Current Status of Mumps Virus Infection: Epidemiology, Pathogenesis, and Vaccine
Abstract
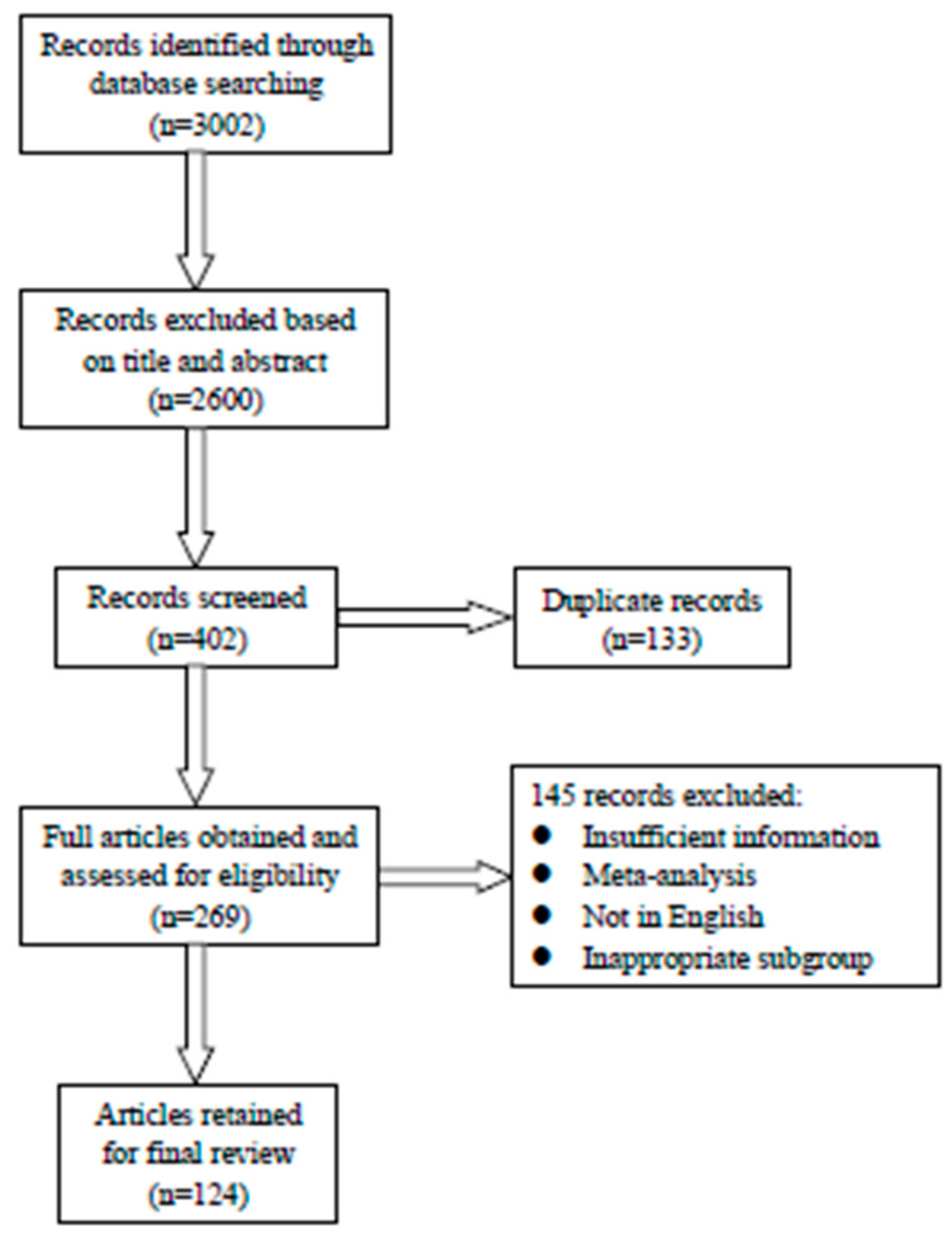
:1. Introduction
2. Epidemiology
3. Pathogenesis
4. Mumps Vaccines
4.1. General Considerations
4.2. Jeryl Lynn Strain Mumps Vaccine
4.3. Leningrad-3 Strain Mumps Vaccine
4.4. Leningrad-3-Zagreb Strain Mumps Vaccine
4.5. Rubini Strain Mumps Vaccine
4.6. Urabe Strain Mumps Vaccine
5. Emerging Mumps Virus Infection
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hviid, A.; Rubin, S.; Muhlemann, K. Mumps. Lancet 2008, 371, 932–944. [Google Scholar] [CrossRef]
- Betáková, T.; Svetlíková, D.; Gocník, M. Overview of measles and mumps vaccine: Origin, present, and future of vaccine production. Acta Virol. 2013, 57, 91–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rubin, S.; Kennedy, R.; Poland, G. Emerging mumps infection. Pediatr. Infect. Dis. J. 2016, 35, 799–801. [Google Scholar] [CrossRef]
- Wellington, K.; Goa, K.L. Measles, mumps, rubella vaccine (Priorix; GSK-MMR): A review of its use in the prevention of measles, mumps and rubella. Drugs 2003, 63, 2107–2126. [Google Scholar] [CrossRef] [PubMed]
- Marlow, M.A.; Marin, M.; Moore, K.; Patel, M. CDC guidance for use of a third dose of MMR vaccine during outbreaks. J. Public Health Manag. Pract. 2020, 26, 109–115. [Google Scholar] [CrossRef]
- Van Loon, F.P.; Holmes, S.J.; Sirotkin, B.I.; Williams, W.W.; Cochi, S.L.; Hadler, S.C.; Lindegren, M.L. Mumps surveillance—United States, 1988–1993. MMWR CDC Surveill. Summ. 1995, 44, 1–14. [Google Scholar]
- Lewnard, J.A.; Grad, Y.H. Vaccine waning and mumps re-emergence in the United States. Sci. Transl. Med. 2018. [Google Scholar] [CrossRef] [Green Version]
- Wagenvoort, J.H.; Harmsen, M.; Boutahar-Trouw, B.J.; Kraaijeveld, C.A.; Winkler, K.C. Epidemiology of mumps in The Netherlands. J. Hyg. 1980, 85, 313–326. [Google Scholar] [CrossRef] [Green Version]
- Anderson, R.M.; Crombie, J.A.; Grenfell, B.T. The epidemiology of mumps in the UK: A preliminary study of virus transmission, herd immunity and the potential impact of immunization. Epidemiol. Infect. 1987, 99, 65–84. [Google Scholar] [CrossRef] [Green Version]
- Gay, N.; Miller, E.; Hesketh, L.; Morgan-Capner, P.; Ramsay, M.; Cohen, B.; Brown, D. Mumps surveillance in England and Wales supports introduction of two dose vaccination schedule. Commun. Dis. Rep. CDR Rev. 1997, 7, R21–R26. [Google Scholar]
- Peltola, H.; Heinonen, O.P.; Valle, M.; Paunio, M.; Virtanen, M.; Karanko, V.; Cantell, K. The elimination of indigenous measles, mumps, and rubella from Finland by a 12-year, two-dose vaccination program. N. Engl. J. Med. 1994, 331, 1397–1402. [Google Scholar] [CrossRef] [PubMed]
- Peltola, H.; Davidkin, I.; Paunio, M.; Valle, M.; Leinikki, P.; Heinonen, O.P. Mumps and rubella eliminated from Finland. JAMA 2000, 284, 2643–2647. [Google Scholar] [CrossRef] [PubMed]
- Falk, W.A.; Buchan, K.; Dow, M.; Garson, J.Z.; Hill, E.; Nosal, M.; Tarrant, M.; Westbury, R.C.; White, F.M. The epidemiology of mumps in southern Alberta, 1980–1982. Am. J. Epidemiol. 1989, 130, 736–749. [Google Scholar] [CrossRef] [PubMed]
- Coffinières, E.; Turbelin, C.; Riblier, D.; Aouba, A.; Levy-Bruhl, D.; Arena, C.; Chiappe, S.G.; Ferry, J.P.; Hanslik, T.; Blanchon, T. Mumps: Burden of disease in France. Vaccine 2012, 30, 7013–7018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slater, P.E.; Roitman, M.; Costin, C. Mumps incidence in Israel—Impact of MMR vaccine. Public Health Rev. 1990, 18, 88–93. [Google Scholar]
- Ngaovithunvong, V.; Wanlapakorn, N.; Tesapirat, L.; Suratannon, N.; Poovorawan, Y. Mumps antibody in the Thai population 17 years after the universal measles mumps rubella vaccination program. J. Infect. Dev. Ctries. 2016, 10, 735–740. [Google Scholar] [CrossRef] [Green Version]
- Cui, A.; Zhu, Z.; Hu, Y.; Deng, X.; Sun, Z.; Zhang, Y.; Mao, N.; Xu, S.; Fang, X.; Gao, H.; et al. Mumps Epidemiology and Mumps Virus Genotypes Circulating in Mainland China during 2013–2015. PLoS ONE 2017, 12. [Google Scholar] [CrossRef]
- Park, S.H. Resurgence of mumps in Korea. Infect. Chemother. 2015, 47, 1–11. [Google Scholar] [CrossRef]
- Aratchige, P.E.; McIntyre, P.B.; Quinn, H.E.; Gilbert, G.L. Recent increases in mumps incidence in Australia: The “forgotten” age group in the 1998 Australian Measles Control Campaign. Med. J. Aust. 2008, 189, 434–437. [Google Scholar] [CrossRef]
- Chen, C.C.; Lu, C.C.; Su, B.H.; Chen, K.T. Epidemiologic features of mumps in Taiwan from 2006 to 2011: A new challenge for public health policy. World J. Pediatr. 2015, 11, 141–147. [Google Scholar] [CrossRef]
- Galazka, A.M.; Robertson, S.E.; Kraigher, A. Mumps and mumps vaccine: A global review. Bull. World Health Organ. 1999, 77, 3–14. [Google Scholar] [PubMed]
- Osborne, K.; Gay, N.; Hesketh, L.; Morgan-Capner, P.; Miller, E. Ten years of serological surveillance in England and Wales: Methods, results, implications and action. Int. J. Epidemiol. 2000, 29, 362–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edmunds, W.J.; Gay, N.J.; Kretzschmar, M.; Pebody, R.G.; Wachmann, H.; ESEN Project. European Sero-epidemiology Network. The pre-vaccination epidemiology of measles, mumps and rubella in Europe: Implications for modelling studies. Epidemiol. Infect. 2000, 125, 635–650. [Google Scholar] [CrossRef] [PubMed]
- Marlow, R.; Kuriyakose, S.; Mesaros, N.; Han, H.H.; Tomlinson, R.; Faust, S.N.; Snape, M.; Pollard, A.J.; Finn, A. A Phase III, open-label, randomized multicenter study to evaluate the immunogeneicity and safety of a booster dose of two different reduced antigen diphtheria-tetanus-acellular pertussis-polio vaccines, when co-administered with measles-mumps-rubella vaccine in 3 and 4 year-old healthy children in the UK. Vaccine 2018, 36, 2300–2306. [Google Scholar]
- Shah, A.P.; Smolensky, M.H.; Burau, K.D.; Cech, I.M.; Lai, D. Seasonality of primarily childhood and young adult infectious diseases in the United States. Chronobiol. Int. 2006, 23, 1065–1082. [Google Scholar] [CrossRef]
- Altizer, S.; Dobson, A.; Hosseini, P.; Hudson, P.; Pascual, M.; Rohani, P. Seasonality and the dynamics of infectious diseases. Ecol. Lett. 2006, 9, 467–484. [Google Scholar] [CrossRef] [Green Version]
- Levitt, L.P.; Mahoney, D.H., Jr.; Casey, H.L.; Bond, J.O. Mumps in a general population. A sero-epidemiologic study. Am. J. Dis. Child. 1970, 120, 134–138. [Google Scholar] [CrossRef]
- Eriksen, J.; Davidkin, I.; Kafatos, G.; Andrews, N.; Barbara, C.; Cohen, D.; Duks, A.; Griskevicius, A.; Johansen, K.; Bartha, K.; et al. Seroepidemiology of mumps in Europe (1996–2008): Why do outbreaks occur in highly vaccinated populations? Epidemiol. Infect. 2013, 141, 651–666. [Google Scholar] [CrossRef] [Green Version]
- Béraud, G.; Abrams, S.; Beutels, P.; Dervaux, B.; Hens, N. Resurgence risk for measles, mumps and rubella in France in 2018 and 2020. Eurosurveillance 2018, 23. [Google Scholar] [CrossRef]
- Metcalf, C.J.E.; Graham, A.L.; Grenfell, B.T. Understanding Herd Immunity. Trends Immunol. 2015, 36, 753–755. [Google Scholar] [CrossRef] [Green Version]
- LeBaron, C.W.; Forghani, B.; Matter, L.; Reef, S.E.; Beck, C.; Bi, D.; Cossen, C.; Sullivan, B.J. Persistence of mumps antibodies after 2 doses of measles-mumps-rubella vaccine. J. Infect. Dis. 2009, 200, 888–899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davidkin, I.; Jokinen, S.; Broman, M.; Leinikki, P.; Peltola, H. Persistence of measles, mumps, and rubella antibodies in an MMR-vaccinated cohort: A 20-year follow-up. J. Infect. Dis. 2008, 197, 950–956. [Google Scholar] [CrossRef]
- Mauldin, J.; Carbone, K.; Hsu, H.; Yolken, R.; Rubin, S. Mumps virus-specific antibody titers from pre-vaccine era sera: Comparison of the plaque reduction neutralization assay and enzyme immunoassays. J. Clin. Microbiol. 2005, 43, 4847–4851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vyse, A.J.; Gay, N.J.; Hesketh, L.M.; Pebody, R.; Morgan-Capner, P.; Miller, E. Interpreting serological surveys using mixture models: The seroepidemiology of measles, mumps and rubella in England and Wales at the beginning of the 21st century. Epidemiol. Infect. 2006, 134, 1303–1312. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.C.; Su, B.H.; Su, H.J.; Chang, H.L.; Lin, C.Y.; Chen, H.; Chen, K.T. The association between the incidence of mumps and meteorological parameters in Taiwan. Hum. Vaccin. Immunother. 2015, 11, 1406–1412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Q.; Yang, Z.; Ding, H.; Zhang, X.; Dong, Z.; Hu, W.; Liu, X.; Wang, M.; Hu, G.; Fu, C. The relationship between meteorological factors and mumps incidence in Guangzhou, China, 2005–2012. Hum. Vaccin. Immunother. 2014, 10, 2421–2432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Onozuka, D.; Hashizume, M. Effect of weather variability on the incidence of mumps in children: A time-series analysis. Epidemiol. Infect. 2011, 139, 1692–1700. [Google Scholar] [CrossRef] [Green Version]
- Fisman, D.N. Seasonality of infectious diseases. Annu. Rev. Public Health 2007, 28, 127–143. [Google Scholar] [CrossRef] [Green Version]
- Zhu, H.; Zhao, H.; Ou, R.; Xiang, H.; Hu, L.; Jing, D.; Sharma, M.; Ye, M. Epidemiological Characteristics and Spatiotemporal Analysis of Mumps from 2004 to 2018 in Chongqing, China. Int. J. Environ. Res. Public Health 2019, 16, 3052. [Google Scholar] [CrossRef] [Green Version]
- Rubin, S.; Eckhaus, M.; Rennick, L.J.; Bamford, C.G.; Duprex, W.P. Molecular biology, pathogenesis and pathology of mumps virus. J. Pathol. 2015, 235, 242–252. [Google Scholar] [CrossRef]
- Centers for Disease Control (CDC). Mumps prevention. MMWR Morb. Mortal. Wkly. Rep. 1989, 38, 388–392. [Google Scholar]
- Peltola, H.; Kulkarni, P.S.; Kapre, S.V.; Paunio, M.; Jadhav, S.S.; Dhere, R.M. Mumps outbreaks in Canada and the United States: Time for new thinking on mumps vaccines. Clin. Infect. Dis. 2007, 45, 459–466. [Google Scholar] [CrossRef] [Green Version]
- Vandermeulen, C.; Leroux-Roels, G.; Hoppenbrouwers, K. Mumps outbreaks in highly vaccinated populations: What makes good even better? Hum. Vaccin. 2009, 5, 494–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boxall, N.; Kubinyiova, M.; Príkazský, V.; Benes, C.; Cástková, J. An increase in the number of mumps cases in the Czech Republic, 2005–2006. Eurosurveillance 2008, 13, 146–149. [Google Scholar]
- Park, D.W.; Nam, M.H.; Kim, J.Y.; Kim, H.J.; Sohn, J.W.; Cho, Y.; Song, K.J.; Kim, M.J. Mumps outbreak in highly vaccinated school population: Assessment of secondary vaccine failure using IgG avidity measurements. Vaccine 2007, 25, 4665–4670. [Google Scholar] [CrossRef]
- Fields, V.S.; Safi, H.; Waters, C.; Dillaha, J.; Capelle, L.; Riklon, S.; Wheeler, J.G.; Haselow, D.T. Mumps in a highly vaccinated Marshallese community in Arkansas, USA: An outbreak report. Lancet Infect. Dis. 2019, 19, 185–192. [Google Scholar] [CrossRef]
- Vygen, S.; Fischer, A.; Meurice, L.; Mounchetrou, N.I.; Gregoris, M.; Ndiaye, B.; Ghenassia, A.; Poujol, I.; Stahl, J.P.; Antona, D.; et al. Waning immunity against mumps in Vaccinated young adults, France 2013. Eurosurveillance 2016, 21. [Google Scholar] [CrossRef] [Green Version]
- Aasheim, E.T.; Inns, T.; Trindall, A.; Emmett, L.; Brown, K.E.; Williams, C.J.; Reacher, M. Outbreak of mumps in a school setting, United Kingdom, 2013. Hum. Vaccin. Immunother. 2014, 10, 2446–2449. [Google Scholar] [CrossRef] [Green Version]
- Westphal, D.W.; Eastwood, A.; Levy, A.; Davies, J.; Huppatz, C.; Gilles, M.; Lyttle, H.; Williams, S.A.; Dowse, G.K. A protracted mumps outbreak in Western Australia despite high vaccine coverage: A population-based surveillance study. Lancet Infect. Dis. 2019, 19, 177–184. [Google Scholar] [CrossRef]
- Dayan, G.H.; Quinlisk, M.P.; Parker, A.A.; Barskey, A.E.; Harris, M.L.; Schwartz, J.M.; Hunt, K.; Finley, C.G.; Leschinsky, D.P.; O’Keefe, A.L.; et al. Recent resurgence of mumps in the United States. N. Engl. J. Med. 2008, 358, 1580–1589. [Google Scholar] [CrossRef] [Green Version]
- Bag, S.K.; Dey, A.; Wang, H.; Beard, F. Australian vaccine preventable disease epidemiological review series: Mumps 2008-2012. Commun. Dis Intell. Q. Report. 2015, 39, E10–E18. [Google Scholar]
- Schwarz, N.G.; Bernard, H.; Melnic, A.; Bucov, V.; Caterinciuc, N.; an der Heiden, M.; Andrews, N.; Pebody, R.; Aidyralieva, C.; Hahné, S. Mumps outbreak in the Republic of Moldova, 2007–2008. Pediatr. Infect. Dis. J. 2010, 29, 703–706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pebody, R.G.; Gay, N.J.; Hesketh, L.M.; Vyse, A.; Morgan-Capner, P.; Brown, D.W.; Litton, P.; Miller, E. Immunogenicity of second dose measles-mumps-rubella (MMR) vaccine and implications for serosurveillance. Vaccine 2002, 20, 1134–1140. [Google Scholar] [CrossRef]
- Castilla, J.; García Cenoz, M.; Arriazu, M.; Fernández-Alonso, M.; Martínez-Artola, V.; Etxeberria, J.; Irisarri, F.; Barricarte, A. Effectiveness of Jeryl Lynn-containing vaccine in Spanish children. Vaccine 2009, 27, 2089–2093. [Google Scholar] [CrossRef]
- Cohen, C.; White, J.M.; Savage, E.J.; Glynn, J.R.; Choi, Y.; Andrews, N.; Brown, D.; Ramsay, M.E. Vaccine effectiveness estimates, 2004–2005 mumps outbreak, England. Emerg. Infect. Dis. 2007, 13, 12–17. [Google Scholar] [CrossRef] [Green Version]
- Dayan, G.H.; Rubin, S. Mumps outbreaks in vaccinated populations: Are available mumps vaccines effective enough to prevent outbreaks? Clin. Infect. Dis. 2008, 47, 1458–1467. [Google Scholar] [CrossRef] [Green Version]
- Gupta, R.K.; Best, J.; MacMahon, E. Mumps and the UK epidemic 2005. BMJ 2005, 330, 1132–1135. [Google Scholar] [CrossRef]
- Foy, H.M.; Cooney, M.K.; Hall, C.E.; Bor, E.; Maletzky, A.J. Isolation of mumps virus from children with acute lower respiratory tract disease. Am J Epidemiol. 1971, 94, 467–472. [Google Scholar] [CrossRef]
- Jin, L.; Örvell, C.; Myers, R.; Rota, P.A.; Nakayama, T.; Forcic, D.; Hiebert, J.; Brown, K.E. Genomic diversity of mumps virus and global distribution of the 12 genotypes. Rev. Med. Virol. 2015, 25, 85–101. [Google Scholar] [CrossRef]
- Sawada, A.; Yamaji, Y.; Nakayama, T. Mumps Hoshino and Torii vaccine strains were distinguished from circulating wild strains. J. Infect. Chemorther. 2013, 19, 480–485. [Google Scholar] [CrossRef]
- Maillet, M.; Bouvat, E.; Robert, N.; Baccard-Longère, M.; Morel-Baccard, C.; Morand, P.; Vabret, A.; Stahl, J.P. Mumps outbreak and laboratory diagnosis. J. Clin. Virol. 2015, 62, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Ennis, F.A.; Jackson, D. Isolation of virus during the incubation period of mumps infection. J. Pediatr. 1968, 72, 536–537. [Google Scholar] [CrossRef]
- Katoh, H.; Nakatsu, Y.; Kubota, T.; Sakata, M.; Takeda, M.; Kidokoro, M. Mumps Virus Is Released from the Apical Surface of Polarized Epithelial Cells, and the Release Is Facilitated by a Rab11-Mediated Transport System. J. Virol. 2015, 89, 12026–12034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Overman, J.R. Viremia in human mumps virus infections. Arch. Intern. Med. 1958, 102, 354–356. [Google Scholar] [CrossRef]
- Cooney, M.K.; Fox, J.P.; Hall, C.E. The Seattle Virus Watch. VI. Observations of infections with and illness due to parainfluenza, mumps and respiratory syncytial viruses and Mycoplasma pneumoniae. Am. J. Epidemiol. 1975, 101, 532–551. [Google Scholar] [CrossRef]
- Levine, D.A. Vaccine-Preventable Diseases In Pediatric Patients: A Review Of Measles, Mumps, Rubella, And Varicella. Pediatr. Emerg. Med. Pract. 2016, 13, 1–20. [Google Scholar]
- Bockelman, C.; Frawley, T.C.; Long, B.; Koyfman, A. Mumps: An Emergency Medicine-Focused Update. J. Emerg. Med. 2018, 54, 207–214. [Google Scholar] [CrossRef]
- Kabakuş, N.; Aydinoğlu, H.; Yekeler, H.; Arslan, I.N. Fatal mumps nephritis and myocarditis. J. Trop. Pediatr. 1999, 45, 358–360. [Google Scholar] [CrossRef] [Green Version]
- Ternavasio-de la Vega, H.G.; Boronat, M.; Ojeda, A.; García-Delgado, Y.; Angel-Moreno, A.; Carranza-Rodríguez, C.; Bellini, R.; Francès, A.; Nóvoa, F.J.; Pérez-Arellano, J.L. Mumps orchitis in the post-vaccine era (1967–2009): A single-center series of 67 patients and review of clinical outcome and trends. Medicine 2010, 89, 96–116. [Google Scholar] [CrossRef]
- Chiba, Y.; Horino, K.; Umetsu, M.; Wataya, Y.; Chiba, S. Virus excretion and antibody response in saliva in natural mumps. Tohoku J. Exp. Med. 1973, 111, 229–238. [Google Scholar] [CrossRef] [Green Version]
- Fleischer, B.; Kreth, H.W. Mumps virus replication in human lymphoid cell lines and in peripheral blood lymphocytes: Preference for T cells. Infect. Immun. 1982, 35, 25–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kowalzik, F.; Faber, J.; Knuf, M. MMR and MMRV vaccines. Vaccine 2018, 36, 5402–5407. [Google Scholar] [CrossRef] [PubMed]
- Schwarzer, S.; Reibel, S.; Lang, A.B.; Struck, M.M.; Finkel, B.; Gerike, E.; Tischer, A.; Gassner, M.; Glück, R.; Stück, B.; et al. Safety and characterization of the immune response engendered by two combined measles, mumps and rubella vaccines. Vaccine 1998, 16, 298–304. [Google Scholar] [CrossRef]
- Fescharek, R.; Quast, U.; Maass, G.; Merkle, W.; Schwarz, S. Measles-mumps vaccination in the FRG: An empirical analysis after 14 years of use. I. Efficacy and analysis of vaccine failures. Vaccine 1990, 8, 333–336. [Google Scholar] [CrossRef]
- Feiterna-Sperling, C.; Brönnimann, R.; Tischer, A.; Stettler, P.; Durrer, P.; Gaedicke, G. Open randomized trial comparing the immunogenicity and safety of a new measles-mumps-rubella vaccine and a licensed vaccine in 12- to 24-month-old children. Pediatr. Infect. Dis. J. 2005, 24, 1083–1088. [Google Scholar] [CrossRef]
- Madsen, K.M.; Hviid, A.; Vestergaard, M.; Schendel, D.; Wohlfahrt, J.; Thorsen, P.; Olsen, J.; Melbye, M. A population-based study of measles, mumps, and rubella vaccination and autism. N. Engl. J. Med. 2002, 347, 1477–1482. [Google Scholar] [CrossRef]
- Cardemil, C.V.; Dahl, R.M.; James, L.; Wannemuehler, K.; Gary, H.E.; Shah, M.; Marin, M.; Riley, J.; Feikin, D.R.; Patel, M.; et al. Effectiveness of a Third Dose of MMR Vaccine for Mumps Outbreak Control. N. Engl. J. Med. 2017, 377, 947–956. [Google Scholar] [CrossRef]
- Giaquinto, C.; Gabutti, G.; Baldo, V.; Villa, M.; Tramontan, L.; Raccanello, N.; Russo, F.; Poma, C.; Scamarcia, A.; Cantarutti, L.; et al. Impact of a vaccination programme in children vaccinated with ProQuad, and ProQuad-specific effectiveness against varicella in the Veneto region of Italy. BMC Infect. Dis. 2018, 18, 103. [Google Scholar] [CrossRef]
- Buynak, E.B.; Hilleman, M.R. Live attenuated mumps virus vaccine. 1. Vaccine development. Proc. Soc. Exp. Biol. Med. 1966, 123, 768–775. [Google Scholar] [CrossRef]
- Bonnet, M.C.; Dutta, A.; Weinberger, C.; Plotkin, S.A. Mumps vaccine virus strains and aseptic meningitis. Vaccine 2006, 24, 7037–7045. [Google Scholar] [CrossRef]
- Jonville-Bera, A.; Autret, E.; Galy-Eyraud, C.; Hessel, L. Aseptic meningitis following mumps vaccine. A retrospective survey by the French Regional Pharmacovigilance centres and by Pasteur-Mérieux Sérums and Vaccines. Pharmacoepidemiol. Drug Saf. 1996, 5, 33–37. [Google Scholar] [CrossRef]
- WHO Expert Committee on Biological Standardization. Forty Third Report; World Health Organization: Geneva, Switzerland, 1994. [Google Scholar]
- Kuter, B.J.; Brown, M.; Wiedmann, R.T.; Hartzel, J.; Musey, L. Safety and Immunogenicity of M-M-RII (Combination Measles-Mumps-Rubella Vaccine) in Clinical Trials of Healthy Children Conducted Between 1988 and 2009. Pediatr. Infect. Dis. J. 2016, 35, 1011–1020. [Google Scholar] [CrossRef] [PubMed]
- Fahlgren, K. Two doses of MMR vaccine—Sufficient to eradicate measles, mumps and rubella? Scand. J. Soc. Med. 1988, 6, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Broliden, K.; Abreu, E.R.; Arneborn, M.; Böttiger, M. Immunity to mumps before and after MMR vaccination at 12 years of age in the first generation offered the two—Dose immunization programme. Vaccine 1998, 16, 323–327. [Google Scholar] [CrossRef]
- Davidkin, I.; Valle, M.; Julkunen, I. Persistence of anti-mumps virus antibodies after a two-dose MMR vaccination: A nine-year follow-up. Vaccine 1995, 13, 1617–1622. [Google Scholar] [CrossRef]
- Ehrenkranz, N.J.; Ventura, A.K.; Medler, E.M.; Jackson, J.E.; Kenny, M.T. Clinical evaluation of a new measles– mumps–rubella combined live virus vaccine in the Dominican Republic. Bull. World Health Organ. 1975, 52, 81–85. [Google Scholar]
- Briss, P.A.; Fehrs, L.J.; Parker, R.A.; Wright, P.F.; Sannella, E.C.; Hutcheson, R.H.; Schaffner, W. Sustained transmission of mumps in a highly vaccinated population: Assessment of primary vaccine failure and waning vaccine–induced immunity. J. Infect. Dis. 1994, 169, 77–82. [Google Scholar] [CrossRef]
- Shah, M.; Quinlisk, P.; Weigel, A.; Riley, J.; James, L.; Patterson, J.; Hickman, C.; Rota, P.A.; Stewart, R.; Clemmons, N.; et al. Mumps Outbreak in a Highly Vaccinated University-Affiliated Setting Before and After a Measles-Mumps-Rubella Vaccination Campaign-Iowa, July 2015–May 2016. Clin. Infect. Dis. 2018, 66, 81–88. [Google Scholar] [CrossRef]
- Hilleman, M.R.; Weibel, R.E.; Buynak, E.B.; Stokes, J., Jr.; Whitman, J.E., Jr. Live attenuated mumps-virus vaccine. IV. Protective efficacy as measured in a field evaluation. N. Engl. J. Med. 1967, 276, 252–258. [Google Scholar] [CrossRef]
- Pugh, R.N.; Akinosi, B.; Pooransingh, S.; Kumar, J.; Grant, S.; Livesley, E.; Linnane, J.; Ramaiah, S. An outbreak of mumps in the metropolitan area of Walsall, UK. Int. J. Infect. Dis. 2002, 6, 283–287. [Google Scholar] [CrossRef] [Green Version]
- May, M.; Rieder, C.A.; Rowe, R. Emergent lineages of mumps virus suggest the need for a polyvalent vaccine. Int. J. Infect. Dis. 2018, 66, 1–4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Centers for Disease Control and Prevention (CDC). Mumps outbreak at a summer camp—New York, 2005. MMWR Morb. Mortal. Wkly. Rep. 2006, 55, 175–177. [Google Scholar]
- Domínguez, A.; Torner, N.; Castilla, J.; Batalla, J.; Godoy, P.; Guevara, M.; Carnicer, D.; Caylà, J.; Rius, C.; Jansà, J.M. Mumps vaccine effectiveness in highly immunized populations. Vaccine 2010, 28, 3567–3570. [Google Scholar] [CrossRef] [PubMed]
- Black, S.; Shinefield, H.; Ray, P.; Lewis, E.; Chen, R.; Glasser, J.; Hadler, S.; Hardy, J.; Rhodes, P.; Swint, E.; et al. Risk of hospitalization because of aseptic meningitis after measles–mumps–rubella vaccination in one- to two-year-old children: An analysis of the Vaccine Safety Datalink (VSD) Project. Pediatr. Infect. Dis. J. 1997, 16, 500–503. [Google Scholar] [CrossRef]
- Reisinger, K.S.; Brown, M.L.; Xu, J.; Sullivan, B.J.; Marshall, G.S.; Nauert, B.; Matson, D.O.; Silas, P.E.; Schödel, F.; Gress, J.O.; et al. A combination measles, mumps, rubella, and varicella vaccine (ProQuad) given to 4- to 6-year-old healthy children vaccinated previously with M-M-RII and Varivax. Pediatrics 2006, 117, 265–272. [Google Scholar] [CrossRef]
- Ma, S.J.; Li, X.; Xiong, Y.Q.; Yao, A.L.; Chen, Q. Combination measles-mumps-rubella-varicella vaccine in healthy children: A Systematic Review and Meta-analysis of Immunogenicity and Safety. Medicine 2015, 94, e1721. [Google Scholar] [CrossRef]
- Tillieux, S.L.; Halsey, W.S.; Sathe, G.M.; Vassilev, V. Comparative analysis of the complete neucleotide sequences of measles, mumps, and rubella strain genomes comtained in Priorix-TetraTM and ProQuadTM live attenuated combined vaccines. Vaccine 2009, 27, 2265–2273. [Google Scholar] [CrossRef]
- Nakayama, T.; Eda, M.; Hirano, M.; Goto, W. Immunogenicity and safety of the new MMR vaccine containing measles AIK-C, rubella Takahashi, and mumps RIT4385 strains in Japanese children: A randomized phase I/II clinical trial. Hum. Vaccin. Immunother. 2019, 15, 1139–1144. [Google Scholar] [CrossRef]
- Smorodintsev, A.A.; Nasibov, M.N.; Jakovleva, N.V. Experience with live rubella virus vaccine combined with live vaccines against measles and mumps. Bull. World Health Organ. 1970, 42, 283–289. [Google Scholar]
- Cizman, M.; Mozetic, M.; Radescek-Rakar, R.; Pleterski-Rigler, D.; Susec-Michieli, M. Aseptic meningitis after vaccination against measles and mumps. Pediatr. Infect. Dis. J. 1989, 8, 302–308. [Google Scholar]
- Beck, M.; Welsz-Malecek, R.; Mesko-Prejac, M.; Radman, V.; Juzbasic, M.; Rajninger-Miholic, M.; Prislin-Musklic, M.; Dobrovsak-Sourek, V.; Smerdel, S.; Stainer, D.W. Mumps vaccine L-Zagreb, prepared in chick fibroblasts: I. production and field trials. J. Biol. Stand. 1989, 17, 85–90. [Google Scholar] [CrossRef]
- Bhargava, I.; Chhaparwal, B.C.; Phadke, M.A.; Irani, S.F.; Chhaparwal, D.; Dhorje, S.; Maheshwari, C.P. Immunogenicity and reactogenicity of indigenously produced MMR vaccine. Indian Pediatr. 1995, 32, 983–988. [Google Scholar] [PubMed]
- Tesovic, G.; Begovac, J.; Bace, A. Aseptic meningitis after measles, mumps, and rubella vaccine. Lancet 1993, 341, 1541. [Google Scholar] [CrossRef]
- Glück, R.; Hoskins, J.M.; Wegmann, A.; Just, M.; Germanier, R. Rubini, a new live attenuated mumps vaccine virus strain for human diploid cells. Dev. Biol. Stand. 1986, 65, 29–35. [Google Scholar] [PubMed]
- Vesikari, T.; André, F.E.; Simoen, E.; Florent, G.; Ala-Laurila, E.L.; Heikkinen, A.; Kuusinen, H.; Terho, A. Evaluation in young children of the Urabe Am 9 strain of live attenuated mumps vaccine in comparison with the Jeryl Lynn strain. Acta Paediatr. Scand. 1983, 72, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Ong, G.; Goh, K.T.; Ma, S.; Chew, S.K. Comparative efficacy of Rubini, Jeryl-Lynn and Urabe mumps vaccine in an Asian population. J. Infect. 2005, 51, 294–298. [Google Scholar] [CrossRef] [PubMed]
- Dias, J.A.; Cordeiro, M.; Afzal, M.A.; Freitas, M.G.; Morgado, M.R.; Silva, J.L.; Nunes, L.M.; Lima, M.G.; Avilez, F. Mumps epidemic in Portugal despite high vaccine coverage—Preliminary report. Eurosurveillance 1996, 1, 25–28. [Google Scholar] [CrossRef]
- Richard, J.L.; Zwahlen, M.; Feuz, M.; Matter, H.C. Swiss Sentinel Surveillance Network. Comparison of the effectiveness of two mumps vaccines during an outbreak in Switzerland in 1999 and 2000: A case-cohort study. Eur. J. Epidemiol. 2003, 18, 569–577. [Google Scholar] [CrossRef]
- Singh, R.; John, T.J.; Cherian, T.; Raghupathy, P. Immune response to measles, mumps and rubella vaccine at 9, 12 and 15 months of age. Indian J. Med. Res. 1994, 100, 155–159. [Google Scholar]
- Huang, L.M.; Lee, C.Y.; Hsu, C.Y.; Huang, S.S.; Kao, C.L.; Wu, F.F.; Lee, C.C.; Chang, H.S.; Huang, L.Y. Effect of monovalent measles and trivalent measles–mumps–rubella vaccines at various ages and concurrent administration with hepatitis B vaccine. Pediatr. Infect. Dis. J. 1990, 9, 461–465. [Google Scholar] [CrossRef]
- Miller, E.; Hill, A.; Morgan-Capner, P.; Forsey, T.; Rush, M. Antibodies to measles, mumps and rubella in UK children 4 years after vaccination with different MMR vaccines. Vaccine 1995, 13, 799–802. [Google Scholar] [CrossRef]
- Brown, E.G.; Furesz, J.; Dimock, K.; Yarosh, W.; Contreras, G. Nucleotide sequence analysis of Urab mumps vaccine strain that caused meningitis in vaccine recipients. Vaccine 1991, 9, 840–842. [Google Scholar] [CrossRef]
- Brown, E.G.; Dimock, K.; Wright, K.E. The Urabe AM9 mumps vaccine is a mixture of viruses differing at amino acid 335 of the hemagglutinin-neuraminidase gene with one form associated with disease. J. Infect. Dis. 1996, 174, 619–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boulianne, N.; De Serres, G.; Ratnam, S.; Ward, B.J.; Joly, J.R.; Duval, B. Measles, mumps, and rubella antibodies in children 5–6 years after immunization: Effect of vaccine type and age of vaccination. Vaccine 1995, 13, 1611–1616. [Google Scholar] [CrossRef]
- Dourado, I.; Cunha, S.; Teixeira, M.G.; Farrington, C.P.; Melo, A.; Lucena, R.; Barreto, M.L. Outbreak of aseptic meningitis associated with mass vaccination with a urabe-containing measles-mumps-rubella vaccine: Implications for immunization programs. Am. J. Epidemiol. 2000, 151, 524–530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amexis, G.; Fineschi, N.; Chumakov, K. Correlation of genetic variability with safety of mumps vaccine Urabe AM9 strain. Virology 2001, 287, 234–241. [Google Scholar] [CrossRef] [Green Version]
- Furesz, J.; Contreras, G. Vaccine–related mumps meningitis—Canada. Can. Dis. Wkly. Rep. 1990, 16, 253–254. [Google Scholar]
- Miller, E.; Goldacre, M.; Pugh, S.; Colville, A.; Farrington, P.; Flower, A.; Nash, J.; MacFarlane, L.; Tettmar, R. Risk of aseptic meningitis after measles, mumps, and rubella vaccine in UK children. Lancet 1993, 341, 979–982. [Google Scholar] [CrossRef]
- Sugiura, A.; Yamada, A. Aseptic meningitis as a complication of mumps vaccination. Pediatr. Infect. Dis. J. 1991, 10, 209–213. [Google Scholar] [CrossRef]
- Ueda, K.; Miyazaki, C.; Hidaka, Y.; Okada, K.; Kusuhara, K.; Kadoya, R. Aseptic meningitis caused by measles-mumps-rubella vaccine in Japan. Lancet 1995, 346, 701–702. [Google Scholar] [CrossRef]
- Rubin, S.A.; Link, M.A.; Sauder, C.J.; Zhang, C.; Ngo, L.; Rima, B.K.; Duprex, W.P. Recent mumps outbreaks in vaccinated populations: No evidence of immune escape. J. Virol. 2012, 86, 615–620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rota, J.S.; Rosen, J.B.; Doll, M.K.; McNall, R.J.; McGrew, M.; Williams, N.; Lopareva, E.N.; Barskey, A.E.; Punsalang, A., Jr.; Rota, P.A.; et al. Comparison of the Sensitivity of Laboratory Diagnostic Methods from a Well-Characterized Outbreak of Mumps in New York City in 2009. Clin. Vaccine Immunol. 2013, 20, 391–396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cortese, M.M.; Jordan, H.T.; Curns, A.T.; Quinlan, P.A.; Ens, K.A.; Denning, P.M.; Dayan, G.H. Mumps vaccine performance among university students during a mumps outbreak. Clin. Infect. Dis. 2008, 46, 1172–1180. [Google Scholar] [CrossRef] [PubMed]
| Pre-vaccine | Postvaccine | |||
|---|---|---|---|---|
| Country [reference] | Years | Annual incidence (per 100,000) | Years | Annual incidence (per 100,000) |
| USA [6] | 1967 | 100 | 1993 | <0.1 |
| Denmark [4] | 1979 | 726 | 1995 | 1 |
| Finland [11,12] | 1982 | 43 | 1995 | <0.1 |
| Slovenia [4] | 1979 | 410 | 1995 | 4 |
| Croatia [9] | 1985 | 101 | 1995 | 12 |
| France [14] | 1986 | 859 | 2011 | 9 |
| England and Wales [10] | 1985 | 40 | 1995 | 5 |
| Eastern Germany [13] | 1986 | 155 | 2016 | 0.62 |
| Israel [15] | 1985 | 102 | 1995 | 10 |
| Thailand [16] | 1996 | 20–70 | 1997 | 10–30 |
| Korea [18] | 1961 | >15,000 | 1981 | <10 |
| Australia [19] | 1969 | 59,000 | 2002 | 60 |
| Taiwan [20] | 1992 | 10 | 2006 | 1.3 |
| Types of vaccines | |||||
|---|---|---|---|---|---|
| Variables | Triviraten | MMR II | Morupar | Priorix | Trimovax |
| Company | Berna | Merck | Sanofi Aventis | GlaxoSmithKline | Sanofi Pasteur |
| Measles | |||||
| Strain | Edmonston-Zagreb, | Enders Edmonston, | Schwarz, | Schwarz, | Schwarz, |
| Strength | >1000 TCID50 | >1000 TCID50 | >1000 TCID50 | >1000 TCID50 | >1000 TCID50 |
| Mumps | |||||
| Strain | Rubini | Jeryl Lynn | Urabe AM9 | RIT 4385 | Urabe AM9 |
| Strength | >5000 TCID50 | >20,000 TCID50 | >5000 TCID50 | >5000 TCID50 | >5000 TCID50 |
| Rubella | |||||
| Strain | Wistar RA 27/3 | Wistar RA 27/3 | Wistar RA 27/3 | Wistar RA 27/3 | Wistar RA 27/3 |
| Strength | >1000 TCID50 | >1000 TCID50 | >1000 TCID50 | >1000 TCID50 | >1000 TCID50 |
| Types of | vaccines | |
|---|---|---|
| Variables | Priorix-Tetra (GlaxoSmithKline) | ProQuad (Merck) |
| Route of administration | Subcutaneous | Subcutaneous |
| Formulation | Lyophilized | Lyophilized |
| Diluent | Water for injection | Water for injection |
| Excipients | Amino acids, lactose (anhydrous), mannitol and sorbitol as stabilizers | Sorbitol, sodium phosphate, sucrose, sodium chloride, hydrolyzed gelatin, recombinant human albumin, fetal bovine serum, and other buffer and medium ingredients |
| Measles | ||
| Strain | Schwarz | Enders’ Edmonston |
| Strength | >103.0 TCID50 | >103.0 TCID50 |
| Mumps | ||
| Strain | Jeryl Lynn RIT 4385, | Jeryl Lynn |
| Strength | >104.4 TCID50 | >104.3 TCID50 |
| Rubella | ||
| Strain | Wistar RA 27/3 | Wistar RA 27/3 |
| Strength | >103.0 TCID50 | >103.0 TCID50 |
| Varicella | ||
| Strain | OKA | OKA/Merck |
| Strength | >103.3 PFU | >104.0 PFU |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, S.-B.; Chang, H.-L.; Chen, K.-T. Current Status of Mumps Virus Infection: Epidemiology, Pathogenesis, and Vaccine. Int. J. Environ. Res. Public Health 2020, 17, 1686. https://doi.org/10.3390/ijerph17051686
Su S-B, Chang H-L, Chen K-T. Current Status of Mumps Virus Infection: Epidemiology, Pathogenesis, and Vaccine. International Journal of Environmental Research and Public Health. 2020; 17(5):1686. https://doi.org/10.3390/ijerph17051686
Chicago/Turabian StyleSu, Shih-Bin, Hsiao-Liang Chang, and Kow-Tong Chen. 2020. "Current Status of Mumps Virus Infection: Epidemiology, Pathogenesis, and Vaccine" International Journal of Environmental Research and Public Health 17, no. 5: 1686. https://doi.org/10.3390/ijerph17051686
APA StyleSu, S.-B., Chang, H.-L., & Chen, K.-T. (2020). Current Status of Mumps Virus Infection: Epidemiology, Pathogenesis, and Vaccine. International Journal of Environmental Research and Public Health, 17(5), 1686. https://doi.org/10.3390/ijerph17051686






