Serum Ghrelin Levels in Saudi Obese Asthmatic School-Children—Correlation with Interleukin-4, Interleukin-5, and Interleukin-21
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Biochemical Evaluation
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mohanan, S.; Tapp, H.; McWilliams, A.; Dulin, M. Obesity and asthma: Pathophysiology and implications for diagnosis and management in primary care. Exp. Boil. Med. 2014, 239, 1531–1540. [Google Scholar] [CrossRef] [PubMed]
- Masoli, M.; Fabian, D.; Holt, S.; Beasley, R. Global Initiative for Asthma (GINA) program: The global burden of asthma: Executive summary of the GINA Dissemination Committee report. Allergy 2004, 59, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Duran-Tauleria, E.; Rona, R.J. Geographical and socioeconomic variation in the prevalence of asthma symptoms in English and Scottish children. Thorax 1999, 54, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Ren, Y.; Li, M.; Zhao, X.; Kong, L.; Kang, J. Prevalence of comorbidities in asthma and non-asthma patients. Medicine (Baltimore) 2016, 95, 1–7. [Google Scholar] [CrossRef]
- Moradi-Lakeh, M.; El Bcheraoui, C.; Daoud, F.; Tuffaha, M.; Kravitz, H.; Al Saeedi, M.; Basulaiman, M.; Memish, Z.A.; Al Mazroa, M.A.; Al Rabeeah, A.A.; et al. Prevalence of asthma in Saudi adults: Findings from a national household survey, 2013. BMC Pulm. Med. 2015, 15, 77. [Google Scholar] [CrossRef]
- Sideleva, O.; Suratt, B.T.; Black, K.E.; Tharp, W.G.; Pratley, R.E.; Forgione, P.; Dienz, O.; Irvin, C.; Dixon, A. Obesity and asthma: An inflammatory disease of adipose tissue not the airway. Am. J. Respir. Crit. Care Med. 2012, 186, 598–605. [Google Scholar] [CrossRef]
- Hancox, R.J.; Milne, B.J.; Poulton, R.; Taylor, D.R.; Greene, J.M.; McLachlan, C.R.; Cowan, J.O.; Flannery, E.M.; Herbison, G.P.; Sears, M.R. Sex Differences in the Relation between Body Mass Index and Asthma and Atopy in a Birth Cohort. Am. J. Respir. Crit. Care Med. 2005, 171, 440–445. [Google Scholar] [CrossRef]
- Umetsu, D.T. Mechanisms by which obesity impacts upon asthma. Thorax 2017, 72, 174–177. [Google Scholar] [CrossRef]
- Sin, D.D.; Jones, R.L.; Man, S.F.P. Obesity is a risk factor for dyspnea but not for airflow obstruction. Arch. Intern. Med. 2002, 162, 1477–1481. [Google Scholar] [CrossRef]
- Taylor, B.; Mannino, D.; Brown, C.; Crocker, D.; Twum-Baah, N.; Holguin, F. Body mass index and asthma severity in the National Asthma Survey. Thorax 2008, 63, 14–20. [Google Scholar] [CrossRef]
- Kojima, M.; Hosoda, H.; Date, Y. Ghrelin is a growth-hormone- releasing acylated peptide from stomach. Nature 1999, 402, 656–660. [Google Scholar] [CrossRef] [PubMed]
- Tolle, V.; Bassant, M.H.; Zizzari, P.; Poindessous-Jazat, F.; Tomasetto, C.; Epelbaum, J.; Pajot, M. Ultradian rhythmicity of ghrelin secretion in relation with gh, feeding behavior, and sleep-wake patterns in rats. Endocrinology 2002, 143, 1353–1361. [Google Scholar] [CrossRef] [PubMed]
- Nakahara, K.; Okame, R.; Katayama, T.; Miyazato, M.; Kangawa, K.; Murakami, N. Nutritional and environmental factors affecting plasma ghrelin and leptin levels in rats. J. Endocrinol. 2010, 207, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Fu, T.; Wang, L.; Zeng, Q.; Zhang, Y.; Sheng, B.; Han, L. Ghrelin ameliorates asthma by inhibiting endoplasmic reticulum stress. Am. J. Med. Sci. 2018, 354, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Salah, A.; Ragab, M.; Masnsour, W.; Taher, M. Leptin and adiponectin are valuable serum markers explaining obesity/bronchial asthma interrelationship. Egypt. J. Chest Dis. Tuberc. 2015, 64, 529–533. [Google Scholar] [CrossRef]
- Toru, Ü.; Ayada, C.; Genç, O.; Şahin, S.; Arık, Ö.; Acat, M.; Bulut, İ.; Çetinkaya, E. Visfatin and ghrelin: Can they be forthcoming biomarkers or new drug targets for asthma? Int. J. Clin. Exp. Med. 2015, 8, 6257–6261. [Google Scholar]
- Mende, F.; Hundahl, C.; Plouffe, B.; Skov, L.J.; Sivertsen, B.; Madsen, A.N.; Lückmann, M.; Diep, T.A.; Offermanns, S.; Frimurer, T.M.; et al. Translating biased signaling in the ghrelin receptor system into differential in vivo functions. Proc. Natl. Acad. Sci. USA 2018, 115, E10255–E10264. [Google Scholar] [CrossRef]
- Okamatsu, Y.; Matsuda, K.; Hiramoto, I.; Tani, H.; Kimura, K.; Yada, Y.; Kakuma, T.; Higuchi, S.; Kojima, M.; Matsuishi, T. Ghrelin and leptin modulate immunity and liver function in overweight children. Pediatr. Int. 2009, 51, 9–13. [Google Scholar] [CrossRef]
- Suresh, K.; Chandrashekara, S. Sample size estimation and power analysis for clinical research studies. J. Hum. Reprod. Sci. 2012, 5, 7–13. [Google Scholar] [CrossRef]
- Al-Moamary, M.S.; Alhaider, S.A.; Idrees, M.M.; Al Ghobain, M.O.; Zeitouni, M.O.; Al-Harbi, A.S.; Yousef, A.A.; Al-Matar, H.; Alorainy, H.S.; Al-Hajjaj, M.S. The Saudi Initiative for Asthma—2016 update: Guidelines for the diagnosis and management of asthma in adultsand children. Ann. Thorac. Med. 2016, 11, 3–42. [Google Scholar] [CrossRef]
- Alsahn, B.; Alshamrani, A.; Alzahrani, A.; Alsahmi, O.; Alqudhybi, A. Asthma Control Assessment Using Asthma Control Test among Pediatric Patients Attending a Tertiary Care Hospital in Saudi Arabia. Egypt. J. Hosp. Med. 2017, 68, 1215–1223. [Google Scholar] [CrossRef]
- Youssef, D.M.; Elbehidy, R.M.; Shokry, D.M.; Elbehidy, E.M. The influence of leptin on Th1/Th2 balance in obese children with asthma. J. Bras. Pneumol. 2013, 39, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Yüksel, H.; Sogut, A.; Yilmaz, O.; Onur, E.; Dinç, G. Role of Adipokines and Hormones of Obesity in Childhood Asthma. Allergy Asthma Immunol. Res. 2011, 4, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Tsaroucha, A.; Daniil, Z.; Malli, F.; Georgoulias, P.; Minas, M.; Kostikas, K.; Bargiota, A.; Zintzaras, E.; Gourgoulianis, K.I. Leptin, Adiponectin, and Ghrelin Levels in Female Patients with Asthma during Stable and Exacerbation Periods. J. Asthma 2012, 50, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Müller, T.D.; Nogueiras, R.; Andermann, M.L.; Andrews, Z.B.; Anker, S.D.; Argente, J.; Batterham, R.L.; Benoit, S.C.; Bowers, C.Y.; Broglio, F.; et al. Ghrelin. Mol. Metab. 2015, 4, 437–460. [CrossRef]
- Taheri, S.; Lin, L.; Austin, D.; Young, T.; Mignot, E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004, 1, e62. [Google Scholar] [CrossRef]
- Sánchez-De-La-Torre, M.; Mediano, O.; Barceló, A.; Pierola, J.; De La Peña, M.; Esquinas, C.; Miro, A.; Durán-Cantolla, J.; Agusti, A.G.; Capote, F.; et al. The influence of obesity and obstructive sleep apnea on metabolic hormones. Sleep Breath. 2011, 16, 649–656. [Google Scholar] [CrossRef]
- Cobanoglu, N.; Galip, N.; Dalkan, C.; Bahceciler, N.N. Leptin, ghrelin and calprotectin: Inflammatory markers in childhood asthma. Multidiscip. Respir. Med. 2013, 8, 62. [Google Scholar] [CrossRef]
- Dixit, V.D.; Taub, D.D. Ghrelin and immunity: A young player in an old field. Exp. Gerontol. 2005, 40, 900–910. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Toyomasu, K.; Uchimura, N.; Ishitake, T. Low-molecular-weight adiponectin is more closely associated with episodes of asthma than high-molecular-weight adiponectin. Endocr. J. 2013, 60, 119–125. [Google Scholar] [CrossRef]
- Moser, R.; Fehr, J.; Bruijnzeel, P.L. IL-4 controls the selective endothelium-driven transmigration of eosinophils from allergic individuals. J. Immunol. 1992, 149, 1432–1438. [Google Scholar] [PubMed]
- Dubucquoi, B.S.; Desreumaux, P.; Janin, S.A.; Klein, O.; Goldman, M.; Tavernier, I.I.J.; Capron, A.; Capron, M. Interleukin 5 synthesis by eosinophils: Association with granules and immunoglobulin-dependent secretion. J. Exp. Med. 1994, 179, 703–708. [Google Scholar] [CrossRef] [PubMed]
- Gong, F.; Su, Q.; Pan, Y.H.; Huang, X.; Shen, W.H. The emerging role of interleukin-21 in allergic diseases (Review). Biomed. Rep. 2013, 1, 837–839. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Parronchi, P.; De Carli, M.; Manetti, R.; Simonelli, C.; Piccinni, M.-P.; Macchia, D.; Maggi, E.; Del Prete, G.; Ricci, M.; Romagnani, S. Aberrant interleukin (IL)-4 and IL-5 productionin vitro by CD4+ helper T cells from atopic subjects. Eur. J. Immunol. 1992, 22, 1615–1620. [Google Scholar] [CrossRef]
- Hiromura, Y.; Kishida, T.; Nakano, H.; Hama, T.; Imanishi, J.; Hisa, Y.; Mazda, O. IL-21 administration into the nostril alleviates murine allergic rhinitis. J. Immunol. 2007, 179, 7157–7165. [Google Scholar] [CrossRef]
- Sivakumar, P.V.; Foster, D.C.; Clegg, C.H. Interleukin-21 is a T-helper cytokine that regulates humoral immunity and cell-mediated anti-tumour responses. Immunol. 2004, 112, 177–182. [Google Scholar] [CrossRef]
- Miethe, S.; Guarino, M.; Alhamdan, F.; Simon, H.-U.; Renz, H.; Dufour, J.-F.; Potaczek, D.P.; Garn, H. The effects of obesity on asthma: Immunometabolic links. Pol. Arch. Intern. Med. 2018. [Google Scholar] [CrossRef]
- Ersahin, M.; Toklu, H.Z.; Erzik, C.; Akakin, D.; Tetik, S.; Şener, G.; Yeğen, B.Ç. Ghrelin alleviates spinal cord injury in rats via its anti-inflammatory effects. Turk. Neurosurg. 2011, 21, 599–605. [Google Scholar] [CrossRef]
- Wu, R.-Q.; Dong, W.; Cui, X.; Zhou, M.; Simms, H.H.; Ravikumar, T.S.; Wang, P. Ghrelin Down-regulates Proinflammatory Cytokines in Sepsis Through Activation of the Vagus Nerve. Ann. Surg. 2007, 245, 480–486. [Google Scholar] [CrossRef]
- Al-Ayed, M.S.Z. Relaxant effect of ghrelin on guinea pig isolated tracheal smooth muscle: Role of epithelial NO and PGE2. Pflügers Archiv. Eur. J. Physiol. 2018, 470, 949–958. [Google Scholar] [CrossRef]
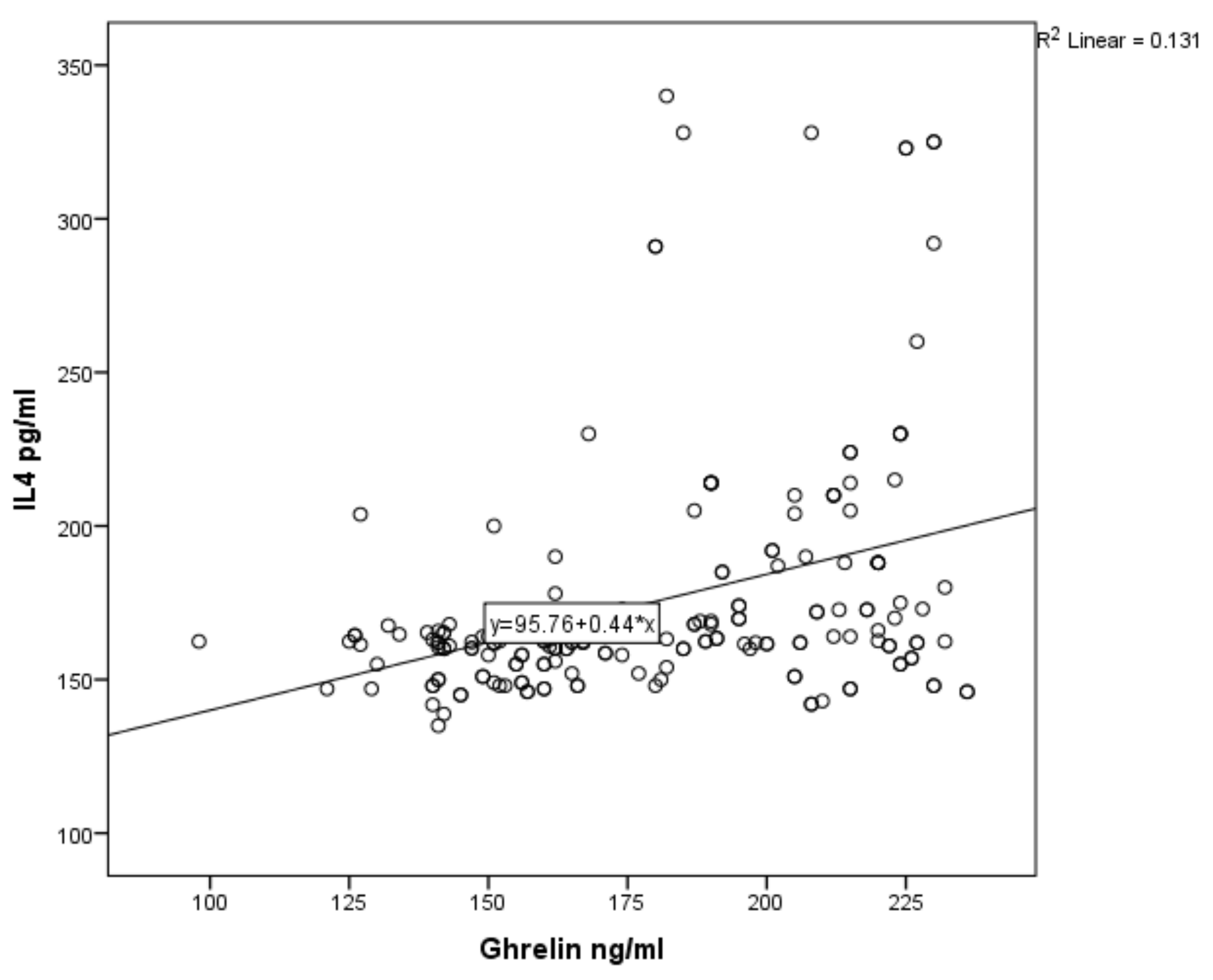
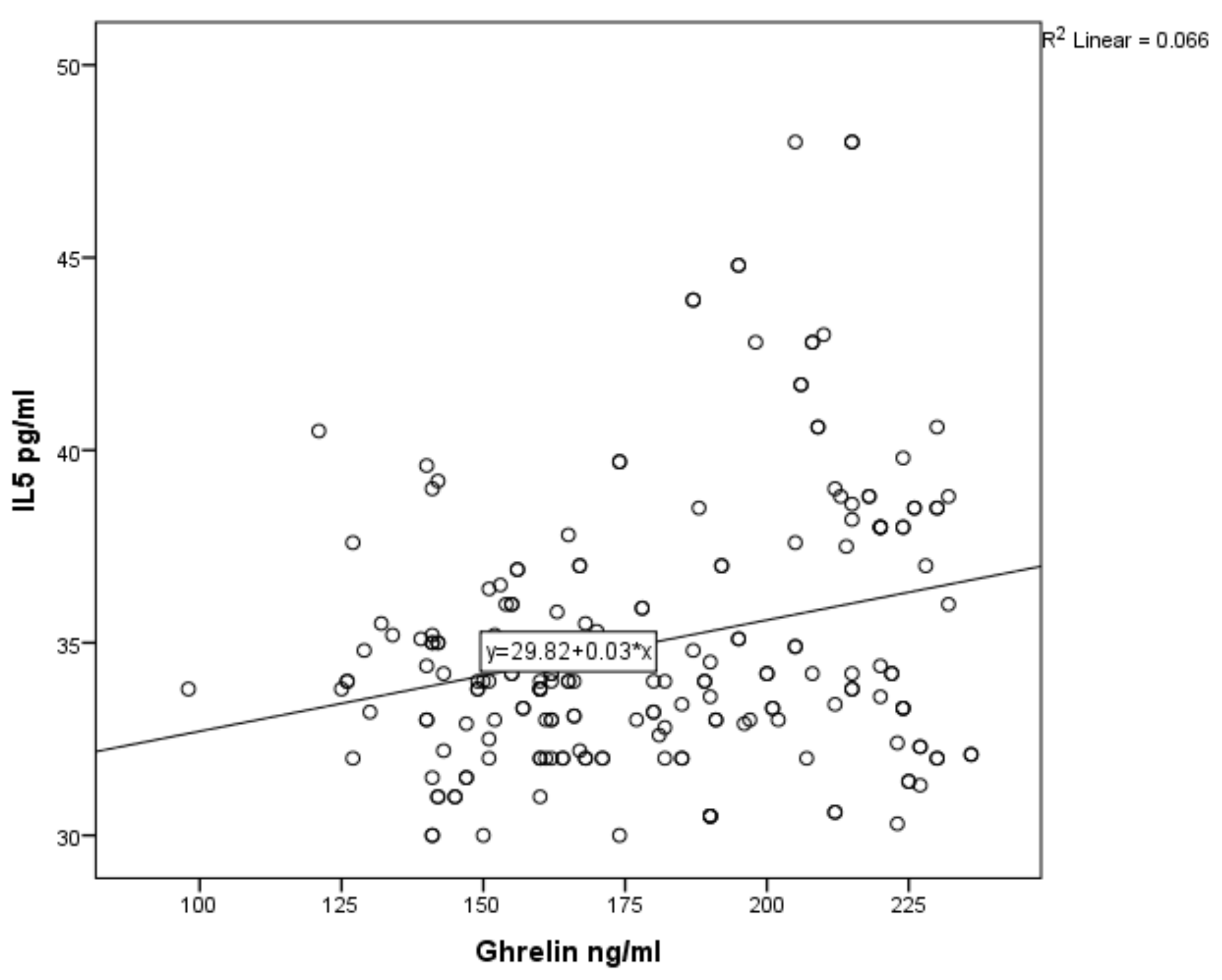
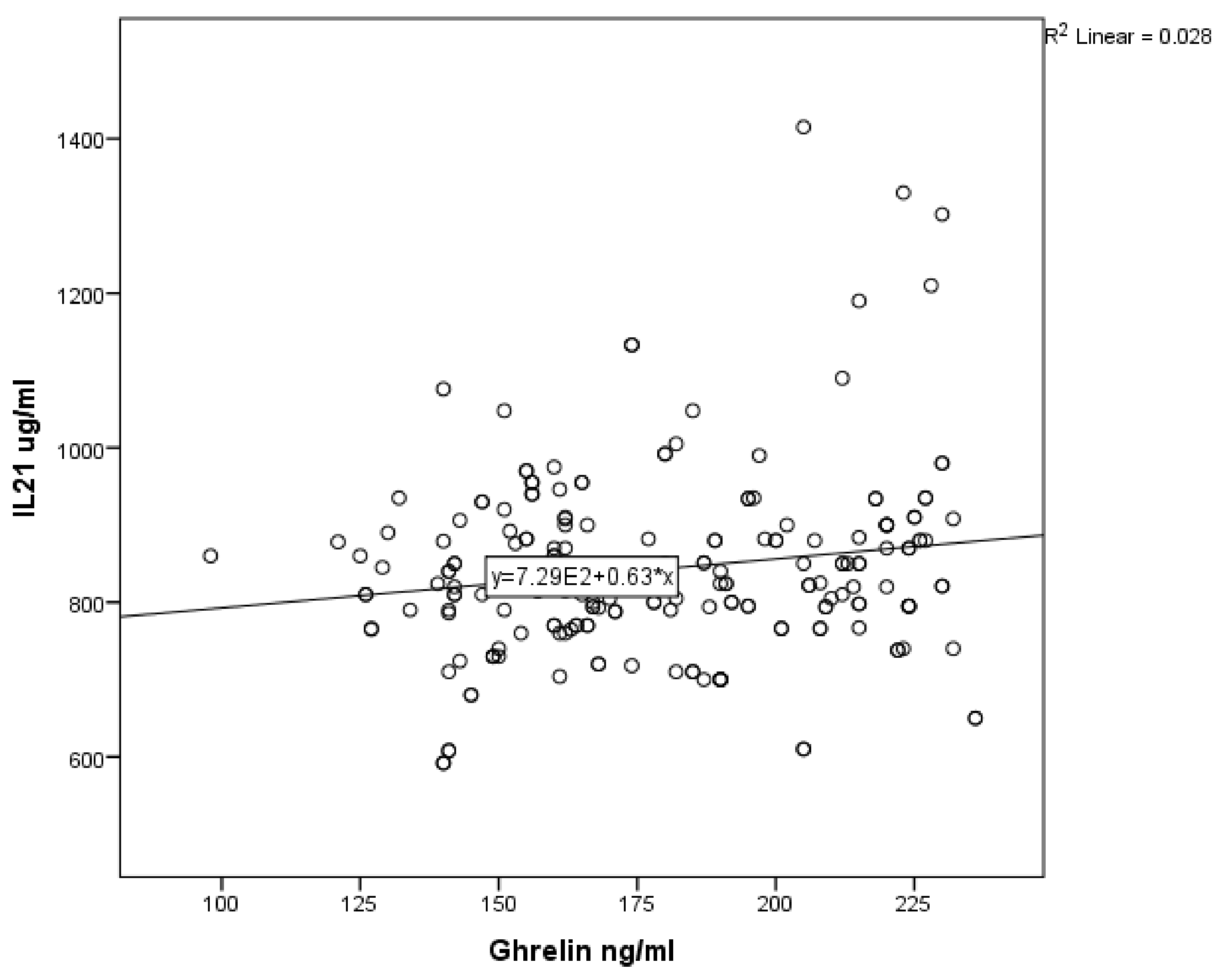
| Variable | Number | Median | Mean | SD |
|---|---|---|---|---|
| Non-obese non-asthmatic | 101 | 166 | 174.02 | 31.23 |
| Non-obese asthmatic | 100 | 212 | 199.25 | 27.13 |
| Obese non-asthmatic | 100 | 190 | 186.21 | 30.99 |
| Obese asthmatic | 100 | 210 | 197.43 | 27.09 |
| Parameter (Median, Mean ± SD) | Asthmatics: N (200) | Non-Asthmatics: N (201) | p Value |
|---|---|---|---|
| Ghrelin, ng/mL | 210, 198.40 ± 27.06 | 178, 180.10 ± 31.63 | 0.001 * |
| IL-4, pg/mL | 172.5, 191.02 ± 45.98 | 163, 175.51 ± 38.52 | 0.001 * |
| IL-5, pg/mL | 35, 36.51 ± 6.14 | 34, 35.08 ± 3.58 | 0.124 |
| IL-21, µg/mL | 880, 896.20 ± 157.66 | 824, 843.45 ± 119.16 | 0.003 * |
| Parameter (Mean ±SD) | Controlled: N (120) | Uncontrolled: N (80) | p Value |
|---|---|---|---|
| Ghrelin, ng/mL | 193.81 ± 27.51 | 204.95 ± 25.12 | 0.002 * |
| IL-4, pg/mL | 190.00 ± 48.45 | 191.10 ± 44.46 | 0.214 |
| IL-5, pg/mL | 35.51 ± 5.47 | 38.02 ± 6.77 | 0.001 * |
| IL-21, µg/mL | 857.51 ± 133.10 | 954.25 ± 173.70 | 0.001 * |
| Variables | aOR | 95% CI | p Value |
|---|---|---|---|
| Sex: males vs. females | 0.449 | 0.179–1.129 | 0.089 |
| Obesity: obese vs. non-obese | 1.057 | 0.669–1.669 | 0.812 |
| Asthmatic: Asthmatics vs. non-asthmatics * | 2.191 | 1.344–3.571 | 0.002 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Ayed, M.S.; Al-Shaibari, K.S.; Alshehri, D.; Alzahrani, M.J.; Nasser, I.; Alaamri, H.S.; Alaseeri, W.A.; Mahfouz, A.A.; Alsareii, S.A.; Asaad, A.M.; et al. Serum Ghrelin Levels in Saudi Obese Asthmatic School-Children—Correlation with Interleukin-4, Interleukin-5, and Interleukin-21. Int. J. Environ. Res. Public Health 2020, 17, 1656. https://doi.org/10.3390/ijerph17051656
Al-Ayed MS, Al-Shaibari KS, Alshehri D, Alzahrani MJ, Nasser I, Alaamri HS, Alaseeri WA, Mahfouz AA, Alsareii SA, Asaad AM, et al. Serum Ghrelin Levels in Saudi Obese Asthmatic School-Children—Correlation with Interleukin-4, Interleukin-5, and Interleukin-21. International Journal of Environmental Research and Public Health. 2020; 17(5):1656. https://doi.org/10.3390/ijerph17051656
Chicago/Turabian StyleAl-Ayed, Mohammed Saeed, Khaled Sadeq Al-Shaibari, Dhafer Alshehri, Mohammed Jamaan Alzahrani, Iman Nasser, Hamdan Saad Alaamri, Wed Ahmad Alaseeri, Ahmed A. Mahfouz, Saeed Ali Alsareii, Ahmed Morad Asaad, and et al. 2020. "Serum Ghrelin Levels in Saudi Obese Asthmatic School-Children—Correlation with Interleukin-4, Interleukin-5, and Interleukin-21" International Journal of Environmental Research and Public Health 17, no. 5: 1656. https://doi.org/10.3390/ijerph17051656
APA StyleAl-Ayed, M. S., Al-Shaibari, K. S., Alshehri, D., Alzahrani, M. J., Nasser, I., Alaamri, H. S., Alaseeri, W. A., Mahfouz, A. A., Alsareii, S. A., Asaad, A. M., Magzoub, A., Qureshi, M. A., Elagab, E., Hassan, E. E., & Shalayel, M. H. F. (2020). Serum Ghrelin Levels in Saudi Obese Asthmatic School-Children—Correlation with Interleukin-4, Interleukin-5, and Interleukin-21. International Journal of Environmental Research and Public Health, 17(5), 1656. https://doi.org/10.3390/ijerph17051656





