Rationale, Study Design, and Cohort Characteristics for the Markers for Environmental Exposures (MEE) Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
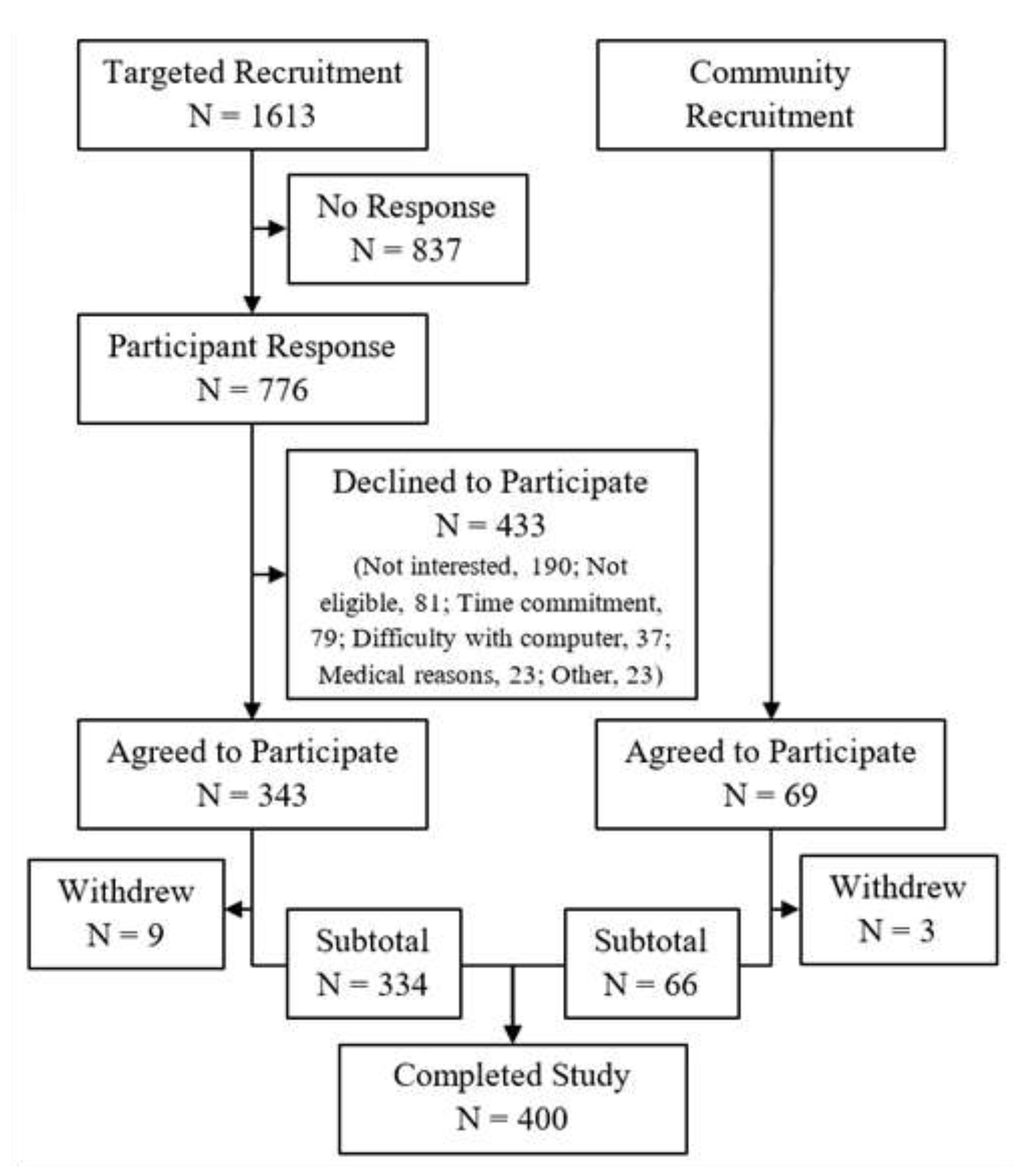
2.2. Study Population
2.3. Specimen and Data Collection
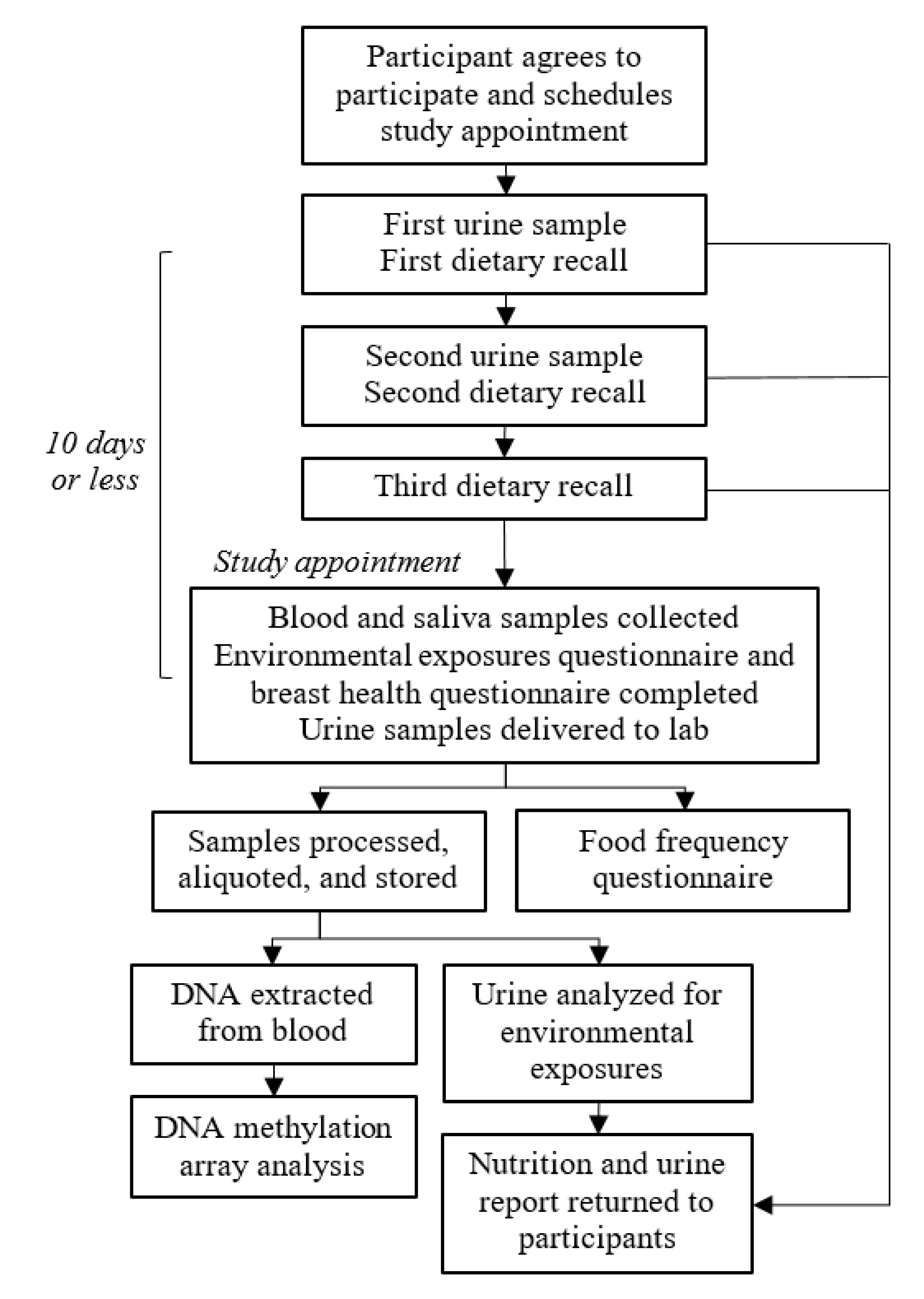
2.3.1. Specimen Collection, Processing, and Storage
2.3.2. Environmental Exposures and Breast Health Data Collection
2.3.3. Dietary Data Collection and Processing
2.3.4. DNA Methylation Data Collection and Processing
2.3.5. Questionnaire Data Curation
2.3.6. Statistical Analysis
3. Results
3.1. Specimen and Questionnaire Collection
3.2. Participant Demographics
3.3. Lifestyle and Health History
3.4. Environmental Exposures
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lichtenstein, P.; Holm, N.V.; Verkasalo, P.K.; Iliadou, A.; Kaprio, J.; Koskenvuo, M.; Pukkala, E.; Skytthe, A.; Hemminki, K. Environmental and heritable factors in the causation of cancer: Analyses of cohorts of twins from Sweden, Denmark, and Finland. N. Engl. J. Med. 2000, 343, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Foulkes, W.D. Inherited susceptibility to common cancers. N. Engl. J. Med. 2008, 359, 2143–2153. [Google Scholar] [CrossRef] [PubMed]
- Tomasetti, C.; Vogelstein, B. Variation in cancer risk among tissues can be explained by the number of stem cell divisions. Science 2015, 347, 78–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mucci, L.A.; Hjelmborg, J.B.; Harris, J.R.; Czene, K.; Havelick, D.J.; Scheike, T.; Graff, R.E.; Holst, K.; Möller, S.; Unger, R.H.; et al. Familial risk and heritability of cancer among twins in Nordic countries. JAMA J. Am. Med. Assoc. 2016, 315, 68–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forman, M.R.; Winn, D.M.; Collman, G.W.; Rizzo, J.; Birnbaum, L.S. Environmental exposures, breast development and cancer risk: Through the looking glass of breast cancer prevention. Reprod. Toxicol. 2015, 54, 6–10. [Google Scholar] [CrossRef]
- Cosselman, K.E.; Navas-Acien, A.; Kaufman, J.D. Environmental factors in cardiovascular disease. Nat. Rev. Cardiol. 2015, 12, 627–642. [Google Scholar] [CrossRef]
- Béjot, Y.; Reis, J.; Giroud, M.; Feigin, V. A review of epidemiological research on stroke and dementia and exposure to air pollution. Int. J. Stroke 2018, 13, 687–695. [Google Scholar] [CrossRef]
- De Miguel-Díez, J.; Hernández-Vázquez, J.; López-De-Andrés, A.; Álvaro-Meca, A.; Hernández-Barrera, V.; Jiménez-García, R. Analysis of environmental risk factors for chronic obstructive pulmonary disease exacerbation: A case-crossover study (2004–2013). PLoS ONE 2019, 14, e0217143. [Google Scholar] [CrossRef] [Green Version]
- Dendup, T.; Feng, X.; Clingan, S.; Astell-Burt, T. Environmental risk factors for developing type 2 diabetes mellitus: A systematic review. Int. J. Environ. Res. Public Health 2018, 15, 78. [Google Scholar] [CrossRef] [Green Version]
- Pizzorno, J. Environmental Toxins and Infertility. Integr. Med. (Encinitas) 2018, 17, 8–11. [Google Scholar]
- Cannon, J.R.; Greenamyre, J.T. The role of environmental exposures in neurodegeneration and neurodegenerative diseases. Toxicol. Sci. 2011, 124, 225–250. [Google Scholar] [CrossRef] [PubMed]
- Dietz, P.M.; Homa, D.; England, L.J.; Burley, K.; Tong, V.T.; Dube, S.R.; Bernert, J.T. Estimates of nondisclosure of cigarette smoking among pregnant and nonpregnant women of reproductive age in the United States. Am. J. Epidemiol. 2011, 173, 355–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shipton, D.; Tappin, D.M.; Vadiveloo, T.; Crossley, J.A.; Aitken, D.A.; Chalmers, J. Reliability of self reported smoking status by pregnant women for estimating smoking prevalence: A retrospective, cross sectional study. BMJ 2009, 339, b4347. [Google Scholar] [CrossRef] [Green Version]
- Leenen, F.A.D.; Muller, C.P.; Turner, J.D. DNA methylation: Conducting the orchestra from exposure to phenotype? Clin. Epigenet. 2016, 8, 92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meehan, R.R.; Thomson, J.P.; Lentini, A.; Nestor, C.E.; Pennings, S. DNA methylation as a genomic marker of exposure to chemical and environmental agents. Curr. Opin. Chem. Biol. 2018, 45, 48–56. [Google Scholar] [CrossRef]
- Ladd-Acosta, C.; Fallin, M.D. DNA methylation signatures as biomarkers of prior environmental exposures. Curr. Epidemiol. Rep. 2019, 6, 1–13. [Google Scholar] [CrossRef]
- Gao, X.; Jia, M.; Zhang, Y.; Breitling, L.P.; Brenner, H. DNA methylation changes of whole blood cells in response to active smoking exposure in adults: A systematic review of DNA methylation studies. Clin. Epigenetics 2015, 7, 113. [Google Scholar] [CrossRef] [Green Version]
- Shenker, N.S.; Polidoro, S.; van Veldhoven, K.; Sacerdote, C.; Ricceri, F.; Birrell, M.A.; Belvisi, M.G.; Brown, R.; Vineis, P.; Flanagan, J.M. Epigenome-wide association study in the European Prospective Investigation into Cancer and Nutrition (EPIC-Turin) identifies novel genetic loci associated with smoking. Hum. Mol. Genet. 2013, 22, 843–851. [Google Scholar] [CrossRef] [Green Version]
- Breitling, L.P.; Yang, R.; Korn, B.; Burwinkel, B.; Brenner, H. Tobacco-smoking-related differential DNA methylation: 27K discovery and replication. Am. J. Hum. Genet. 2011, 88, 450–457. [Google Scholar] [CrossRef] [Green Version]
- Philibert, R.; Hollenbeck, N.; Andersen, E.; Osborn, T.; Gerrard, M.; Gibbons, F.X.; Wang, K. A quantitative epigenetic approach for the assessment of cigarette consumption. Front. Psychol. 2015, 6, 656. [Google Scholar] [CrossRef] [Green Version]
- Shenker, N.S.; Ueland, P.M.; Polidoro, S.; van Veldhoven, K.; Ricceri, F.; Brown, R.; Flanagan, J.M.; Vineis, P. DNA methylation as a long-term biomarker of exposure to tobacco smoke. Epidemiology 2013, 24, 712–716. [Google Scholar] [CrossRef] [PubMed]
- Plusquin, M.; Guida, F.; Polidoro, S.; Vermeulen, R.; Raaschou-Nielsen, O.; Campanella, G.; Hoek, G.; Kyrtopoulos, S.A.; Georgiadis, P.; Naccarati, A.; et al. DNA methylation and exposure to ambient air pollution in two prospective cohorts. Environ. Int. 2017, 108, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Chi, G.C.; Liu, Y.; MacDonald, J.W.; Barr, R.G.; Donohue, K.M.; Hensley, M.D.; Hou, L.; McCall, C.E.; Reynolds, L.M.; Siscovick, D.S.; et al. Long-term outdoor air pollution and DNA methylation in circulating monocytes: Results from the Multi-Ethnic Study of Atherosclerosis (MESA). Environ. Health 2016, 15, 119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pavanello, S.; Bollati, V.; Pesatori, A.C.; Kapka, L.; Bolognesi, C.; Bertazzi, P.A.; Baccarelli, A. Global and gene-specific promoter methylation changes are related to anti-B[a]PDE-DNA adduct levels and influence micronuclei levels in polycyclic aromatic hydrocarbon-exposed individuals. Int. J. Cancer 2009, 125, 1692–1697. [Google Scholar] [CrossRef] [PubMed]
- White, A.J.; Chen, J.; Teitelbaum, S.L.; McCullough, L.E.; Xu, X.; Hee Cho, Y.; Conway, K.; Beyea, J.; Stellman, S.D.; Steck, S.E.; et al. Sources of polycyclic aromatic hydrocarbons are associated with gene-specific promoter methylation in women with breast cancer. Environ. Res. 2016, 145, 93–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horvath, S. DNA methylation age of human tissues and cell types. Genome Biol. 2013, 14, 3156. [Google Scholar] [CrossRef] [Green Version]
- Hannum, G.; Guinney, J.; Zhao, L.; Zhang, L.; Hughes, G.; Sadda, S.V.; Klotzle, B.; Bibikova, M.; Fan, J.B.; Gao, Y.; et al. Genome-wide methylation profiles reveal quantitative views of human aging rates. Mol. Cell 2013, 49, 359–367. [Google Scholar] [CrossRef] [Green Version]
- McPherson, K.; Steel, C.M.; Dixon, J.M. Breast cancer—Epidemiology, risk factors, and genetics. BMJ 2000, 321, 624–628. [Google Scholar] [CrossRef] [Green Version]
- Levine, M.E.; Lu, A.T.; Chen, B.H.; Hernandez, D.G.; Singleton, A.B.; Ferrucci, L.; Bandinelli, S.; Salfati, E.; Manson, J.A.E.; Quach, A.; et al. Menopause accelerates biological aging. Proc. Natl. Acad. Sci. USA 2016, 113, 9327–9332. [Google Scholar] [CrossRef] [Green Version]
- Park, H.L.; Tran, S.M.; Lee, J.; Goodman, D.; Ziogas, A.; Kelly, R.; Larsen, K.M.; Alvarez, A.; Tannous, C.; Strope, J.; et al. Clinical implementation of a breast cancer risk assessment program in a multiethnic patient population: Which risk model to use? Breast J. 2015, 21, 562–564. [Google Scholar] [CrossRef] [Green Version]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benbow, J.L.; Clarke, C.A.; Park, H.L.; Duffy, C.N.; Chung, N.T.; Brankin, A.E.; Angell-Mendez, K.; Ziogas, A.; Pinder, R.; Deapen, D.; et al. Novel Implementation of Cloud Computing and Mobile Technology to Transform Data Collection and Management in Cancer Epidemiology; American Association for Cancer Research (AACR): Philadelphia, PA, USA, 2014; p. 302. [Google Scholar]
- Curl, C.L.; Beresford, S.A.A.; Hajat, A.; Kaufman, J.D.; Moore, K.; Nettleton, J.A.; Diez-Roux, A. V Associations of organic produce consumption with socioeconomic status and the local food environment: Multi-Ethnic Study of Atherosclerosis (MESA). PLoS ONE 2013, 8, e69778. [Google Scholar] [CrossRef] [PubMed]
- Baudry, J.; Méjean, C.; Allès, B.; Péneau, S.; Touvier, M.; Hercberg, S.; Lairon, D.; Galan, P.; Kesse-Guyot, E. Contribution of organic food to the diet in a large sample of French adults (The NutriNet-Santé cohort study). Nutrients 2015, 7, 8615–8632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, I.; Tseng, C.; Wu, J.; Yang, J.; Conroy, S.M.; Shariff-Marco, S.; Li, L.; Hertz, A.; Gomez, S.L.; Le Marchand, L.; et al. Association between ambient air pollution and breast cancer risk: The multiethnic cohort study. Int. J. Cancer 2020, 146, 699–711. [Google Scholar] [CrossRef] [PubMed]
- McCormack, V.A.; Dos Santos Silva, I. Breast density and parenchymal patterns as markers of breast cancer risk: A meta-analysis. Cancer Epidemiol. Biomark. Prev. 2006, 15, 1159–1169. [Google Scholar] [CrossRef] [Green Version]
- Boyd, N.F.; Martin, L.J.; Yaffe, M.J.; Minkin, S. Mammographic density and breast cancer risk: Current understanding and future prospects. Breast Cancer Res. 2011, 13, 223. [Google Scholar] [CrossRef] [Green Version]
- McTiernan, A.; Martin, C.F.; Peck, J.D.; Aragaki, A.K.; Chlebowski, R.T.; Pisano, E.D.; Wang, C.Y.; Brunner, R.L.; Johnson, K.C.; Manson, J.E.; et al. Estrogen-plus-progestin use and mammographic density in postmenopausal women: Women’s Health Initiative randomized trial. J. Natl. Cancer Inst. 2005, 97, 1366–1376. [Google Scholar] [CrossRef] [Green Version]
- Hart, V.; Reeves, K.W.; Sturgeon, S.R.; Reich, N.G.; Sievert, L.L.; Kerlikowske, K.; Ma, L.; Shepherd, J.; Tice, J.A.; Mahmoudzadeh, A.P.; et al. The effect of change in body mass index on volumetric measures of mammographic density. Cancer Epidemiol. Biomark. Prev. 2015, 24, 1724–1730. [Google Scholar] [CrossRef] [Green Version]
- Subar, A.F.; Kirkpatrick, S.I.; Mittl, B.; Zimmerman, T.P.; Thompson, F.E.; Bingley, C.; Willis, G.; Islam, N.G.; Baranowski, T.; McNutt, S.; et al. The Automated Self-Administered 24-Hour Dietary Recall (ASA24): A resource for researchers, clinicians, and educators from the National Cancer Institute. J. Acad. Nutr. Diet. 2012, 112, 1134–1137. [Google Scholar] [CrossRef] [Green Version]
- Kirkpatrick, S.I.; Subar, A.F.; Douglass, D.; Zimmerman, T.P.; Thompson, F.E.; Kahle, L.L.; George, S.M.; Dodd, K.W.; Potischman, N. Performance of the Automated Self-Administered 24-hour Recall relative to a measure of true intakes and to an interviewer-administered 24-h recall. Am. J. Clin. Nutr. 2014, 100, 233–240. [Google Scholar] [CrossRef]
- Thompson, F.E.; Dixit-Joshi, S.; Potischman, N.; Dodd, K.W.; Kirkpatrick, S.I.; Kushi, L.H.; Alexander, G.L.; Coleman, L.A.; Zimmerman, T.P.; Sundaram, M.E.; et al. Comparison of interviewer-administered and automated self-administered 24-hour dietary recalls in 3 diverse integrated health systems. Am. J. Epidemiol. 2015, 181, 970–978. [Google Scholar] [CrossRef] [Green Version]
- National Cancer Institute. Diet History Questionnaire, Version 2.0. In National Institutes of Health, Epidemiology and Genomics Research Program; National Cancer Institute: Bethesda, MD, USA, 2010. [Google Scholar]
- Thompson, F.E.; Subar, A.F.; Brown, C.C.; Smith, A.F.; Sharbaugh, C.O.; Jobe, J.B.; Mittl, B.; Gibson, J.T.; Ziegler, R.G. Cognitive research enhances accuracy of food frequency questionnaire reports: Results of an experimental validation study. J. Am. Diet. Assoc. 2002, 102, 212–225. [Google Scholar] [CrossRef]
- Subar, A.F.; Thompson, F.E.; Kipnis, V.; Midthune, D.; Hurwitz, P.; McNutt, S.; McIntosh, A.; Rosenfeld, S. Comparative validation of the Block, Willett, and National Cancer Institute food frequency questionnaires: The Eating at America’s Table Study. Am. J. Epidemiol. 2001, 154, 1089–1099. [Google Scholar] [CrossRef] [PubMed]
- Subar, A.F.; Kipnis, V.; Troiano, R.P.; Midthune, D.; Schoeller, D.A.; Bingham, S.; Sharbaugh, C.O.; Trabulsi, J.; Runswick, S.; Ballard-Barbash, R.; et al. Using intake biomarkers to evaluate the extent of dietary misreporting in a large sample of adults: The OPEN study. Am. J. Epidemiol. 2003, 158, 1–13. [Google Scholar] [CrossRef]
- National Cancer Institute. Diet*Calc Analysis Program, Version 1.4.3. In Epidemiology and Genomics Research Program; National Cancer Institute: Bethesda, MD, USA, 2005. [Google Scholar]
- Rhee, J.J.; Sampson, L.; Cho, E.; Hughes, M.D.; Hu, F.B.; Willett, W.C. Comparison of methods to account for implausible reporting of energy intake in epidemiologic studies. Am. J. Epidemiol. 2015, 181, 225–233. [Google Scholar] [CrossRef]
- Moran, S.; Arribas, C.; Esteller, M. Validation of a DNA methylation microarray for 850,000 CpG sites of the human genome enriched in enhancer sequences. Epigenomics 2016, 8, 389–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dedeurwaerder, S.; Defrance, M.; Bizet, M.; Calonne, E.; Bontempi, G.; Fuks, F. A comprehensive overview of Infinium Human Methylation450 data processing. Brief. Bioinform. 2013, 15, 929–941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- U.S. Department of Labor Bureau of Labor Statistics Standard Occupational Classification (SOC) System. Available online: https://www.bls.gov/soc/2018/major_groups.htm (accessed on 23 August 2019).
- U.S. Department of Labor, Employment and Training Administration. O*NET, the Occupational Information Network. Available online: https://www.onetonline.org/ (accessed on 23 August 2019).
- UCI Community and Labor Project, UCLA Labor Center. Orange County on the Cusp of Change; UCI Community and Labor Project, UCLA Labor Center: Los Angeles, CA, USA, 2014. [Google Scholar]
- U.S. Department of Health and Human Services. Physical Activity Guidelines for Americans, 2nd ed.; U.S. Department of Health and Human Services: Washington, DC, USA, 2018.
- U.S. Census Bureau 2013–2017 American Community Survey 5-Year Estimates. Available online: https://data.census.gov/cedsci/ (accessed on 14 January 2020).
- Schoenborn, C.A.; Adams, P.F.; Peregoy, J.A. Health behaviors of adults: United States, 2008–2010. Vital Health Stat. Ser. 10 Data Natl. Health Surv. 2013, 257, 1–184. [Google Scholar]
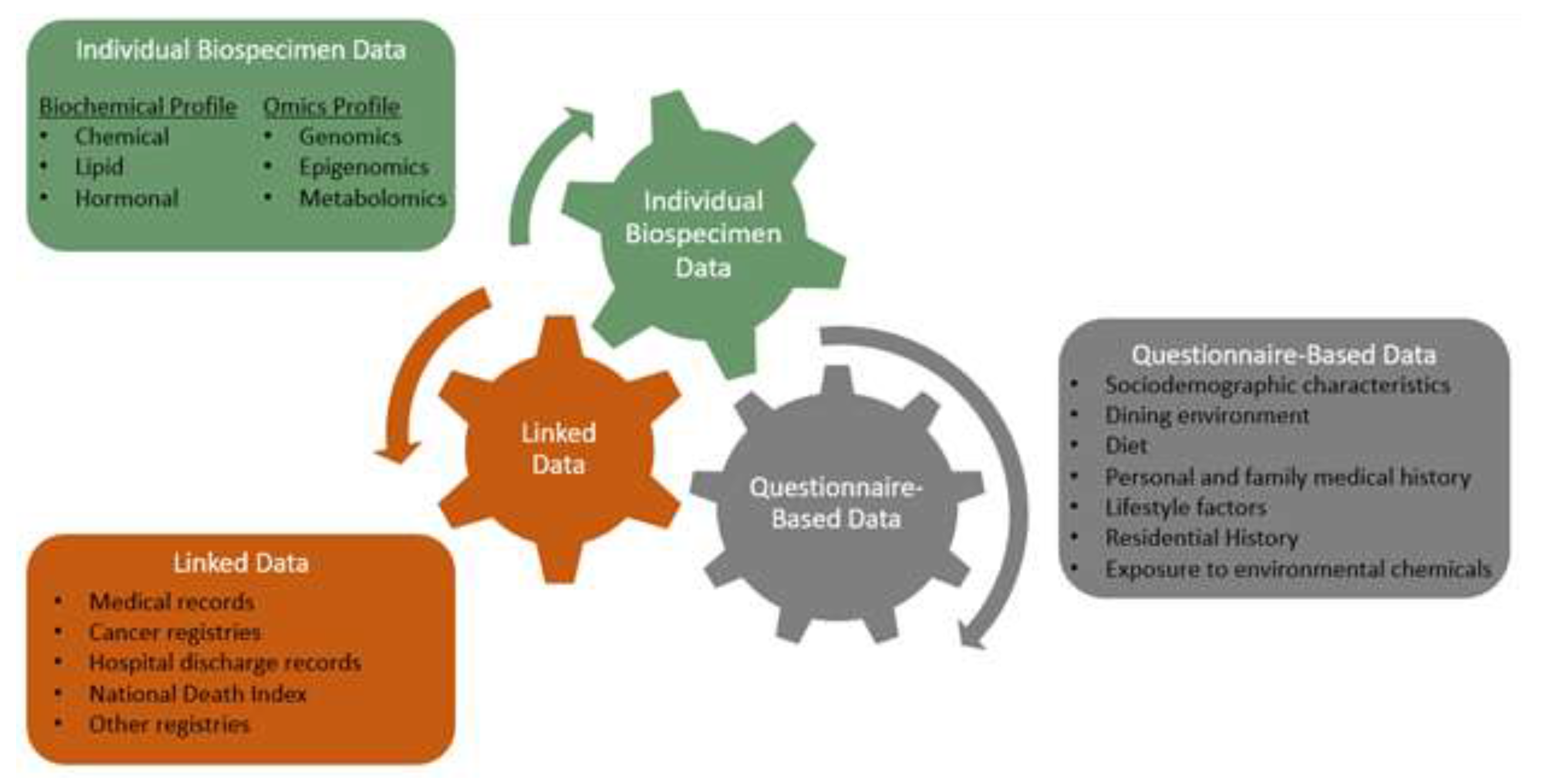
| Data Sources | Description |
| Environmental exposures questionnaire | Environmental exposures, source of drinking water, organic eating behaviors, residence history, occupation |
| Breast health questionnaire | Personal medical history, reproductive history, family history of cancer, demographic data |
| Dietary recalls (ASA24) | Complete report of all food, drink, and supplements consumed the previous day (3 recalls were requested) |
| Food frequency questionnaire (DHQII) | Summary of dietary intake frequencies over the previous year |
| Electronic medical record | Mammogram reports |
| Specimens Collected | Description |
| Blood | Peripheral blood, separated into serum, plasma, and buffy coat; DNA extracted from buffy coat |
| Urine | Two first-void urine samples |
| Saliva | Optional saliva sample |
| Characteristic | Mean (SD) or N (%) |
|---|---|
| Age, years, mean (SD) | 56.7 (4.6) |
| Race/Ethnicity, N (%) | |
| Non-Hispanic white | 257 (64.3) |
| Hispanic | 74 (18.5) |
| Asian | 43 (10.8) |
| Other/Unknown | 26 (6.5) |
| Education, N (%) | |
| High school graduate or less | 34 (8.5) |
| Some college or technical school | 87 (21.8) |
| College graduate or more | 277 (69.3) |
| Missing | 2 (0.5) |
| Occupation, N (%), out of 250 | |
| Unemployed or disabled | 11 (4.4) |
| Homemaker | 12 (4.8) |
| Retired | 23 (9.2) |
| Employed (Full-time or Part-time) | 204 (81.6) |
| Current residence, N (%) | |
| Los Angeles County | 27 (6.8) |
| North Orange County | 185 (46.2) |
| South Orange County | 178 (44.5) |
| Other | 10 (2.6) |
| Diet questionnaires completed, N (%) | |
| ASA24 dietary recall | |
| None | 21 (5.3) |
| 1 | 33 (8.3) |
| 2 | 76 (19.0) |
| ≥3 | 270 (67.5) |
| Paired urine and ASA dietary recall * | |
| None | 56 (14) |
| 1 | 110 (27.5) |
| 2 | 234 (58.5) |
| Food frequency questionnaire | 263 (65.8) |
| Characteristic | Mean (SD) or N (%) |
|---|---|
| Smoking status, N (%) | |
| Current smoker | 17 (4.3) |
| Former smoker | 90 (22.5) |
| Never-smoker | 292 (73.0) |
| Missing | 1 (0.3) |
| Alcohol consumption, N (%) | |
| Never | 106 (26.5) |
| Less than 2 drinks per week | 166 (41.5) |
| 2–7 drinks per week | 74 (18.5) |
| More than 7 per week | 51 (12.8) |
| Missing | 3 (0.8) |
| Weekly physical activity meets the Physical Activity Guidelines for Americans, N (%) | |
| No | 226 (56.5) |
| Yes | 156 (39.0) |
| Missing | 18 (4.5) |
| BMI, kg/m2, mean (SD) | 26.8 (6.5) |
| Age of menarche, mean (SD) | 12.8 (1.5) |
| Pregnancy history | |
| Number of live births, N (%) | |
| 0 | 87 (21.8) |
| 1 | 74 (18.5) |
| 2 | 141 (35.3) |
| 3 | 74 (18.5) |
| More than 3 | 24 (6.0) |
| Age at first birth, mean (SD) | 27.7 (6.2) |
| Age of menopause, mean (SD) | 48.7 (6.1) |
| History of gynecologic surgery, N (%) | |
| Oophorectomy | 84 (21.0) |
| Hysterectomy | 99 (24.8) |
| Hormone replacement therapy use, N (%) | |
| Never | 255 (63.8) |
| Previous | 62 (15.5) |
| Current | 82 (20.5) |
| Missing | 1 (0.3) |
| Mammographic breast density, N (%) | |
| Almost entirely fatty | 42 (10.5) |
| Scattered fibroglandular densities | 110 (27.5) |
| Heterogeneously dense | 163 (40.8) |
| Extremely dense | 77 (19.3) |
| Missing | 8 (2.0) |
| Family history of cancer in first-degree relatives, N (%) | |
| Breast cancer (invasive or ductal carcinoma in situ [DCIS]) | 83 (21.1) |
| Ovarian cancer | 13 (3.3) |
| Characteristic | Mean (SD) or N (%) |
|---|---|
| Organic food consumption frequency, N (%) | |
| Often or always | 127 (31.8) |
| Sometimes | 114 (28.5) |
| Seldom or never | 158 (39.5) |
| Missing | 1 (0.3) |
| Source of drinking water, N (%) | |
| Tap water (without filter) | 36 (9.0) |
| Bottled water | 158 (39.5) |
| Filtered water | 204 (51.0) |
| Don’t know or not sure | 1 (0.3) |
| Missing | 1 (0.3) |
| History of living on a farm, N (%) | |
| > 10 years | 22 (5.5) |
| ≤ 10 years | 32 (8.0) |
| None | 346 (86.5) |
| Age when started living on a farm, mean (SD) | 8.8 (11.2) |
| Used pesticides at home or workplace within past 7 days, N (%), out of 250 | |
| No | 174 (69.6) |
| Yes | 50 (20.0) |
| Don’t know or not sure | 26 (10.4) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lucia, R.M.; Huang, W.-L.; Alvarez, A.; Thampy, D.; Elyasian, M.; Hidajat, A.; Yang, K.; Forman, D.; Pebdani, A.; Masunaka, I.; et al. Rationale, Study Design, and Cohort Characteristics for the Markers for Environmental Exposures (MEE) Study. Int. J. Environ. Res. Public Health 2020, 17, 1774. https://doi.org/10.3390/ijerph17051774
Lucia RM, Huang W-L, Alvarez A, Thampy D, Elyasian M, Hidajat A, Yang K, Forman D, Pebdani A, Masunaka I, et al. Rationale, Study Design, and Cohort Characteristics for the Markers for Environmental Exposures (MEE) Study. International Journal of Environmental Research and Public Health. 2020; 17(5):1774. https://doi.org/10.3390/ijerph17051774
Chicago/Turabian StyleLucia, Rachel McFarland, Wei-Lin Huang, Andrea Alvarez, Daphne Thampy, Melodie Elyasian, Amanda Hidajat, Kailynn Yang, Danielle Forman, Asana Pebdani, Irene Masunaka, and et al. 2020. "Rationale, Study Design, and Cohort Characteristics for the Markers for Environmental Exposures (MEE) Study" International Journal of Environmental Research and Public Health 17, no. 5: 1774. https://doi.org/10.3390/ijerph17051774
APA StyleLucia, R. M., Huang, W.-L., Alvarez, A., Thampy, D., Elyasian, M., Hidajat, A., Yang, K., Forman, D., Pebdani, A., Masunaka, I., Brain, S., Heditsian, D., Lee, V., Goodman, D., Norden-Krichmar, T. M., Odegaard, A. O., Ziogas, A., & Park, H. L. (2020). Rationale, Study Design, and Cohort Characteristics for the Markers for Environmental Exposures (MEE) Study. International Journal of Environmental Research and Public Health, 17(5), 1774. https://doi.org/10.3390/ijerph17051774






