Qualitative Phenotyping of Obstructive Sleep Apnea and Its Clinical Usefulness for the Sleep Specialist
Abstract
1. Introduction
2. Methods
3. Anatomical Collapsibility and Passive Critical Occlusion Pressure (Pcrit)
4. Muscular Upper Airway Gain (UAG)
- Muscles that regulate the position of the hyoid bone, mainly the geniohyoid and sternohyoid muscles.
- Tongue base muscles, predominantly the genioglossus and other pharyngeal constrictors.
- Muscles of the soft palate, in particular the tensor palatini and elevator palatini muscles.
- Neurons modulating wakefulness/sleep state (active during wakefulness and less active or inactive during sleep).
- Neurons in the respiratory centers located in the ventro-lateral medulla oblongata (inspiratory phasic premotor activity, usually active 50–100 ms prior to activation of the diaphragm).
- UA mechanoreceptors triggered by negative endopharyngeal pressure (inspiratory phasic activity).
- Patients in whom the neuromuscular compensation offsets the anatomic collapsibility in all positions and in all sleep stages.
- Patients affected by OSA because neuromuscular compensation does not offset the anatomic collapsibility in all positions and in all sleep stages.
- Several additional mechanisms may cause a further reduction of the neuromuscular compensation during sleep:
5. Loop Gain (LG)
6. Arousal Threshold (AT)
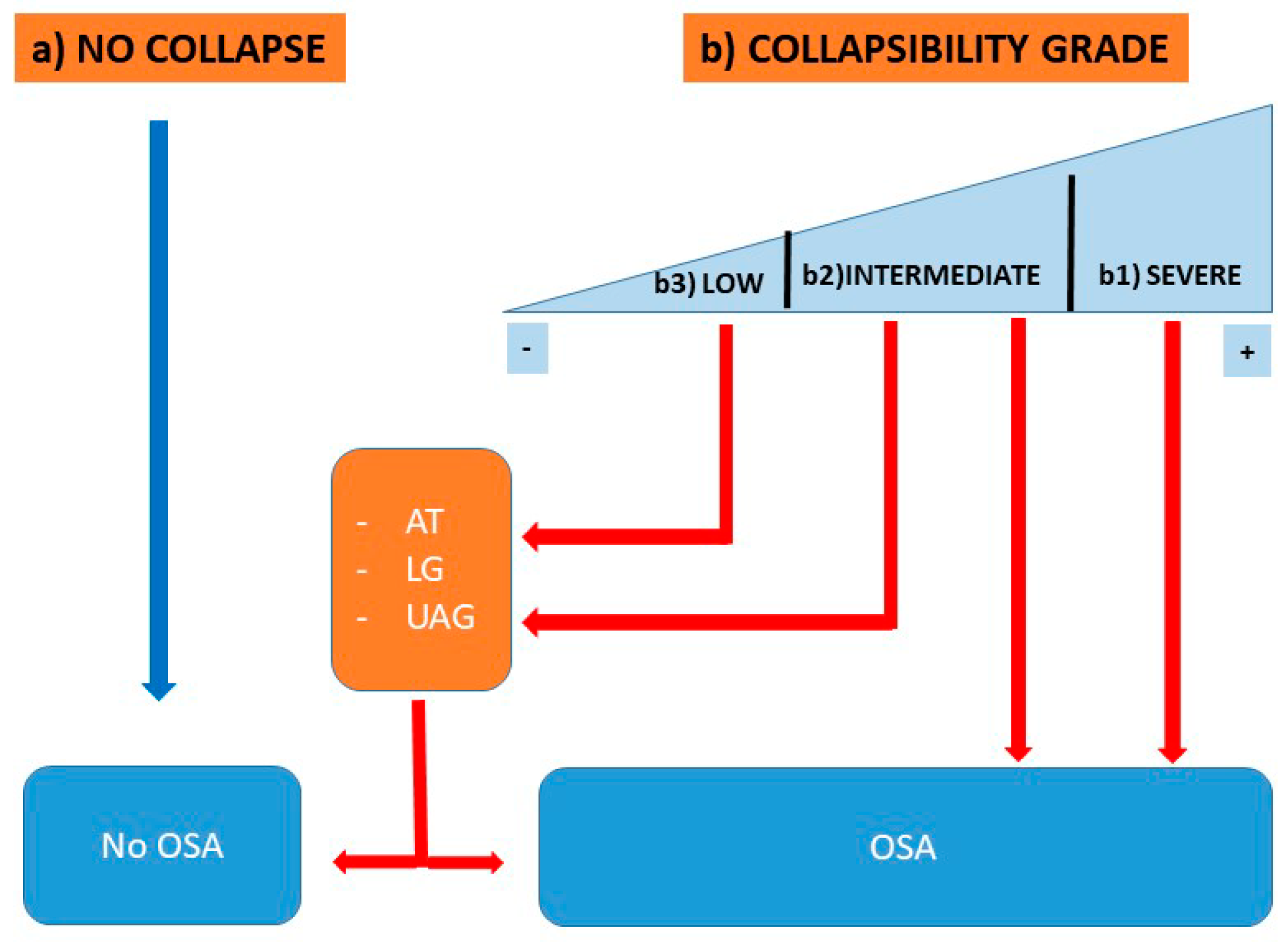
7. Qualitative Phenotyping Model of OSA
- >2.5 cmH2O—“High Collapsibility”
- +2.5– 2.5 cmH2O—“Intermediate Collapsibility”
- <−2.5 cmH2O—“Low Collapsibility”
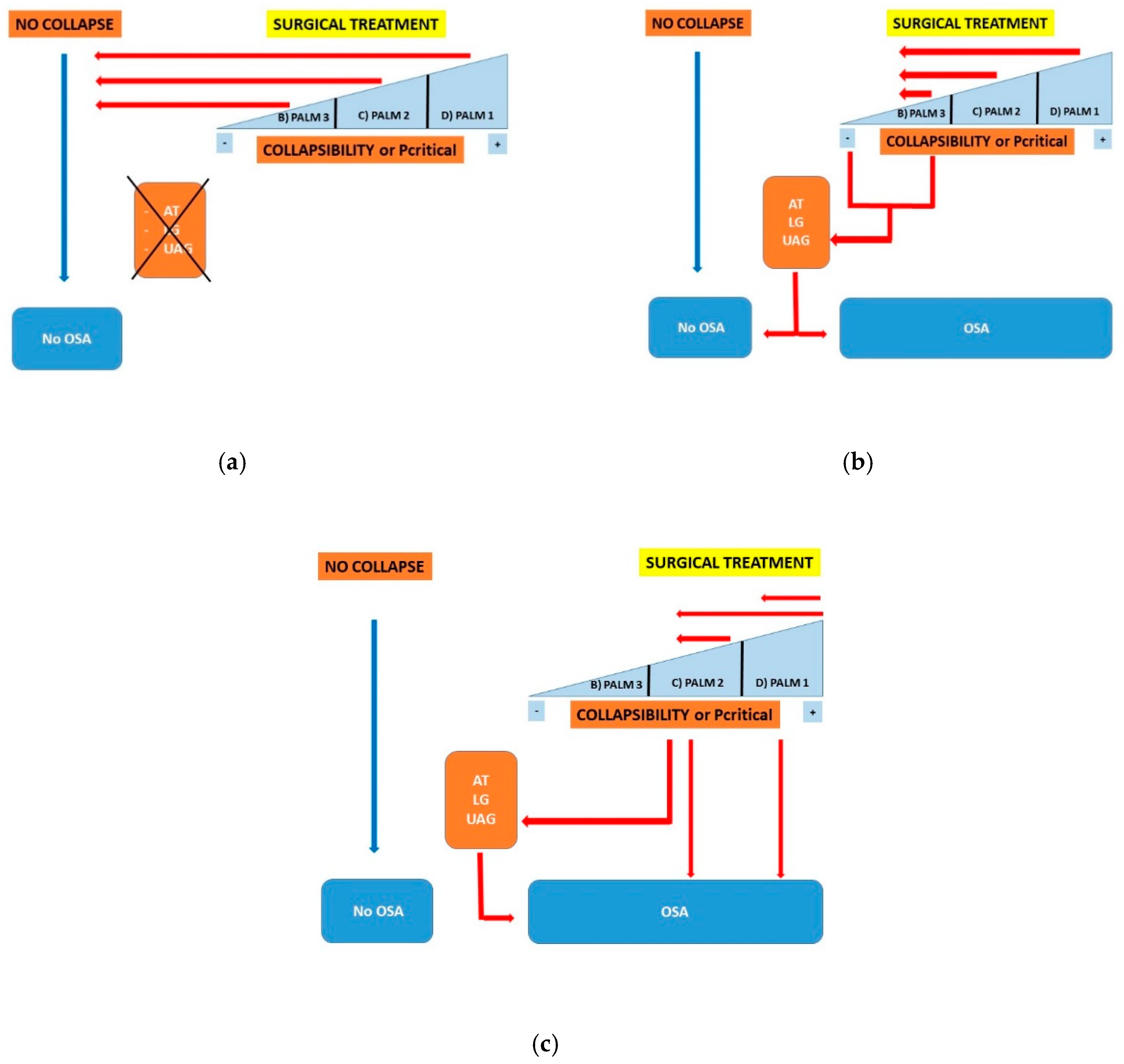
- PALM 1: This group involves about 23% of OSA patients and is characterized by a high anatomical collapsibility (Pcrit higher than +2.5 cmH2O). Weight loss, positional therapy, oral appliance (OA), CPAP, and upper airway surgery are the 1st line treatment as these treatments focus on anatomical factors (anatomical treatments).
- PALM 2: This is the largest subgroup and involves about 57% of patients with OSA; it is characterized by an intermediate collapsibility (Pcrit between +2.5 and −2.5 cmH2O). These patients are potential candidates for anatomical treatments (subgroup 2a) or for a combination of anatomical and non-anatomical treatments (subgroup 2b).
- PALM 3: This subgroup involves approximately 19% of OSA patients with low collapsibility (Pcrit less than −2.5 cmH2O), associated with abnormal non-anatomical PT. These patients are potential candidates for non-CPAP treatment options such as weight loss, OA, oxygen, and drugs which target the loop gain or the AT.
7.1. Polysomnographic Pattern
7.2. Therapeutic level of CPAP
7.3. Severity of OSA measured by the apnea hypopnea index (AHI)
- (A)
- Patients with a Pcrit> 2.5 cmH2O (high collapsibility, PALM 1) typically have a high AHI > 40/h.
- (B)
- Most patients with a Pcrit less than −2.5 cmH2O (low collapsibility, PALM 3) have an AHI < 40/h.
- (C)
- Patients with a Pcrit between –2.5 and +2.5 cmH2O (moderate collapsibility, PALM 2) can stratify at each level of AHI severity.
- (1)
- High collapsibility of the UA: In these cases, the presence of impaired LG and AT represents a negative predictive factor for any anatomical surgical outcome, as compared to patients in which the UA collapse represents the only PT impaired.
- (2)
- Low UA collapsibility: Patients with low anatomic collapsibility may benefit from targeted non-CPAP therapy (weight loss, mandibular advancement device (MAD), sleep stabilizing drugs, and oxygen). Any surgical approach in these patients should be reserved as a second-line therapeutic option. This subset of patients can reliably be identified.
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Young, T.; Palta, M.; Dempsey, J.; Skatrud, S.; Weber, J.; Badr, S. The occurrence of sleep disordered breathing among middle-aged adults. N. Engl. J. Med. 1993, 328, 1230–1235. [Google Scholar] [CrossRef] [PubMed]
- Roehrs, T.; Zorick, F.; Wittig, R.; Conway, W.A.; Roth, T. Predictors of level of daytime sleepiness in patients with sleep- related breathing disorders. Chest 1989, 95, 1202–1206. [Google Scholar] [CrossRef]
- Colt, H.; Haas, H.; Rich, G. Hypoxemia vs sleep fragmenta- tion as cause of excessive daytime sleepiness in obstructive apnea. Chest 1991, 100, 1542–1548. [Google Scholar] [CrossRef] [PubMed]
- Fulda, S.; Shulz, H. Cognitive dysfunction in sleep disorders. Sleep Med. Rev. 2001, 5, 423–445. [Google Scholar] [CrossRef] [PubMed]
- Beebe, D.; Gozal, D. Obstructive sleep apnea and the pre- frontal cortex: Towards a comprehensive model linking nocturnal upper airway obstruction to daytime cognitive and behavioral deficits. J. Sleep Res. 2002, 11, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Verstraeten, E.; Cluydts, R.; Pevernagie, D.; Hoffmann, G. Executive function in sleep apnea: Controlling for attentional capacity in assessing executive attention. Sleep 2004, 27, 685–693. [Google Scholar]
- Jenkinson, C.; Davies, R.; Mullins, R.; Stradling, J. Comparison of therapeutic and subtherapeutic nasal continuous positive air- way pressure for obstructive sleep apnoea: A randomized prospective parallel trial. Lancet 1999, 353, 2100–2105. [Google Scholar] [CrossRef]
- Montserrat, J.; Ferrer, F.; Hernadez, L.; Farré, R.; Vilagut, G.; Navajas, D.; Badia, J. Effectiveness of CPAP treatment in daytime function in sleep apnea syndrome: A randomized con-trolled Study with an optimized placebo. Am. J. Respir. Crit. Care Med. 2001, 164, 608–613. [Google Scholar] [CrossRef]
- Sassani, A.; Findley, L.; Kryger, M.; Goldlust, E.; George, C.; Davidson, T. Reducing motor-vehicle collisions, costs, and fatalities by treating obstructive sleep apnea syndrome. Sleep 2004, 27, 453–458. [Google Scholar] [CrossRef]
- He, J.; Kryger, M.; Zorick, F.; Conway, W.; Roth, T. Mortality and apnea index in obstructive sleep apnea. Experience in 385 male patients. Chest 1988, 94, 9–14. [Google Scholar] [CrossRef]
- Marin, J.; Carrizzo, S.; Vincente, E.; Agusti, A. Long-term cardiovascular outcomes in men with obstructive sleep apnea—hypopnea with or without treatment with continuous positive airway pressure: An observational study. Lancet 2005, 365, 1046–1053. [Google Scholar] [CrossRef]
- Croce, D.; Porazzi, E.; Castiglioni Rusconi, M. Impatto Socio-SanitarioDell’osas in Italia: The Cost of Illness. A Cura del Centro di Ricerca in Economia e Management in Sanità dell’Università Carlo Cattaneo; IstitutoSuperioredellaSanità: Roma, Italy, 1988. [Google Scholar]
- Osman, A.M.; Carter, S.G.; Carberry, J.C.; Eckert, D.J. Obstructive sleep apnea: Current perspectives. Nat. Sci. Sleep 2018, 10, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Arens, R.; Marcus, C. Pathophysiology of upper airway obstruction: A developmental perspective. Sleep 2004, 27, 997–1019. [Google Scholar] [CrossRef] [PubMed]
- Riha, R.L.; Brander, P.; Vennelle, M.; Douglas, N.J. A cephalometric comparison of patients with the sleep apnea/hypo-apnea syndrome and their siblings. Sleep 2005, 28, 315–320. [Google Scholar]
- Bacon, W.H.; Turlot, J.C.; Krieger, J.; Stierle, J.L. Cephalometric evaluation of pharyngeal obstructive factors in patients with sleep apneas syndrome. Angle Orthod. 1990, 60, 115–122. [Google Scholar]
- Riley, R.; Guilleminault, C.; Herran, J.; Powell, N. Cephalo-metric analyses and flow-volume loops in obstructive sleep apnea patients. Sleep 1983, 6, 303–311. [Google Scholar] [CrossRef]
- Rivlin, J.; Hoffstein, V.; Kalbfleisch, J.; McNicholas, W.; Zamel, N.; Bryan, A.C. Upper airway morphology in patients with idiopathic obstructive sleep apnea. Am. Rev. Respir. Dis. 1984, 129, 355–360. [Google Scholar] [CrossRef]
- Malhotra, A.; Huang, Y.; Fogel, R.B.; Pillar, G.; Edwards, J.K.; Kikinis, R.; Loring, S.H.; White, D.P. The male predisposition to obstructive sleep apnoea. Sleep 2018, 31, 1543–1549. [Google Scholar]
- Pae, E.K.; Lowe, A.A.; Fleetham, J.A. A role of pharyngeal length in obstructive sleep apnea patients. Am. J. Orthod. Dentofac. Orthop. 1997, 111, 12–17. [Google Scholar] [CrossRef]
- Gaudette, E.; Kimoff, R.J. Pathophysiology of OSA sleep apnoea. Eur. Respir. Mon. 2010, 1, 31–50. [Google Scholar]
- Pevernagie, D.A.; Stanson, A.W.; Sheedy, P.F.; Daniels, B.K. Shepard Effects of body position on the upper airway of patients with obstructive sleep apnea. Am. J. Respir. Crit. Care Med. 1995, 152, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Schwab, R.J.; Gupta, K.B.; Gefter, W.B.; Metzger, L.J.; Hoffman, E.A.; Pack, A.I. Upper airway and soft tissue anatomy in normal subjects and patients with sleep-disordered breathing. Significance of the lateral pharyngeal walls. Am. J. Respir. Crit. Care Med. 1995, 152, 1673–1689. [Google Scholar] [CrossRef] [PubMed]
- Schwab, J.; Pasirstein, M.; Pierson, R.; Mackley, A.; Hachadoorian, R.; Arens, R.; Maislin, G. Identification of upper airway anatomic risk factors for obstructive sleep apnea with volumetric magnetic resonance imaging. Am. J. Respir. Crit. Care Med. 2003, 168, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Stauffer, J.L.; Buick, M.K.; Bixler, E.O.; Sharkey, F.E.; Abt, A.B.; Manders, E.K.; Kales, A. Morphology of the uvula in obstructive sleep apnea. Am. J. Respir. Crit. Care Med. 1989, 140, 724–728. [Google Scholar] [CrossRef] [PubMed]
- Horner, R.L.; Shea, S.A.; Mcivor, J.; Guz, A. Pharyngeal size and shape during wakefulness and sleep in patients with obstructive sleep apnoea. QJM Int. J. Med. 1989, 72, 719–735. [Google Scholar]
- Steier, J.; Jolley, C.J.; Seymour, J. Increased load on the respiratory muscles in obstructive apnea. Respir. Physiol. Neurobiol. 2010, 171, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Steier, J.; Jolley, C.J.; Seymour, J. Neural respiratory drive in obesity. Thorax 2009, 64, 719–725. [Google Scholar] [CrossRef] [PubMed]
- Steier, J.; Lunt, A.; Hart, N. Observational study of effect of obesity on lung volumes. Thorax 2014, 69, 752–759. [Google Scholar] [CrossRef]
- Series, F.; Cormier, Y.; Desmeules, M. Influence of passive changes of lung volume on upper airways. J. Appl. Physiol. 1990, 68, 2159–2164. [Google Scholar] [CrossRef]
- Series, F.; Marc, I. Influence of lung volume dependence of upper airway resistance during continuous negative airway pressure. J. Appl. Physiol. 1994, 77, 840–844. [Google Scholar] [CrossRef]
- Findley, L.J.; Ries, A.L.; Tisi, G.M.; Wagner, P.D. Hypoxemia during apnoea in normal subjects: Mechanisms and impact of lung volume. J. Appl. Physiol. 1983, 55, 1777–1783. [Google Scholar] [CrossRef] [PubMed]
- Walsh, J.H.; Leigh, M.S.; Paduch, A.; Maddison, K.J.; Armstrong, J.J.; Sampson, D.D.; Hillman, D.R. Eastwood PR Effect of body posture on pharyngeal shape and size in adults with and without obstructive sleep apnoea. Sleep 2008, 31, 1543–1549. [Google Scholar] [CrossRef] [PubMed]
- Oksenberg, A.; Silverberg, D.S. The effect of body posture on sleep-related breathing disorders: Facts and therapeutic implications. Sleep Med. Rev. 1998, 2, 139–162. [Google Scholar] [CrossRef]
- Series, F.; Cote’, C.; St Pierre, S. Dysfunctional mechanical coupling of upper airway tissues in sleep apnoea syndrome. Am. J. Respir. Crit. Care Med. 1999, 159, 1551–1555. [Google Scholar] [CrossRef]
- Saboisky, J.P.; Butler, J.E.; Gandevia, S.C.; Eckert, D.J. Functional role of neural injury in obstructive sleep apnea. Front. Neurol. 2012, 3, 95. [Google Scholar] [CrossRef]
- Saboisky, J.P.; Stashuk, D.W.; Hamilton-Wright, A.; Carusona, A.L.; Campana, L.M.; Trinder, J.; Eckert, D.J.; Jordan, A.S.; McSharry, D.G.; White, D.P.; et al. Neurogenic changes in the upper airway of patients with obstructive sleep apnea. Am. J. Respir. Crit. Care Med. 2012, 185, 322–329. [Google Scholar] [CrossRef]
- Redolfi, S.; Yumino, D.; Ruttanaumpawan, P.; Yau, B.; Su, M.C.; Lam, J.; Bradley, T.D. Relationship between overnight rostral fluid shift and obstructive sleep apnea in nonobese men. Am. J. Respir. Crit. Care Med. 2009, 179, 241–246. [Google Scholar] [CrossRef]
- Kent, B.D.; Steier, J. A brief history of fluid and sleep. Comment on Effect of ultrafiltration on sleep apnea and sleep structure in patients with end-stage renal disease. Am. J. Respir. Crit. Care Med. 2015, 191, 1219–1220. [Google Scholar] [CrossRef]
- Lyons, O.D.; Chan, C.T.; Yadollahi, A.; Bradley, T.D. Effect of ultrafiltration on sleep apnea and sleep structure in patients with end-stage renal disease. Am. J. Respir. Crit. Care Med. 2015, 191, 1287–1294. [Google Scholar] [CrossRef]
- De Vito, A.; Berrettini, S.; Carabelli, A.; Sellari-Franceschini, S. The importance of nasal resistance in obstructive sleep apnea syndrome: A study with positional rhinomanometry. Sleep Breath 2001, 5, 3–11. [Google Scholar] [CrossRef]
- McNicholas, W.T.; Tarlo, S.; Cole, P.; Zamel, N.; Rutherford, R.; Griffin, D.; Phillipson, E.A. Obstructive apnoeas during sleep in patients with seasonal allergic rhinitis. Am. Rev. Respir. Dis. 1982, 126, 625–628. [Google Scholar] [PubMed]
- Kiely, J.L.; Nolan, P.; McNicholas, W.T. Intranasal corticosteroid therapy for obstructive sleep apnoea in patients with coexisting rhinitis. Thorax 2004, 59, 50–55. [Google Scholar] [PubMed]
- Wijesuriya, N.S.; Lewis, C.; Butler, J.E.; Lee, B.B.; Jordan, A.S.; Berlowitz, D.J.; Eckert, D.J. High nasal resistance is stable over time but poorly perceived in people with tetraplegia and obstructive sleep apnoea. Respir. Physiol. Neurobiol. 2017, 235, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Gainche, L.; Berlowitz, D.J.; LeGuen, M.; Ruehland, W.R.; O’Donoghue, F.J.; Trinder, J.; Graco, M.; Schembri, R.; Eckert, D.J.; Rochford, P.D.; et al. Nasal Resistance Is Elevated in People with Tetraplegia and Is Reduced by Topical Sympathomimetic Administration. J. Clin. Sleep Med. 2016, 12, 1487–1492. [Google Scholar] [CrossRef]
- Pien, G.W.; Keenan, B.T.; Marcus, C.L. An examination of methodological paradigms for calculating upper airway critical pressures during sleep. Sleep 2016, 39, 977–987. [Google Scholar] [CrossRef]
- White, D.P. Sleep apnea. Proc. ATS 2006, 3, 124–128. [Google Scholar] [CrossRef]
- Mezzanotte, W.S.; Tangel, D.J.; White, D.P. Influence of sleep onset on upper-airway muscle activity in apnea patients versus normal controls. Am. J. Respir. Crit. Care Med. 1996, 153, 1880–1887. [Google Scholar] [CrossRef]
- Lu, J.W.; Mann, G.L.; Ross, R.J.; Morrison, A.R.; Kubin, L. Differential effect of sleep-wake states on lingual and dorsal neck muscle activity in rats. Respir. Physiol. Neurobiol. 2005, 147, 191–203. [Google Scholar] [CrossRef]
- Lo, Y.L.; Jordan, A.S.; Malhotra, A.; Wellman, A.; Heinzer, R.A.; Eikermann, M.; Schory, K. Influence of wakefulness on pharyngeal airway muscle activity. Thorax 2007, 62, 799–805. [Google Scholar] [CrossRef]
- Fogel, R.B.; Malhotra, A.; White, D.P. Sleep 2: Pathophysiology of obstructive sleep apnoea/hypopnoea syndrome. Thorax 2004, 59, 159–163. [Google Scholar] [CrossRef]
- Pierce, R.; White, D.; Malhotra, A.; Edwards, J.K. Upper airway collapsibility, dilator muscle activation and resistance in sleep apnoea. Eur. Respir. J. 2007, 30, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Fogel, R.J.; Trinder, J.; White, D.P.; Malhotra, A.; Raneri, J.; Schory, K.; Kleverlaan, D. The effect of sleep onset on upper airway muscle activity in patients with sleep apnoea versus controls. J. Physiol. 2005, 564, 549–562. [Google Scholar] [CrossRef] [PubMed]
- Fogel, R.B.; Malhotra, A.; Pillar, G.; Edwards, J.K.; Beauregard, J.; Shea, S.A.; White, D.P. Genioglossal activation in patients with obstructive sleep apnea versus control subjects. Mechanisms of muscle control. Am. J. Respir. Crit. Care Med. 2001, 164, 2025–2030. [Google Scholar] [CrossRef] [PubMed]
- Mezzanotte, W.S.; Tangel, D.J.; White, D.P. Waking genioglossal electromyogram in sleep apnea patients versus normal controls (a neuromuscular compensatory mechanism). J. Clin. Investig. 1992, 89, 1571–1579. [Google Scholar] [CrossRef] [PubMed]
- Kirkness, J.P.; Peterson, L.A.; Squier, S.B. Performance characteristics of upper airway critical collapsing pressure measurements during sleep. Sleep 2011, 34, 459–467. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Boyd, J.H.; Petrof, B.J.; Hamid, Q.; Fraser, R.; Kimoff, R.J. Upper airway muscle inflammation and denervation changes in obstructive sleep apnea. Am. J. Respir. Crit. Care Med. 2004, 170, 541–546. [Google Scholar] [CrossRef]
- Lindman, R.; Stal, P.S. Abnormal palatopharyngeal muscle morphology in sleep-disordered breathing. J. Neurol. Sci. 2002, 195, 11–23. [Google Scholar] [CrossRef]
- Svanborg, E. Impact of obstructive apnea syndrome on upper airway respiratory muscles. Respir. Physiol. Neurobiol. 2005, 147, 263–272. [Google Scholar] [CrossRef]
- Friberg, D.; Ansved, T.; Borg, C.; Carlsson-Nordlander, B.; Larsson, H.; Svanborg, E. Histological indications of a progressive snorers disease in an upper airway muscle. Am. J. Respir. Crit. Care Med. 1998, 157, 586–593. [Google Scholar] [CrossRef]
- Carrera, M.; Barbe´, F.; Sauleda, J.; Toma´s, M.; Go´mez, C.; Agustı´, A. Patients with obstructive sleep apnea exhibit genioglossus dysfunction that is normalized after treatment with continuous positive airway pressure. Am. J. Respir. Crit. Care Med. 1999, 159, 1960–1966. [Google Scholar] [CrossRef]
- Owens, R.L.; Edwards, B.A.; Eckert, D.J. An Integrative model of physiological traits can be used to predict Obstructive Sleep Apnea and response to non-positive airway pressure therapy. Sleep 2015, 38, 961–970. [Google Scholar] [CrossRef] [PubMed]
- Wellman, A.; Genta, P.R.; Owens, R.L. Test of starling resistor model in the human upper airway during sleep. J. Appl. Physiol. 1985, 117, 1478–1485. [Google Scholar] [CrossRef] [PubMed]
- Eckert, D.J.; White, D.P.; Jordan, A.S. Defining phenotypic causes of obstructive sleep apnoea: Identification of novel therapeutic targets. Am. J. Respir. Crit. Care Med. 2013, 188, 996–1004. [Google Scholar] [CrossRef] [PubMed]
- Azarbarzin, A.; Sands, S.A.; Marques, M. Palatal prolapse as a signature of expiratory flow limitation and inspiratory palatal collapse in patients with obstructive sleep apnoea. Eur. Respir. J. 2018, 51, 1701419. [Google Scholar] [CrossRef] [PubMed]
- Genta, P.R.; Sands, S.A.; Butler, J.P. Airflow shape is associated with the pharyngeal structure causing OSA. Chest 2017, 152, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Azarbarzin, A.; Marques, M.; Sands, S.A. Predicting epiglottic collapse in patients with obstructive sleep apnoea. Am. Eur. Respir. J. 2017, 50, 1700345. [Google Scholar] [CrossRef]
- Sands, S.A.; Owens, R.L. Congestive heart failure and central sleep apnea. Sleep Med. Clin. 2016, 11, 127–142. [Google Scholar] [CrossRef]
- Eckert, D.J. Phenotypic approaches to obstructive sleep apnoea—New pathways for targeted therapy. Sleep Med. Rev. 2018, 37, 45–59. [Google Scholar] [CrossRef]
- Meza, S.; Younes, M. Ventilatory stability during sleep studied with proportional assist ventilation (PAV). Sleep 1996, 19, S164–S166. [Google Scholar] [CrossRef]
- Wellman, A.; Edwards, B.A.; Sands, S.A. A simplified method for determining phenotypic traits in patients with obstructive sleep apnoea. J. Appl. Physiol. 2013, 114, 911–922. [Google Scholar] [CrossRef]
- Terrill, P.I.; Edwards, B.A.; Nemati, S. Quantifying the ventilatory control contribution to sleep apnoea using polysomnography. Eur. Respir. J. 2015, 45, 408–418. [Google Scholar] [CrossRef] [PubMed]
- Bosi, M.; De Vito, A.; Kotecha, B. Phenotyping the pathophysiology of obstructive sleep apnea using polygraphy/polysomnography: A review of the literature. Sleep Breath. 2018. [Google Scholar] [CrossRef] [PubMed]
- Sands SA, Owens RL, Malhotra A New approaches to diagnosis sleep-disordered breathing. Sleep Med. Clin. 2016, 11, 143–152. [CrossRef] [PubMed]
- Berry, R.B.; Gleeson, K. Respiratory arousal from sleep: Mechaninsm and significance. Sleep 1997, 20, 654–675. [Google Scholar] [CrossRef] [PubMed]
- Americam Academy Sleep Medicine. International Classification of Sleep Disorders; AASM: Darien, IL, USA, 2014. [Google Scholar]
- Luo, Y.M.; Wu, H.D.; Tang, J.; Jolley, C.; Steier, J.; Moxham, J.; Zhong, N.S.; Polkey, M.I. Neural respiratory drive during apnoeic events in obstructive sleep apnoea. Eur. Respir. J. 2008, 31, 650–657. [Google Scholar] [CrossRef] [PubMed]
- Younes, M.J. Role of respiratory control mechanisms in the pathogenesis of obstructive sleep disorders. Appl. Physiol. 2008, 105, 1389–1405. [Google Scholar] [CrossRef]
- Xiao, S.C.; He, B.T.; Steier, J.; Moxham, J.; Polkey, M.I.; Luo, Y.M. Neural respiratory drive and arousal in patients with obstructive sleep apnea hypopnea. Sleep 2015, 38, 941–949. [Google Scholar] [CrossRef][Green Version]
- Eckert, D.J.; Younes, M.K. Arousal from sleep: Implications for obstructive sleep apnea pathogenesis and treatment. Appl. Physiol. 2014, 116, 302–313. [Google Scholar] [CrossRef]
- Younes M Role of arousals in the pathogenesis of obstructive sleep apnea. Am. J. Respir. Crit. Care Med. 2004, 169, 623–633. [CrossRef]
- Dempsey, J.A.; Veasey, S.C. Morgan BJ Pathophysiology of sleep apnea. Physiol. Rev. 2010, 90, 47–112. [Google Scholar] [CrossRef]
- Sands, S.A.; Terrill, P.I.; Edwards, B.A. Quantifying the Arousal Threshold Using Polysomnography in Obstructive Sleep Apnea. Sleep 2018, 41, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Edwards, B.A.; Eckert, D.J.; McSharry, D.G.; Sands, S.A.; Desai, A.; Kehlmann, G.; Bakker, J.P.; Genta, P.R.; Owens, R.L.; White, D.P.; et al. Clinical predictors of the respiratory arousal threshold in patients with obstructive sleep apnea. Am. J. Respir. Crit. Care Med. 2014, 190, 1293–1300. [Google Scholar] [CrossRef] [PubMed]
- Gray, E.L.; McKenzie, D.K.; Eckert, D.J. Obstructive Sleep Apnea without Obesity Is Common and Difficult to Treat: Evidence for a Distinct Pathophysiological Phenotype. J. Clin. Sleep Med. 2017, 13, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.W.W.; Sutherland, K.; Sands, S.A.; Edwards, B.A.; Chan, T.O.; SS Ng, S.; Hui, D.S.; Cistulli, P.A. Differences in respiratory arousal threshold in Caucasian and Chinese patients with obstructive sleep apnoea. Respirology 2017, 22, 1015–1021. [Google Scholar] [CrossRef]
- Carberry, J.C.; Amatoury, J.; Eckert, D. Personalized Management Approach for OSA. Chest 2018, 153, 744–755. [Google Scholar] [CrossRef]
- Schwartz, A.R.; Schubert, N.; Rothman, W. Effect of uvulopalatopharyngoplasty on upper airway collapsibility in obstructive sleep apnea. Am. Rev. Respir. Dis. 1992, 145, 527–532. [Google Scholar] [CrossRef]
- Sands, S.A.; Edwards, B.A.; Terrill, P.I. Phenotyping Pharyngeal Pathophysiology using Polysomnography in Patients with Obstructive Sleep Apnea. Am. J. Respir. Crit. Care Med. 2018, 197, 1187–1192. [Google Scholar] [CrossRef]
- Gold, A.R.; Schwartz, A.R. The pharyngeal critical pressure. The whys and hows of using nasal continuous positive airway pressure diagnostically. Chest 1996, 110, 1077–1088. [Google Scholar] [CrossRef]
- Gleadhill, I.C.; Schwartz, A.R.; Schubert, N. Upper airway collapsibility in snorers and in patients with obstructive hypopnea and apnea. Am. Rev. Respir. Dis. 1991, 143, 1300–1303. [Google Scholar] [CrossRef]
- Gold, A.R.; Marcus, C.L.; Dipalo, F. Gold MS Upper airway collapsibility during sleep in upper airway resistance syndrome. Chest 2002, 121, 1531–1540. [Google Scholar] [CrossRef]
- Landry, S.A.; Joosten, S.A.; Eckert, D.J. Therapeutic CPAP Level Predicts Upper Airway Collapsibility in Patients with Obstructive Sleep Apnea. Sleep 2017, 40. [Google Scholar] [CrossRef] [PubMed]
| (I) ANATOMIC COLLAPSIBILITY | (II) AROUSAL THRESHOLD |
| PALM 1 or 2 (1) Obstructive Apnea pattern (High Pcrit) (2) Severe AHI (High Pcrit) PALM 3 (1) UARS pattern (Low Pcrit) (2) CPAP value ≤ 8cm H2O (Low Pcrit) | LOW AT (1) At least 2 out of 3 PSG VARIABLES: AHI <3O Hypopnea/Apnea > 58.3% Nadir > 82.5% (2) UARS pattern HIGH AT Duration and severity of desaturations |
| (III) VENTILATORY INSTABILITY | (IV) MUSCULAR RECOVERY |
| (1) Coexistence of OSA and CSR (2)High proportion of central/mixed event (3) N-REM predominant patterns | (1) Starling resistor pattern (2)Intra-breath negative dependence pattern (3) Intra-event negative dependence pattern |
| Collapsibility. | AHI | CPAP Value | PALM Classification |
|---|---|---|---|
| less than −2.5 cmH2O | <40 | ≤8 cmH2O | PALM 3 |
| between −2.5 and +2.5 cmH2O | <40 | >8 cmH2O | PALM 2 |
| more than −2.5 cmH2O | >40 | >8 cmH2O | PALM 2 or 1 |
| Ventilatory Instability | Arousal Threshold (AT) |
|---|---|
| (1) Coexistence of OSA and CSR (2) High proportion of central/mixed respiratory events (3) NREM dominant pattern (CAP dominant OSA) | Low AT (1) At least 2/3 variables (AHI<30, H/A> 58.3%, SaO2 Nadir >82.5%)High AT (2) Long event duration and severe desaturations (e.g., OHS) |
| UA COLLAPSIBILITY | LOW AROUSAL THRESHOLD | HIGH LOOP GAIN |
|---|---|---|
| high collapsibility | yes/no | yes/no |
| intermediate collapsibility | yes/no | yes/no |
| low collapsibility | yes/no | yes/no |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bosi, M.; De Vito, A.; Eckert, D.; Steier, J.; Kotecha, B.; Vicini, C.; Poletti, V. Qualitative Phenotyping of Obstructive Sleep Apnea and Its Clinical Usefulness for the Sleep Specialist. Int. J. Environ. Res. Public Health 2020, 17, 2058. https://doi.org/10.3390/ijerph17062058
Bosi M, De Vito A, Eckert D, Steier J, Kotecha B, Vicini C, Poletti V. Qualitative Phenotyping of Obstructive Sleep Apnea and Its Clinical Usefulness for the Sleep Specialist. International Journal of Environmental Research and Public Health. 2020; 17(6):2058. https://doi.org/10.3390/ijerph17062058
Chicago/Turabian StyleBosi, Marcello, Andrea De Vito, Danny Eckert, Joerg Steier, Bhik Kotecha, Claudio Vicini, and Venerino Poletti. 2020. "Qualitative Phenotyping of Obstructive Sleep Apnea and Its Clinical Usefulness for the Sleep Specialist" International Journal of Environmental Research and Public Health 17, no. 6: 2058. https://doi.org/10.3390/ijerph17062058
APA StyleBosi, M., De Vito, A., Eckert, D., Steier, J., Kotecha, B., Vicini, C., & Poletti, V. (2020). Qualitative Phenotyping of Obstructive Sleep Apnea and Its Clinical Usefulness for the Sleep Specialist. International Journal of Environmental Research and Public Health, 17(6), 2058. https://doi.org/10.3390/ijerph17062058






