Coipan, E.C.; Vladimirescu, A.F.; Ciolpan, O.; Teodorescu, I. Tick Species (Acari: Ixodoidea) Distribution, Seasonality and Host Associations in Romania. Trav. Mus. Natl. Hist. Nat. Grigore Antipa 2011, 54, 301–317. [Google Scholar] [CrossRef]
- Mărcuţan, I.D.; Kalmár, Z.; Ionică, A.M.; D’Amico, G.; Mihalca, A.D.; Vasile, C.; Sándor, A.D. Spotted fever group rickettsiae in ticks of migratory birds in Romania. Parasit. Vectors 2016, 9, 294. [Google Scholar] [CrossRef] [PubMed]
- Sándor, A.D.; Kalmár, Z.; Matei, I.; Ionică, A.M.; Mărcuţan, I.D. Urban breeding corvids as disseminators of ticks and emerging tick-borne pathogens. Vector Borne Zoonotic Dis. 2017, 17, 152–154. [Google Scholar] [CrossRef] [PubMed]
- Filippova, N.A. Ixodes eldaricus i ego rasprostranenie na yuge SSSR. Parazitologiya 1974, 8, 504–514. [Google Scholar]
- Keskin, A.; Erciyas-Yavuz, K. Ticks (Acari: Ixodidae) parasitizing passerine birds in Turkey with new records and new tick-host associations. J. Med. Entomol. 2019, 56, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Laakkonen, J.; Terhivuo, J.; Huhtamo, E.; Vapalahti, O.; Uzcàtegui, N.Y. First report of Ixodes frontalis (Acari: Ixodidae) in Finland, an example of foreign tick species transported by a migratory bird. Memo. Soc. Fauna Flora Fenn. 2009, 85, 16–19. [Google Scholar]
- Palomar, A.M.; Portillo, A.; Santibáñez, P.; Mazuelas, D.; Roncero, L.; Gutiérrez, Ó.; Oteo, J.A. Presence of Borrelia turdi and Borrelia valaisiana (Spirochaetales: Spirochaetaceae) in ticks removed from birds in the north of Spain, 2009–2011. J. Med. Entomol. 2017, 54, 243–246. [Google Scholar] [CrossRef]
- Graham, R.I.; Mainwaring, M.C.; Du Feu, R. Detection of spotted fever group Rickettsia spp. from bird ticks in the U.K. Med. Vet. Entomol. 2010, 24, 340–343. [Google Scholar] [CrossRef]
- Jameson, L.J.; Morgan, P.J.; Medlock, J.M.; Watola, G.; Vaux, A.G. Importation of Hyalomma marginatum, vector of Crimean-Congo haemorrhagic fever virus, into the United Kingdom by migratory birds. Ticks Tick Borne Dis. 2012, 3, 95–99. [Google Scholar] [CrossRef]
- Capek, M.; Literak, I.; Kocianova, E.; Sychra, O.; Najer, T.; Trnka, A.; Kverek, P. Ticks of the Hyalomma marginatum complex transported by migratory birds into Central Europe. Ticks Tick Borne Dis. 2014, 5, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Alekseev, A.N.; Dubinina, H.V.; Semenov, A.V.; Bolshakov, C.V. Evidence of ehrlichiosis agents found in ticks (Acari: Ixodidae) collected from migratory birds. J. Med. Entomol. 2001, 38, 471–474. [Google Scholar] [CrossRef] [PubMed]
- Comstedt, P.; Bergström, S.; Olsen, B.; Garpmo, U.; Marjavaara, L.; Mejlon, H.; Barbour, A.G.; Bunikis, J. Migratory passerine birds as reservoirs of Lyme borreliosis in Europe. Emerg. Infect. Dis. 2006, 12, 1087–1095. [Google Scholar] [CrossRef] [PubMed]
- Franke, J.; Meier, F.; Moldenhauer, A.; Straube, E.; Dorn, W.; Hildebrandt, A. Established and emerging pathogens in Ixodes ricinus ticks collected from birds on a conservation island in the Baltic Sea. Med. Vet. Entomol. 2010, 24, 425–432. [Google Scholar] [CrossRef]
- Dubska, L.; Literak, I.; Kocianova, E.; Taragelova, V.; Sychra, O. Differential role of passerine birds in distribution of Borrelia spirochetes, based on data from ticks collected from birds during the postbreeding migration period in Central Europe. Appl. Environ. Microbiol. 2009, 75, 596–602. [Google Scholar] [CrossRef]
- Capligina, V.; Salmane, I.; Keišs, O.; Vilks, K.; Japina, K.; Baumanis, V.; Ranka, R. Prevalence of tick-borne pathogens in ticks collected from migratory birds in Latvia. Ticks Tick Borne Dis. 2014, 5, 75–81. [Google Scholar] [CrossRef]
- Elfving, K.; Olsen, B.; Bergström, S.; Waldenström, J.; Lundkvist, A.; Sjöstedt, A.; Mejlon, H.; Nilsson, K. Dissemination of spotted fever rickettsia agents in Europe by migrating birds. PLoS ONE 2010, 5, e8572. [Google Scholar] [CrossRef]
- Kazarina, A.; Japina, K.; Keišs, O.; Salmane, I.; Bandere, D.; Capligina, V.; Ranka, R. Detection of tick-borne encephalitis virus in I. ricinus ticks collected from autumn migratory birds in Latvia. Ticks Tick Borne Dis. 2015, 6, 178–180. [Google Scholar] [CrossRef]
- Hildebrandt, A.; Fritzsch, J.; Franke, J.; Sachse, S.; Dorn, W.; Straube, E. Co-circulation of emerging tick-borne pathogens in Middle Germany. Vector Borne Zoonotic Dis. 2011, 11, 533–537. [Google Scholar] [CrossRef]
- Flaisz, B.; Sulyok, K.M.; Kováts, D.; Kontschán, J.; Csörgő, T.; Csipak, Á.; Gyuranecz, M.; Hornok, S. Babesia genotypes in Haemaphysalis concinna collected from birds in Hungary reflect phylogeographic connections with Siberia and the Far East. Ticks Tick Borne Dis. 2017, 8, 666–670. [Google Scholar] [CrossRef]
- Moutailler, S.; Valiente Moro, C.; Vaumourin, E.; Michelet, L.; Tran, F.H.; Devillers, E.; Cosson, J.F.; Gasqui, P.; Van, V.T.; Mavingui, P.; et al. Co-infection of ticks: The rule rather than the exception. PLoS Negl. Trop. Dis. 2016, 10, e0004539. [Google Scholar] [CrossRef] [PubMed]
- Raileanu, C.; Moutailler, S.; Pavel, I.; Porea, D.; Mihalca, A.D.; Savuta, G.; Vayssier-Taussat, M. Borrelia Diversity and co-infection with other tick borne pathogens in ticks. Front. Cell. Infect. Microbiol. 2017, 7, 36. [Google Scholar] [CrossRef] [PubMed]
- Norte, A.C.; de Carvalho, I.L.; Núncio, M.S.; Ramos, J.A.; Gern, L. Blackbirds Turdus merula as competent reservoirs for Borrelia turdi and Borrelia valaisiana in Portugal: Evidence from a xenodiagnostic experiment. Environ. Microbiol. Rep. 2013, 5, 604–607. [Google Scholar] [CrossRef] [PubMed]
- La Sorte, F.A.; Jetz, W. Avian distributions under climate change: Towards improved projections. J. Exp. Biol. 2010, 213, 862–869. [Google Scholar] [CrossRef]
- Cohen, E.B.; Moore, F.R.; Fischer, R.A. Experimental evidence for the interplay of exogenous and endogenous factors on the movement ecology of a migrating songbird. PLoS ONE 2012, 7, e41818. [Google Scholar] [CrossRef]
- Bókony, V.; Barta, Z.; Végvári, Z. Changing migratory behaviors and climatic responsiveness in birds. Front. Ecol. Evol. 2019, 7, 89. [Google Scholar] [CrossRef]
- Hüppop, O.; Hüppop, K. North Atlantic oscillation and timing of spring migration in birds. Proc. Biol. Sci. 2003, 270, 233–240. [Google Scholar] [CrossRef]
- Usui, T.; Butchart, S.H.; Phillimore, A.B. Temporal shifts and temperature sensitivity of avian spring migratory phenology: A phylogenetic meta-analysis. J. Anim. Ecol. 2017, 86, 250–261. [Google Scholar] [CrossRef]
- Cotton, P.A. Avian migration phenology and global climate change. Proc. Natl. Acad. Sci. USA 2003, 100, 12219–12222. [Google Scholar] [CrossRef]
- Gordo, O.; Brotons, L.; Ferrer, X.; Comas, P. Do changes in climate patterns in wintering areas affect the timing of the spring arrival of trans-Saharan migrant birds? Glob. Chang. Biol. 2005, 11, 12–21. [Google Scholar] [CrossRef]
- Marra, P.P.; Francis, C.M.; Mulvihill, R.S.; Moore, F.R. The influence of climate on the timing and rate of spring bird migration. Oecologia 2005, 142, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Nourani, E.; Yamaguchi, N.M. The effects of atmospheric currents on the migratory behavior of soaring birds: A review. Ornithol. Sci. 2017, 16, 5–15. [Google Scholar] [CrossRef]
- Haest, B.; Hüppop, O.; Bairlein, F. The influence of weather on avian spring migration phenology: What, where and when? Glob. Chang. Biol. 2018, 24, 5769–5788. [Google Scholar] [CrossRef] [PubMed]
- Møller, A.P.; Jokimäki, J.; Skorka, P.; Tryjanowski, P. Loss of migration and urbanization in birds: A case study of the blackbird (Turdus merula). Oecologia 2014, 175, 1019–1027. [Google Scholar] [CrossRef]
- Parkins, K.L.; Elbin, S.B.; Barnes, E. Light, glass, and bird-building collisions in an urban park. Northeast. Nat. 2015, 22, 84–94. [Google Scholar] [CrossRef]
- Watson, M.J.; Wilson, D.R.; Mennill, D.J. Anthropogenic light is associated with increased vocal activity by nocturnally migrating birds. Condor 2016, 118, 338–344. [Google Scholar] [CrossRef]
- Cabrera-Cruz, S.A.; Smolinsky, J.A.; Buler, J.J. Light pollution is greatest within migration passage areas for nocturnally-migrating birds around the world. Sci. Rep. 2018, 8, 3261. [Google Scholar] [CrossRef]
- de Jong, M.; Jeninga, L.; Ouyang, J.Q.; van Oers, K.; Spoelstra, K.; Visser, M.E. Dose-dependent responses of avian daily rhythms to artificial light at night. Physiol. Behav. 2016, 155, 172–179. [Google Scholar] [CrossRef]
- McWilliams, S.R.; Guglielmo, C.; Pierce, B.; Klaassen, M. Flying, fasting, and feeding in birds during migration: A nutritional and physiological ecology perspective. J. Avian Biol. 2004, 35, 377–393. [Google Scholar] [CrossRef]
- Dutta, H. Insights into the impacts of four current environmental problems on flying birds. Energ. Ecol. Environ. 2017, 2, 329–349. [Google Scholar] [CrossRef]
- Pease, M.L.; Rose, R.K.; Butler, M.J. Effects of human disturbances on the behavior of wintering ducks. Wildl. Society Bull. 2005, 33, 103–112. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buczek, A.M.; Buczek, W.; Buczek, A.; Bartosik, K. The Potential Role of Migratory Birds in the Rapid Spread of Ticks and Tick-Borne Pathogens in the Changing Climatic and Environmental Conditions in Europe. Int. J. Environ. Res. Public Health 2020, 17, 2117. https://doi.org/10.3390/ijerph17062117
Buczek AM, Buczek W, Buczek A, Bartosik K. The Potential Role of Migratory Birds in the Rapid Spread of Ticks and Tick-Borne Pathogens in the Changing Climatic and Environmental Conditions in Europe. International Journal of Environmental Research and Public Health. 2020; 17(6):2117. https://doi.org/10.3390/ijerph17062117
Chicago/Turabian StyleBuczek, Alicja M., Weronika Buczek, Alicja Buczek, and Katarzyna Bartosik. 2020. "The Potential Role of Migratory Birds in the Rapid Spread of Ticks and Tick-Borne Pathogens in the Changing Climatic and Environmental Conditions in Europe" International Journal of Environmental Research and Public Health 17, no. 6: 2117. https://doi.org/10.3390/ijerph17062117
APA StyleBuczek, A. M., Buczek, W., Buczek, A., & Bartosik, K. (2020). The Potential Role of Migratory Birds in the Rapid Spread of Ticks and Tick-Borne Pathogens in the Changing Climatic and Environmental Conditions in Europe. International Journal of Environmental Research and Public Health, 17(6), 2117. https://doi.org/10.3390/ijerph17062117