Time-Cumulative Toxicity of Neonicotinoids: Experimental Evidence and Implications for Environmental Risk Assessments
Abstract
1. Introduction
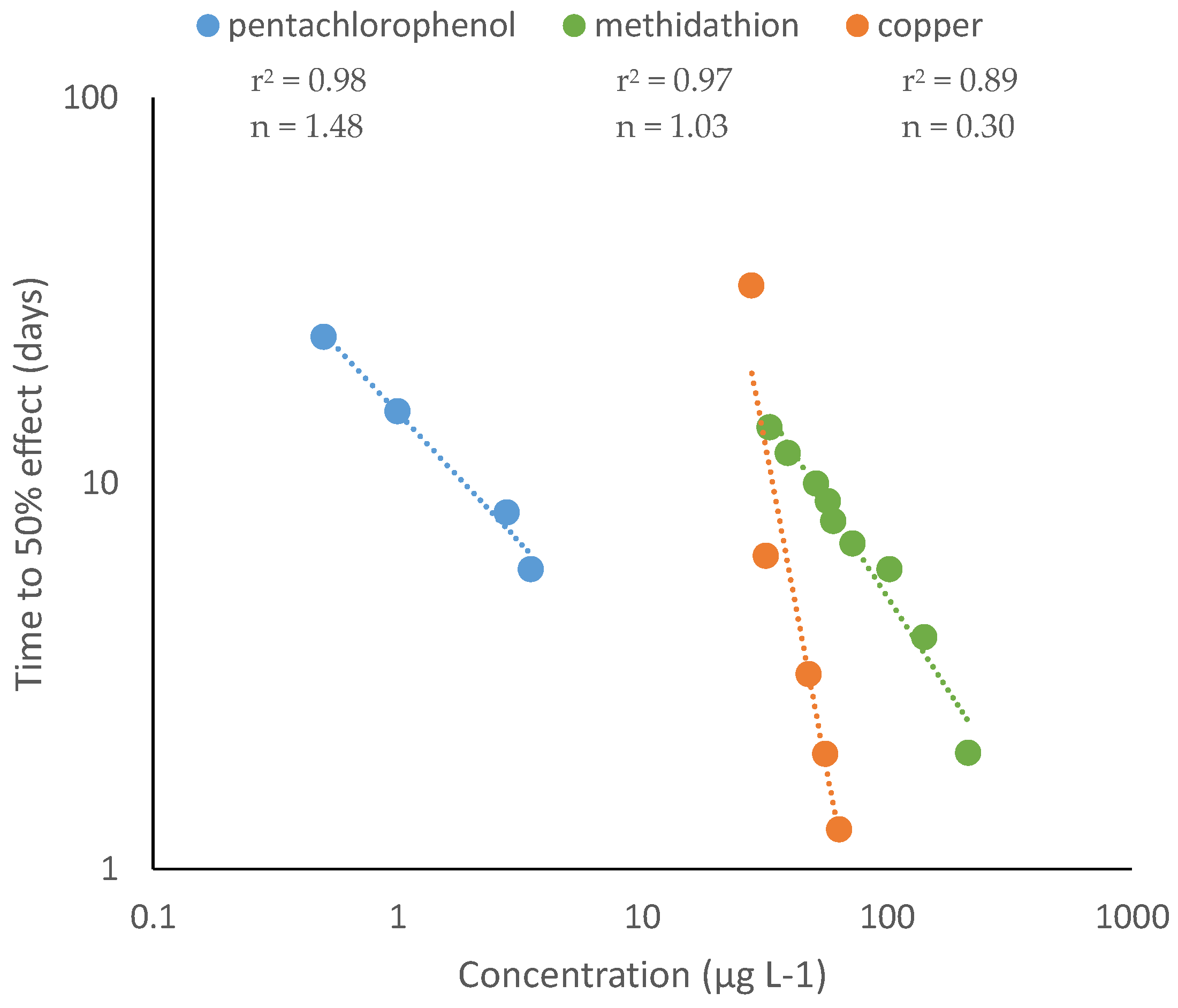
2. Time-Dependent Toxicity
3. How to Identify Chemicals with Time-Dependent Toxicity
4. Experimental Evidence for Neonicotinoids
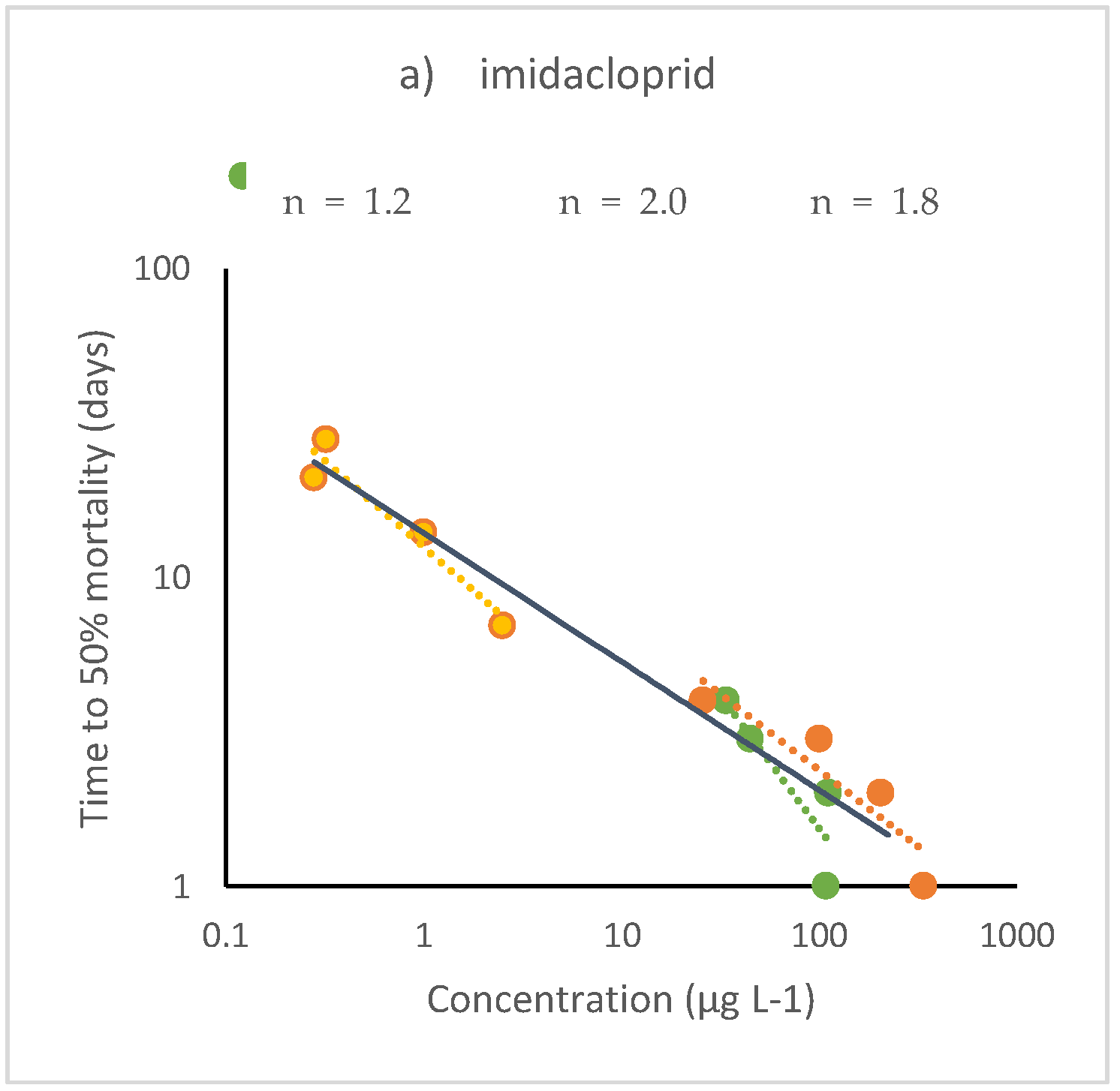
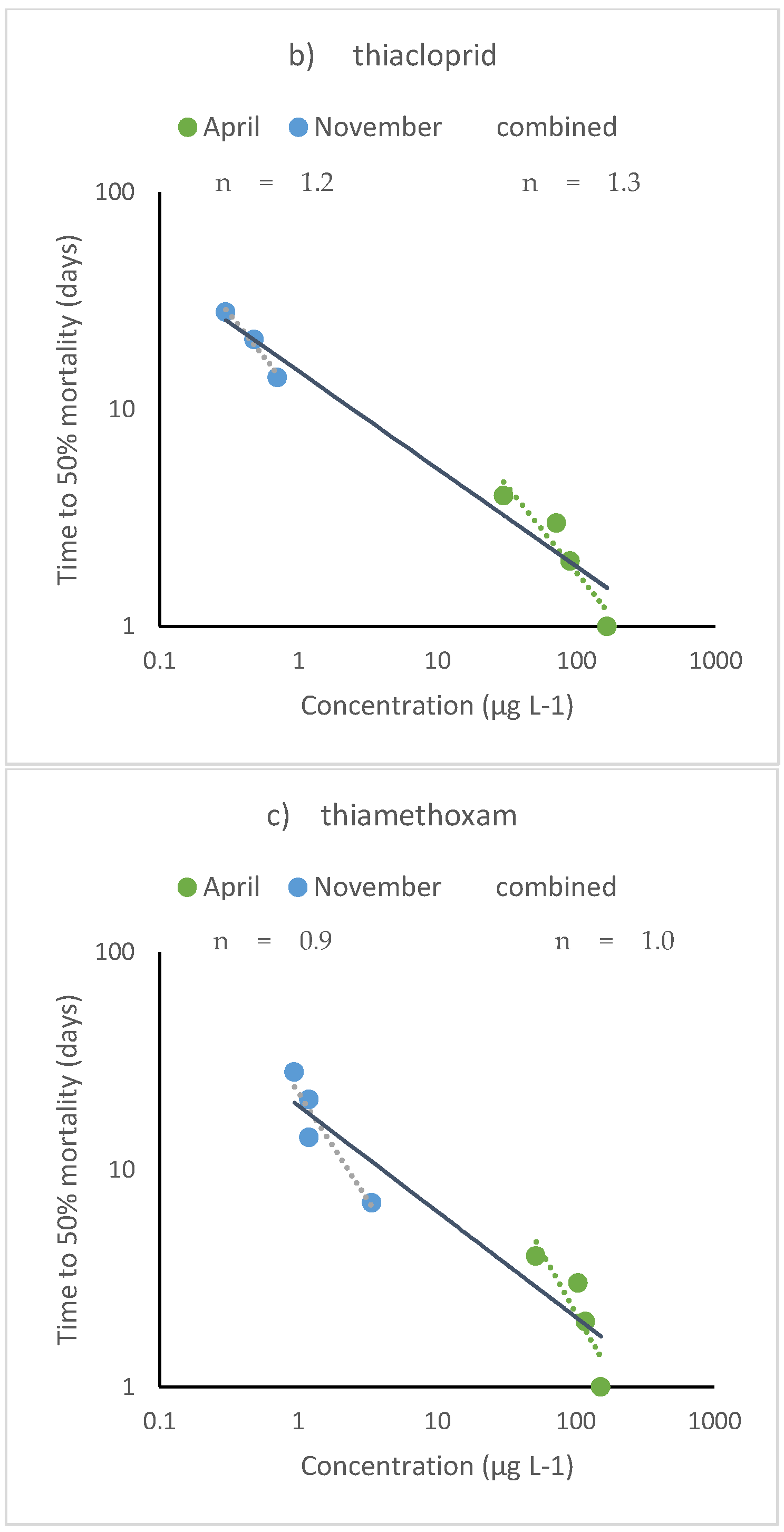
4.1. Aquatic Organisms
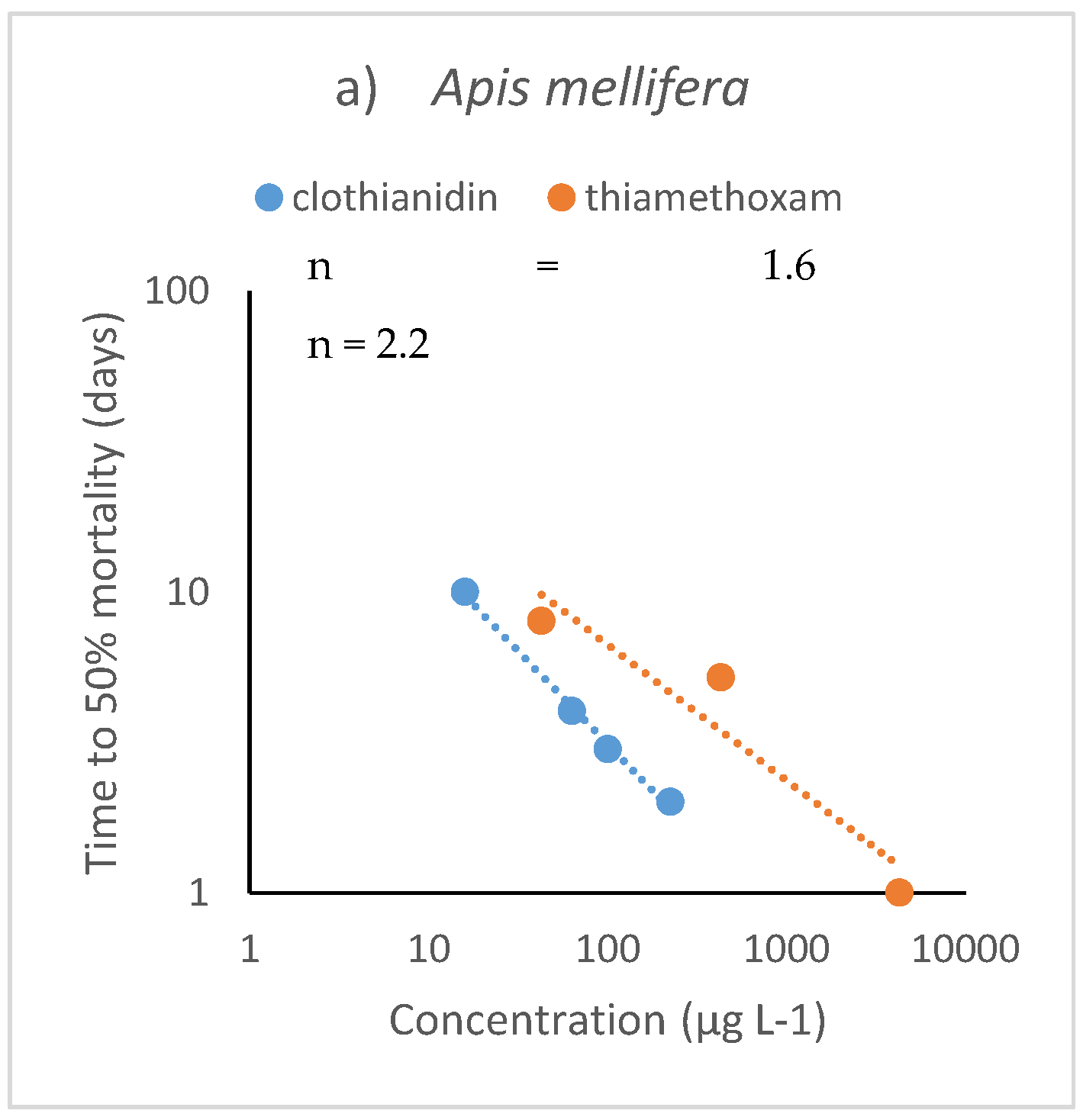
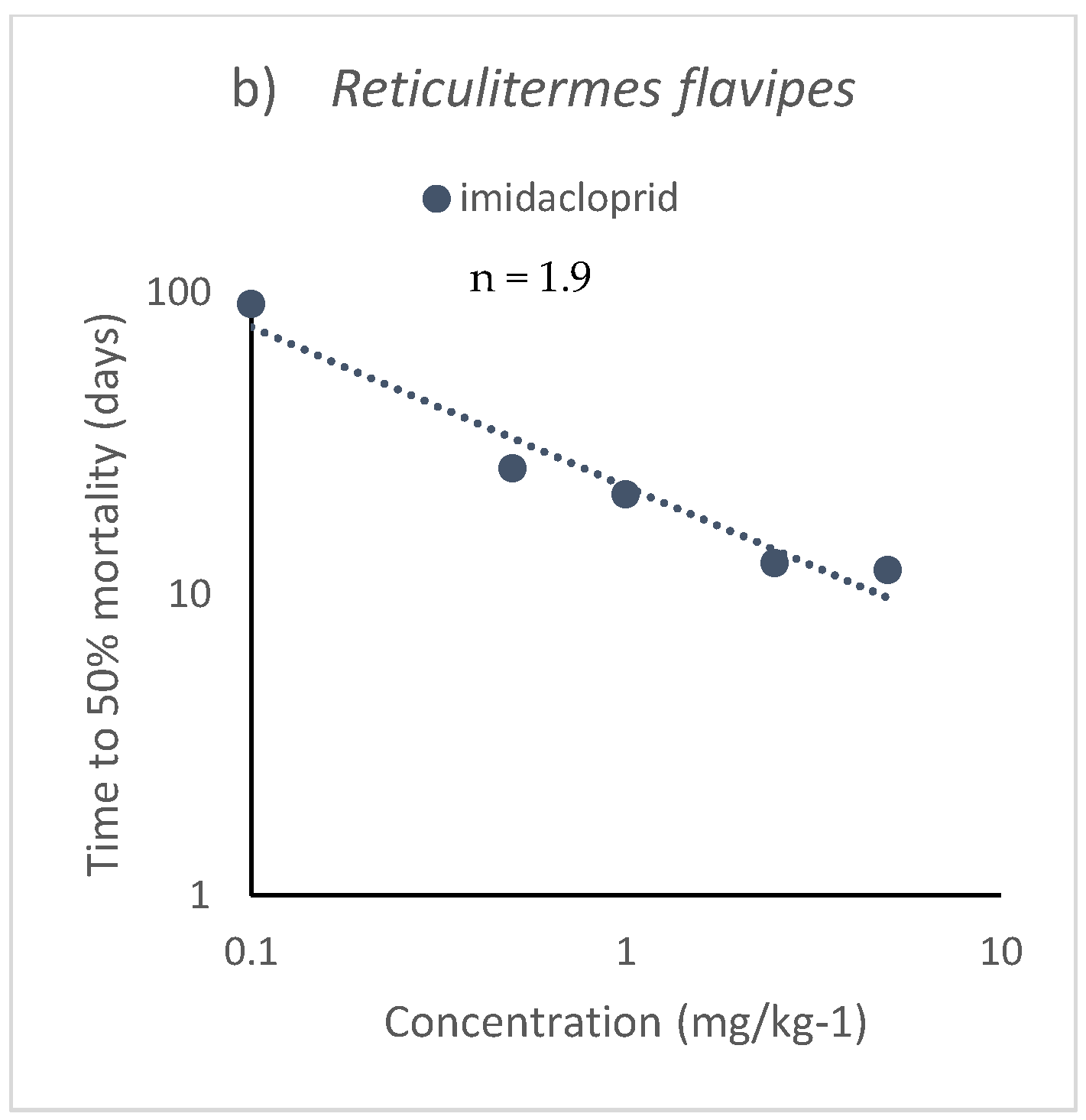
4.2. Terrestrial Organisms
5. Implications for Risk Assessment of Neonicotinoids
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Simon-Delso, N.; Amaral-Rogers, V.; Belzunces, L.P.; Bonmatin, J.M.; Chagnon, M.; Downs, C.; Furlan, L.; Gibbons, D.W.; Giorio, C.; Girolami, V.; et al. Systemic insecticides (neonicotinoids and fipronil): Trends, uses, mode of action and metabolites. Environ. Sci. Pollut. Res. 2015, 22, 5–34. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, P.; Nauen, R.; Schindler, M.; Elbert, A. Overview of the status and global strategy for neonicotinoids. J. Agric. Food Chem. 2011, 59, 2897–2908. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, P.; Nauen, R. Neonicotinoids—From zero to hero in insecticide chemistry. Pest Manag. Sci. 2008, 64, 1084–1098. [Google Scholar] [CrossRef] [PubMed]
- Hladik, M.L.; Main, A.R.; Goulson, D. Environmental risks and challenges associated with neonicotinoid insecticides. Environ. Sci. Technol. 2018, 52, 3329–3335. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, K.; Ihara, M.; Sattelle, D.B. Neonicotinoid insecticides: Molecular targets, resistance, and toxicity. Annu. Rev. Pharmacol. Toxicol. 2020, 60, 241–255. [Google Scholar] [CrossRef]
- Tomizawa, M.; Lee, D.L.; Casida, J.E. Neonicotinoid insecticides: Molecular features conferring selectivity for insect versus mammalian nicotinic receptors. J. Agric. Food Chem. 2000, 48, 6016–6024. [Google Scholar] [CrossRef]
- Mehlhorn, H.; Mencke, N.; Hansen, O. Effects of imidacloprid on adult and larval stages of the flea Ctenocephalides felis after in vivo and in vitro application: A light- and electron-microscopy study. Parasitol. Res. 1999, 85, 625–637. [Google Scholar] [CrossRef]
- Nauen, R. Behaviour modifying effects of low systemic concentrations of imidacloprid on Myzus persicae with special reference to an antifeeding response. Pestic. Sci. 1995, 44, 145–153. [Google Scholar] [CrossRef]
- Beketov, M.A.; Liess, M. Acute and delayed effects of the neonicotinoid insecticide thiacloprid on seven freshwater arthropods. Environ. Toxicol. Chem. 2008, 27, 461–470. [Google Scholar] [CrossRef]
- Tennekes, H.A.; Sánchez-Bayo, F. The molecular basis of simple relationships between exposure concentration and toxic effects with time. Toxicology 2013, 309, 39–51. [Google Scholar] [CrossRef]
- Rondeau, G.; Sánchez-Bayo, F.; Tennekes, H.A.; Decourtye, A.; Ramírez-Romero, R.; Desneux, N. Delayed and time-cumulative toxicity of imidacloprid in bees, ants and termites. Sci. Rep. 2014, 4, 5566. [Google Scholar] [CrossRef] [PubMed]
- Tennekes, H.A.; Sánchez-Bayo, F. Time-dependent toxicity of neonicotinoids and other toxicants: Implications for a new approach to risk assessment. J. Environ. Anal. Toxicol. 2012, S4, S4-001. [Google Scholar] [CrossRef]
- Druckrey, H. Quantitative Grundlagen der Krebserzeugung. Klinische Wochenschriften 1943, 22, 532. [Google Scholar] [CrossRef]
- Druckrey, H.; Schildbach, A.; Schmaehl, D.; Preussmann, R.; Ivankovic, S. Quantitative analysis of the carcinogenic effect of diethylnitrosamine. Arzneimittelforschung 1963, 13, 841–851. [Google Scholar]
- Druckrey, H.; Schagen, B.; Ivankovic, S. Induction of neurogenic malignancies by one single dose of ethyl-nitrosourea (ENU) given to newborn and juvenile BD IX-strain rats. Z. Krebsforsch 1970, 74, 141–161. [Google Scholar] [CrossRef]
- Rattner, B.A.; Horak, K.E.; Warner, S.E.; Day, D.D.; Meteyer, C.U.; Volker, S.F.; Eisemann, J.D.; Johnston, J.J. Acute toxicity, histopathology, and coagulopathy in American kestrels (Falco sparverius) following administration of the rodenticide diphacinone. Environ. Toxicol. Chem. 2011, 30, 1213–1222. [Google Scholar] [CrossRef]
- Simon-Delso, N.; Martin, G.S.; Bruneau, E.; Hautier, L. Time-to-death approach to reveal chronic and cumulative toxicity of a fungicide for honeybees not revealed with the standard ten-day test. Sci. Rep. 2018, 8, 7241. [Google Scholar] [CrossRef]
- Pletz, J.; Sánchez-Bayo, F.; Tennekes, H.A. Dose-response analysis indicating time-dependent neurotoxicity caused by organic and inorganic mercury—Implications for toxic effects in the developing brain. Toxicology 2016, 347, 1–5. [Google Scholar] [CrossRef]
- Tennekes, H.A. The significance of the Druckrey-Küpfmüller equation for risk assessment—The toxicity of neonicotinoid insecticides to arthropods is reinforced by exposure time. Toxicology 2010, 276, 1–4. [Google Scholar] [CrossRef]
- Walker, C.H.; Hopkin, S.P.; Sibly, R.M.; Peakall, D.B. Principles of Ecotoxicology, 2nd ed.; Taylor & Francis: Glasgow, UK, 2001; p. 309. [Google Scholar]
- Jager, T.; Klok, C. Extrapolating toxic effects on individuals to the population level: The role of dynamic energy budgets. Phil. Trans. R. Soc. London B 2010, 365, 3531–3540. [Google Scholar] [CrossRef]
- Legierse, K.C.H.M.; Verhaar, H.J.M.; Vaes, W.H.J.; Bruijn, J.H.M.d.; Hermens, J.L.M. Analysis of the time-dependent acute aquatic toxicity of organophosphorus pesticides: The critical target occupation model. Environ. Sci. Technol. 1999, 33, 917–925. [Google Scholar] [CrossRef]
- Jager, T.; Kooijman, S.A.L.M. Modeling receptor kinetics in the analysis of survival data for organophosphorus pesticides. Environ. Sci. Technol. 2005, 39, 8307–8314. [Google Scholar] [CrossRef] [PubMed]
- Soderlund, D.M.; Clark, J.M.; Sheets, L.P.; Mullin, L.S.; Piccirillo, V.J.; Sargent, D.; Stevens, J.T.; Weiner, M.L. Mechanisms of pyrethroid neurotoxicity: Implications for cumulative risk assessment. Toxicology 2002, 171, 3–59. [Google Scholar] [CrossRef]
- Müller, R.; Berghahn, R.; Hilt, S. Herbicide effects of metazachlor on duckweed (Lemna minor and Spirodela polyrhiza) in test systems with different trophic status and complexity. J. Environ. Sci. Health B 2010, 45, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Druckrey, H.; Dischler, W. Dosis-Wirkungsbeziehungen bei der Krebserzeugung durch 4-dimethylaminostilben bei Ratten. Z. Krebsforsch 1963, 65, 272. [Google Scholar] [CrossRef]
- Newman, M.C.; McCloskey, J.T. Time-to-event analyses of ecotoxicity data. Ecotoxicology 1996, 5, 187–196. [Google Scholar] [CrossRef]
- Kooijman, S.A.L.M. Parametric analyses of mortality rates in bioassays. Water Res. 1981, 15, 107–119. [Google Scholar]
- Sánchez-Bayo, F. From simple toxicological models to prediction of toxic effects in time. Ecotoxicology 2009, 18, 343–354. [Google Scholar] [CrossRef]
- Ashauer, R.; Boxall, A.B.A.; Brown, C.D. Simulating toxicity of carbaryl to Gammarus pulex after aequential pulsed exposure. Environ. Sci. Technol. 2007, 41, 5528–5534. [Google Scholar] [CrossRef]
- Hoang, T.C.; Gallagher, J.S.; Tomasso, J.R.; Klaine, S.J. Toxicity of two pulsed metal exposures to Daphnia magna: Relative effects of pulsed duration-concentration and influence of interpulse period. Arch. Environ. Contam. Toxicol. 2007, 53, 579–589. [Google Scholar] [CrossRef]
- Hano, T.; Ito, K.; Ohkubo, N.; Sakaji, H.; Watanabe, A.; Takashima, K.; Sato, T.; Sugaya, T.; Matsuki, K.; Onduka, T.; et al. Occurrence of neonicotinoids and fipronil in estuaries and their potential risks to aquatic invertebrates. Environ. Pollut. 2019, 252, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.A.I.; Matsumura, F. Synergistic actions of formamidine insecticides on the activity of pyrethroids and neonicotinoids against Aedes aegypti (Diptera: Culicidae). J. Med. Entomol. 2012, 49, 1405–1410. [Google Scholar] [CrossRef] [PubMed]
- Cavallaro, M.C.; Morrissey, C.A.; Headley, J.V.; Peru, K.M.; Liber, K. Comparative chronic toxicity of imidacloprid, clothianidin, and thiamethoxam to Chironomus dilutus and estimation of toxic equivalency factors. Environ. Toxicol. Chem. 2017, 36, 372–382. [Google Scholar] [CrossRef] [PubMed]
- Macaulay, S.J.; Hageman, K.J.; Alumbaugh, R.E.; Lyons, S.M.; Piggott, J.J.; Matthaei, C.D. Chronic toxicities of neonicotinoids to nymphs of the common New Zealand mayfly Deleatidium spp. Environ. Toxicol. Chem. 2019, 38, 2459–2471. [Google Scholar] [CrossRef]
- Stoughton, S.J.; Liber, K.; Culp, J.; Cessna, A. Acute and chronic toxicity of imidacloprid to the aquatic invertebrates Chironomus tentans and Hyalella azteca under constant- and pulse-exposure conditions. Arch. Environ. Contam. Toxicol. 2008, 54, 662–673. [Google Scholar] [CrossRef]
- Ieromina, O.; Peijnenburg, W.J.G.M.; de Snoo, G.; Müller, J.; Knepper, T.P.; Vijver, M.G. Impact of imidacloprid on Daphnia magna under different food quality regimes. Environ. Toxicol. Chem. 2014, 33, 621–631. [Google Scholar] [CrossRef]
- Roessink, I.; Merga, L.B.; Zweers, H.J.; van den Brink, P.J. The neonicotinoid imidacloprid shows high chronic toxicity to mayfly nymphs. Environ. Toxicol. Chem. 2013, 32, 1096–1100. [Google Scholar] [CrossRef]
- van den Brink, P.J.; Smeden, J.M.V.; Bekele, R.S.; Dierick, W.; Gelder, D.D.; Noteboom, M.; Roessink, I. Acute and chronic toxicity of neonicotinoids to nymphs of a mayfly species and some notes on seasonal differences. Environ. Toxicol. Chem. 2016, 35, 128–133. [Google Scholar] [CrossRef]
- Uğurlu, P.; Ünlü, E.; Satar, E.İ. The toxicological effects of thiamethoxam on Gammarus kischineffensis (Schellenberg 1937) (Crustacea: Amphipoda). Environ. Toxicol. Pharmacol. 2015, 39, 720–726. [Google Scholar] [CrossRef]
- Prosser, R.S.; de Solla, S.R.; Holman, E.A.M.; Osborne, R.; Robinson, S.A.; Bartlett, A.J.; Maisonneuve, F.J.; Gillis, P.L. Sensitivity of the early-life stages of freshwater mollusks to neonicotinoid and butenolide insecticides. Environ. Pollut. 2016, 218, 428–435. [Google Scholar] [CrossRef]
- Chandran, N.N.; Fojtova, D.; Blahova, L.; Rozmankova, E.; Blaha, L. Acute and (sub)chronic toxicity of the neonicotinoid imidacloprid on Chironomus riparius. Chemosphere 2018, 209, 568–577. [Google Scholar] [CrossRef] [PubMed]
- Demirci, Ö.; Güven, K.; Asma, D.; Öğüt, S.; Uğurlu, P. Effects of endosulfan, thiamethoxam, and indoxacarb in combination with atrazine on multi-biomarkers in Gammarus kischineffensis. Ecotoxicol. Environ. Saf. 2018, 147, 749–758. [Google Scholar] [CrossRef] [PubMed]
- Hesketh, H.; Lahive, E.; Horton, A.A.; Robinson, A.G.; Svendsen, C.; Rortais, A.; Dorne, J.L.; Baas, J.; Spurgeon, D.J.; Heard, M.S. Extending standard testing period in honeybees to predict lifespan impacts of pesticides and heavy metals using dynamic energy budget modelling. Sci. Rep. 2016, 6, 37655. [Google Scholar] [CrossRef] [PubMed]
- Heard, M.S.; Baas, J.; Dorne, J.-L.; Lahive, E.; Robinson, A.G.; Rortais, A.; Spurgeon, D.J.; Svendsen, C.; Hesketh, H. Comparative toxicity of pesticides and environmental contaminants in bees: Are honey bees a useful proxy for wild bee species? Sci. Total Environ. 2017, 578, 357–365. [Google Scholar] [CrossRef]
- Laurino, D.; Porporato, M.; Patetta, A.; Manino, A. Toxicity of neonicotinoid insecticides to honey bees: Laboratory tests. Bull. Insectol. 2011, 64, 107–113. [Google Scholar]
- Iwasa, T.; Motoyama, N.; Ambrose, J.T.; Roe, R.M. Mechanism for the differential toxicity of neonicotinoid insecticides in the honey bee, Apis mellifera. Crop Protection 2004, 23, 371–378. [Google Scholar] [CrossRef]
- Preetha, G.; Stanley, J.; Suresh, S.; Samiyappan, R. Risk assessment of insecticides used in rice on miridbug, Cyrtorhinus lividipennis Reuter, the important predator of brown planthopper, Nilaparvata lugens (Stal.). Chemosphere 2010, 80, 498–503. [Google Scholar] [CrossRef]
- Alkassab, A.T.; Kirchner, W.H. Impacts of chronic sublethal exposure to clothianidin on winter honeybees. Ecotoxicology 2016, 25, 1000–1010. [Google Scholar] [CrossRef]
- Martínez, L.C.; Plata-Rueda, A.; Zanuncio, J.C.; Serrao, J.E. Comparative toxicity of six insecticides on the rhinoceros beetle (Coleoptera: Scarabaeidae). Fl. Entomol. 2014, 97, 1056–1062. [Google Scholar] [CrossRef]
- Charpentier, G.; Louat, F.; Bonmatin, J.-M.; Marchand, P.A.; Vanier, F.; Locker, D.; Decoville, M. Lethal and sublethal effects of imidacloprid, after chronic exposure, on the insect model Drosophila melanogaster. Environ. Sci. Technol. 2014, 48, 4096–4102. [Google Scholar] [CrossRef]
- D’Ávila, V.A.; Barbosa, W.F.; Guedes, R.N.C.; Cutler, G.C. Effects of spinosad, imidacloprid, and lambda-cyhalothrin on survival, parasitism, and reproduction of the aphid parasitoid Aphidius colemani. J. Econ. Entomol. 2018, 111, 1096–1103. [Google Scholar] [CrossRef] [PubMed]
- Husain, D.; Qasim, M.; Saleem, M.; Akhter, M.; Khan, K.A. Bioassay of insecticides against three honey bee species in laboratory conditions. Cercetari Agronomice Moldova 2014, XLVII, 69–79. [Google Scholar] [CrossRef]
- Suchail, S.; Guez, D.; Belzunces, L.P. Discrepancy between acute and chronic toxicity induced by imidacloprid and its metabolites in Apis mellifera. Environ. Toxicol. Chem. 2001, 20, 2482–2486. [Google Scholar] [CrossRef] [PubMed]
- DEFRA. Assessment of the risk posed to honeybees by systemic pesticides; Department for Environment, Food and Rural Affairs: London, UK, 2007.
- Preetha, G.; Manoharan, T.; Stanley, J.; Kuttalam, S. Impact of chloronicotinyl insecticide, imidacloprid on egg, egg-larval and larval parasitoids under laboratory conditions. J. Plant Protect. Res. 2010, 50, 535–540. [Google Scholar] [CrossRef]
- Carrillo, D.; Peña, J.E.; Rogers, M.E. Relative susceptibility of Haeckeliania sperata (Hymenoptera: Trichogrammatidae) to pesticides used in citrus and ornamental systems in Florida. J. Econ. Entomol. 2009, 102, 905–912. [Google Scholar] [CrossRef]
- Rust, M.K.; Reierson, D.A.; Klotz, J.H. Delayed toxicity as a critical factor in the efficacy of aqueous baits for controlling Argentine ants (Hymenoptera: Formicidae). J. Econ. Entomol. 2004, 97, 1017–1024. [Google Scholar] [CrossRef]
- Ramakrishnan, R.; Suiter, D.R.; Nakatsu, C.H.; Bennett, G.W. Feeding inhibition and mortality in Reticulitermes flavipes (Isoptera: Rhinotermitidae) after exposure to imidacloprid-treated soils. J. Econ. Entomol. 2000, 93, 422–428. [Google Scholar] [CrossRef]
- Niassy, S.; Maniania, N.K.; Subramanian, S.; Gitonga, M.L.; Maranga, R.; Obonyo, A.B.; Ekesi, S. Compatibility of Metarhizium anisopliae isolate ICIPE 69 with agrochemicals used in French bean production. Int. J. Pest Control 2012, 58, 131–137. [Google Scholar] [CrossRef]
- Oliveira, R.A.; Roat, T.C.; Carvalho, S.M.; Malaspina, O. Side-effects of thiamethoxam on the brain and midgut of the africanized honeybee Apis mellifera (Hymenopptera: Apidae). Environ. Toxicol. 2014, 29, 1122–1133. [Google Scholar] [CrossRef]
- Frantzios, G.; Paptsiki, K.; Sidiropoulou, B.; Lazaridis, I.; Theophilidis, G.; Mavragani-Tsipidou, P. Evaluation of insecticidal and genotoxic effects of imidacloprid and acetochlor in Drosophila melanogaster. J. Appl. Entomol. 2008, 132, 583–590. [Google Scholar] [CrossRef]
- Bliss, C.I. The size factor in the action of arsenic upon silkworm larvae. Exp. Biol. 1936, 13, 95–110. [Google Scholar]
- Zaller, J.G.; Brühl, C.A. Editorial: Non-target effects of pesticides on organisms inhabiting agroecosystems. Front. Environ. Sci. 2019, 7. [Google Scholar] [CrossRef]
- Sánchez-Bayo, F.; Goka, K.; Hayasaka, D. Contamination of the aquatic environment with neonicotinoids and its implication for ecosystems. Front. Environ. Sci. 2016, 4, 71. [Google Scholar] [CrossRef]
- Sgolastra, F.; Medrzycki, P.; Bortolotti, L.; Maini, S.; Porrini, C.; Simon-Delso, N.; Bosch, J. Bees and pesticide regulation: Lessons from the neonicotinoid experience. Biol. Conserv. 2020, 241, 108356. [Google Scholar] [CrossRef]
- Sánchez-Bayo, F.; Goka, K. Influence of light in acute toxicity bioassays of imidacloprid and zinc pyrithione to zooplankton crustaceans. Aquat. Toxicol. 2006, 78, 262–271. [Google Scholar]
- Daam, M.A.; Santos Pereira, A.C.; Silva, E.; Caetano, L.; Cerejeira, M.J. Preliminary aquatic risk assessment of imidacloprid after application in an experimental rice plot. Ecotoxicol. Environ. Saf. 2013, 97, 78–85. [Google Scholar] [CrossRef]
- Morrissey, C.A.; Mineau, P.; Devries, J.H.; Sánchez-Bayo, F.; Liess, M.; Cavallaro, M.C.; Liber, K. Neonicotinoid contamination of global surface waters and associated risk to aquatic invertebrates: A review. Environ. Int. 2015, 74, 291–303. [Google Scholar] [CrossRef]
- Pisa, L.W.; Amaral-Rogers, V.; Belzunces, L.P.; Bonmatin, J.M.; Downs, C.A.; Goulson, D.; Kreutzweiser, D.P.; Krupke, C.; Liess, M.; McField, M.; et al. Effects of neonicotinoids and fipronil on non-target invertebrates. Environ. Sci. Pollut. Res. 2015, 22, 68–102. [Google Scholar] [CrossRef]
- Gibbons, D.; Morrissey, C.; Mineau, P. A review of the direct and indirect effects of neonicotinoids and fipronil on vertebrate wildlife. Environ. Sci. Pollut. Res. 2015, 22, 103–118. [Google Scholar] [CrossRef]
- Rinkevich, F.D.; Margotta, J.W.; Pittman, J.M.; Danka, R.G.; Tarver, M.R.; Ottea, J.A.; Healy, K.B. Genetics, synergists, and age affect insecticide sensitivity of the honey bee, Apis mellifera. PLoS ONE 2015, 10, e0139841. [Google Scholar] [CrossRef]
- Paine, T.D.; Hanlon, C.C.; Byrne, F.J. Potential risks of systemic imidacloprid to parasitoid natural enemies of a cerambycid attacking Eucalyptus. Biol. Control 2011, 56, 175–178. [Google Scholar] [CrossRef]
- Tremolada, P.; Mazzoleni, M.; Saliu, F.; Colombo, M.; Vighi, M. Field trial for evaluating the effects on honeybees of corn sown using cruiser® and Celest® treated seeds. Bull. Environ. Contam. Toxicol. 2010, 85, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Preetha, G.; Stanley, J.; Suresh, S.; Kuttalam, S.; Samiyappan, R. Toxicity of selected insecticides to Trichogramma chilonis: Assessing their safety in the rice ecosystem. Phytoparasitica 2009, 37, 209–215. [Google Scholar] [CrossRef]
- Rill, S.M.; Grafton-Cardwell, E.E.; Morse, J.G. Effects of two insect growth regulators and a neonicotinoid on various life stages of Aphytis melinus (Hymenoptera: Aphelinidae). BioControl 2008, 53, 579. [Google Scholar] [CrossRef]
- Prabhaker, N.; Castle, S.J.; Naranjo, S.E.; Toscano, N.C.; Morse, J.G. Compatibility of two systemic neonicotinoids, imidacloprid and thiamethoxam, with various natural enemies of agricultural pests. J. Econ. Entomol. 2011, 104, 773–781. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, R.; Zhao, X.; Chen, L.; Wu, C.; Cang, T.; Wang, Q. Susceptibility of adult Trichogramma nubilale (Hymenoptera: Trichogrammatidae) to selected insecticides with different modes of action. Crop Protection 2012, 34, 76–82. [Google Scholar] [CrossRef]
- Furlan, L.; Pozzebon, A.; Duso, C.; Simon-Delso, N.; Sánchez-Bayo, F.; Marchand, P.A.; Codato, F.; Lexmond, M.B.v.; Bonmatin, J.-M. An update of the Worldwide Integrated Assessment (WIA) on systemic insecticides. Part 3: Alternatives to systemic insecticides. Environ. Sci. Pollut. Res. 2018, in press. [Google Scholar] [CrossRef]
- Benton, E.P.; Grant, J.F.; Nichols, R.J.; Webster, R.J.; Schwartz, J.S.; Bailey, J.K. Risk assessment of imidacloprid use in forest settings on the aquatic macroinvertebrate community. Environ. Toxicol. Chem. 2017, 36, 3108–3119. [Google Scholar] [CrossRef]
- Aslund, M.W.; Winchell, M.; Bowers, L.; McGee, S.; Tang, J.; Padilla, L.; Greer, C.; Knopper, L.; Moore, D.R.J. Ecological risk assessment for aquatic invertebrate communities exposed to imidacloprid due to labeled agricultural and non-agricultural uses in the United States. Environ. Toxicol. Chem. 2017, 36, 1375–1388. [Google Scholar] [CrossRef]
- van Dijk, T.C.; van Staalduinen, M.A.; van der Sluijs, J.P. Macro-invertebrate decline in surface water polluted with imidacloprid. PLoS ONE 2013, 8, e62374. [Google Scholar] [CrossRef]
- Yamamuro, M.; Komuro, T.; Kamiya, H.; Kato, T.; Hasegawa, H.; Kameda, Y. Neonicotinoids disrupt aquatic food webs and decrease fishery yields. Science 2019, 366, 620–623. [Google Scholar] [CrossRef] [PubMed]
- Pisa, L.; Goulson, D.; Yang, E.-C.; Gibbons, D.; Sánchez-Bayo, F.; Mitchell, E.; Aebi, A.; Sluijs, J.v.d.; MacQuarrie, C.J.K.; Giorio, C.; et al. An update of the Worldwide Integrated Assessment (WIA) on systemic insecticides. Part 2: Impacts on organisms and ecosystems. Environ. Sci. Pollut. Res. 2017, in press. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Bayo, F.; Tennekes, H.A. Assessment of ecological risks of agrochemicals requires a new framework. Environ. Risk Assess. Remed. 2017, 1, 20–28. [Google Scholar] [CrossRef]
- Douglas, M.R.; Sponsler, D.B.; Lonsdorf, E.V.; Grozinger, C.M. County-level analysis reveals a rapidly shifting landscape of insecticide hazard to honey bees (Apis mellifera) on US farmland. Sci. Rep. 2020, 10, 797. [Google Scholar] [CrossRef] [PubMed]
- Goulson, D.; Thompson, J.; Croombs, A. Rapid rise in toxic load for bees revealed by analysis of pesticide use in Great Britain. PeerJ 2018, 6, e5255. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Bayo, F.; Wyckhuys, K.A.G. Worldwide decline of the entomofauna: A review of its drivers. Biol. Conserv. 2019, 232, 8–27. [Google Scholar] [CrossRef]
- Beketov, M.A.; Kefford, B.J.; Schäfer, R.B.; Liess, M. Pesticides reduce regional biodiversity of stream invertebrates. PNAS 2013, 110, 11039–11043. [Google Scholar] [CrossRef]
- Munz, N.A.; Burdon, F.J.; de Zwart, D.; Junghans, M.; Melo, L.; Reyes, M.; Schönenberger, U.; Singer, H.P.; Spycher, B.; Hollender, J.; et al. Pesticides drive risk of micropollutants in wastewater-impacted streams during low flow conditions. Water Res. 2017, 110, 366–377. [Google Scholar] [CrossRef]
- Agostini, M.G.; Roesler, I.; Bonetto, C.; Ronco, A.E.; Bilenca, D. Pesticides in the real world: The consequences of GMO-based intensive agriculture on native amphibians. Biol. Conserv. 2020, 241, 108355. [Google Scholar] [CrossRef]
- Nakanishi, K.; Uéda, T.; Yokomizo, H.; Hayashi, T.I. Effects of systemic insecticides on the population dynamics of the dragonfly Sympetrum frequens in Japan: Statistical analyses using field census data from 2009 to 2016. Sci. Total Environ. 2020, 703, 134499. [Google Scholar] [CrossRef]
- Hallmann, C.A.; Foppen, R.P.B.; van Turnhout, C.A.M.; de Kroon, H.; Jongejans, E. Declines in insectivorous birds are associated with high neonicotinoid concentrations. Nature 2014, 511, 341–343. [Google Scholar] [CrossRef] [PubMed]
- Brühl, C.A.; Zaller, J.G. Biodiversity decline as a consequence of an inappropriate environmental risk assessment of pesticides. Front. Environ. Sci. 2019, 7, 177. [Google Scholar] [CrossRef]
- Schafer, R.B.; Liess, M.; Altenburger, R.; Filser, J.; Hollert, H.; Roß-Nickoll, M.; Schaffer, A.; Scheringer, M. Future pesticide risk assessment: Narrowing the gap between intention and reality. Environ. Sci. Eur. 2019, 31, 21. [Google Scholar] [CrossRef]
- Topping, C.J.; Aldrich, A.; Berny, P. Overhaul environmental risk assessment for pesticides. Science 2020, 367, 360. [Google Scholar] [CrossRef] [PubMed]
| Bound Receptors in Relation to Toxicant Concentration | Receptor Binding | Effect | Effect in Relation to Bound Receptors | Effect in Relation to Toxicant Concentration | Characteristics * | Value of Exponent n |
|---|---|---|---|---|---|---|
| CR~ C | Reversible TR → 0 | Reversible | E ~ CR | E ~ C | Dose-dependent | n < 1 |
| Irreversible | Haber’s rule C·t = constant | n = 1 | ||||
| Irreversible TR → ∞ | Reversible | E ~ CR | ||||
| reversible | Time-reinforced C·tn = constant | n > 1 |
| Diethylnitrosamine 1 | Phosmet 2 | CdCl2 3 | ||||||
|---|---|---|---|---|---|---|---|---|
| Rattus sp. | Poecilia reticulata | Daphnia magna | ||||||
| n = 2.3, r2 = 1.0 | n = 1.0, r2 = 0.96 | n = 0.6, r2 = 0.98 | ||||||
| Daily dose | T50 | Total dose | Concentration (C) | T50 | C × T50 | Concentration (C) | T50 | C × T50 |
| mg·kg−1 | days | mg·kg−1 | μM | days | μM | μg·L−1 | days | μg·L−1 |
| 9.6 | 101 | 963 | 8 | 1 | 8.0 | 56 | 2 | 105 |
| 4.8 | 137 | 660 | 5.2 | 2 | 10.4 | 32 | 6 | 181 |
| 2.4 | 192 | 460 | 3.2 | 3 | 9.6 | 18 | 11 | 203 |
| 1.2 | 238 | 285 | 2.7 | 4 | 10.8 | 10 | 38 | 375 |
| 0.6 | 355 | 213 | 2.4 | 5 | 12.0 | 5.6 | 58 | 325 |
| 0.3 | 457 | 137 | 1.8 | 6 | 10.8 | 3.2 | 292 * | 935 |
| 0.15 | 609 | 91 | 1.6 | 7 | 11.2 | |||
| 0.075 | 840 | 64 | 0.93 | 8 | 7.4 | |||
| 0.8 | 10 | 8.0 | ||||||
| Taxa | Species | Chemical | n (1/slope) | Regression Parameters | ΔLC50 | No. c tested | Exposure Time (days) | Reference | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Intercept | Slope | r2 | ||||||||
| Diptera | Aedes aegypti | CLO | 1.70 | 3.835 | −0.588 | 0.98 | 7 | 5 | 3 | Ahmed and Matsumura 2012 [33] |
| Diptera | Chironomus dilutus | CLO | 3.11 | 2.922 | −0.322 | 1.0 | 9 | 5 | 40 | Cavallaro et al. 2017 [34] |
| Ephemeroptera | Deleatidium sp. | CLO | 1.59 | 3.515 | −0.628 | 1.0 | 3 | 10 | 28 | Macaulay et al. 2019 [35] |
| Amphipoda | Hyalella azteca | IMI | 1.58 | 4.085 | −0.634 | 0.65 | 8 | 5 | 28 | Stoughton et a. 2008 [36] |
| Cladocera | Daphnia magna | IMI | 2.41 | 6.540 | −0.410 | 0.89 | 5 | 6 | 10 | Sanchez-Bayo 2009 [29] |
| Cladocera | Daphnia magna | IMI | 1.91 | 6.646 | −0.523 | 0.99 | 21 | 6 | 4 | Sanchez-Bayo (unpublished) |
| Cladocera | Daphnia magna | IMI | 2.56 | 5.999 | −0.390 | 0.99 | na | 6 | 21 | Ieromina et al. 2014 [37] |
| Diptera | Aedes aegypti | IMI | 2.90 | 2.771 | -0.345 | 0.99 | 23 | 5 | 3 | Ahmed and Matsumura 2012 [33] |
| Diptera | Chaoborus obscuripes | IMI | 1.62 | 4.897 | −0.618 | 1.0 | 23 | 5 | 28 | Roessink et al. 2013 [38] |
| Diptera | Chironomus dilutus | IMI | 1.21 | 3.254 | −0.825 | 1.0 | na | 5 | 28 | Stoughton et al 2008 [36] |
| Diptera | Chironomus dilutus | IMI | 1.30 | 2.962 | −0.772 | 1.0 | na | 5 | 40 | Cavallaro et al. 2017 [34] |
| Ephemeroptera | Cloeon dipterum | IMI | 2.52 | 2.684 | −0.397 | 1.0 | 135 | 5 | 28 | Roessink et al. 2013 [38] |
| Ephemeroptera | Cloeon dipterum | IMI | 2.40 * | 2.634 | −0.416 | 0.96 | 700 | 7 | 28 | Van den Brink et al. 2016 [39] |
| Ephemeroptera | Cloeon dipterum | IMI | 2.03 | 3.137 | −0.493 | 0.84 | 13 | 7 | 4 | Van den Brink et al. 2016 [39] |
| Ephemeroptera | Cloeon dipterum | IMI | 1.79 | 2.531 | −0.559 | 0.92 | 8 | 7 | 28 | Van den Brink et al. 2016 [39] |
| Ephemeroptera | Cloeon dipterum | IMI | 2.11 * | 3.037 | −0.473 | 0.80 | 187 | 7 | 28 | Van den Brink et al. 2016 [39] |
| Ephemeroptera | Cloeon dipterum | IMI | 1.38 | 3.862 | −0.726 | 0.99 | 7 | 7 | 4 | Van den Brink et al. 2016 [39] |
| Ephemeroptera | Coenis horaria | IMI | 1.57 | 2.597 | −0.638 | 1.0 | 21 | 5 | 28 | Roessink et al. 2013 [38] |
| Ephemeroptera | Deleatidium sp. | IMI | 2.05 | 2.620 | −0.489 | 0.95 | 14 | 10 | 28 | Macaulay et al. 2019 [35] |
| Isopoda | Asellus aquaticus | IMI | 1.41 | 5.466 | −0.709 | 1.0 | 16 | 5 | 28 | Roessink et al. 2013 [38] |
| Megaloptera | Sialis lutaria | IMI | 2.94 | 4.515 | −0.340 | 1.0 | 308 | 5 | 28 | Roessink et al. 2013 [38] |
| Ostracoda | Cypridopsis vidua | IMI | 4.67 | 5.110 | −0.210 | 0.88 | na | 6 | 4 | Sanchez-Bayo 2009 [29] |
| Amphipoda | Gammarus pulex | THC | 1.30 | 1.729 | −0.767 | 0.72 | na | 5 | 15 | Beketov & Liess 2008 [9] |
| Diptera | Aedes aegypti | THC | 1.54 | 4.166 | −0.648 | 1.0 | 5 | 5 | 3 | Ahmed and Matsumura 2012 [33] |
| Ephemeroptera | Cloeon dipterum | THC | 2.23 * | 2.707 | −0.449 | 0.96 | 557 | 7 | 28 | Van den Brink et al. 2016 [39] |
| Ephemeroptera | Cloeon dipterum | THC ** | 1.83 * | 2.353 | −0.547 | 0.95 | 190 | 7 | 28 | Van den Brink et al. 2016 [39] |
| Ephemeroptera | Cloeon dipterum | THC | 1.25 | 2.398 | −0.798 | 0.98 | na | 7 | 28 | Van den Brink et al. 2016 [39] |
| Ephemeroptera | Cloeon dipterum | THC | 1.25 | 3.166 | −0.801 | 0.97 | 6 | 7 | 4 | Van den Brink et al. 2016 [39] |
| Ephemeroptera | Cloeon dipterum | THC | 1.26 | 4.242 | −0.797 | 0.89 | 6 | 7 | 4 | Van den Brink et al. 2016 [39] |
| Isopoda | Asellus aquaticus | THC | 1.25 | 0.932 | −0.802 | 0.94 | na | 3 | 19 | Beketov & Liess 2008 [9] |
| Odonata | Sympetrum striolatum | THC | 1.53 | 7.430 | −0.650 | 1.0 | na | 4 | 11 | Beketov & Liess 2008 [9] |
| Amphipoda | Gammarus kischineffensis | TMX | 2.41 | 4.768 | −0.416 | 1.0 | 28 | 6 | 4 | Ugurlu et al. 2015 [40] |
| Diptera | Chironomus dilutus | TMX | 2.51 | 3.896 | −0.398 | 1.0 | na | 5 | 40 | Cavallaro et al. 2017 [34] |
| Ephemeroptera | Cloeon dipterum | TMX | 2.05 * | 2.980 | −0.487 | 0.91 | 163 | 7 | 28 | Van den Brink et al. 2016 [39] |
| Ephemeroptera | Cloeon dipterum | TMX ** | 1.70 * | 2.949 | −0.589 | 0.96 | 131 | 7 | 28 | Van den Brink et al. 2016 [39] |
| Mollusca | Planorbella pilsbryi | TMX | 1.33 | 8.521 | −0.753 | 1.0 | 6 | 5 | 28 | Prosser et al. 2016 [41] |
| Taxa | Species | Comments | Chemical | n (1/slope) | Regression Parameters | ΔLC50 | No. c tested | Exposure Time (days) | Reference | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Intercept | Slope | R2 | |||||||||
| Hemiptera | Cyrtorhinus lividipennis | CLO | 3.74 | 1.173 | −0.268 | 1.0 | 13 | 6 | 2 | Preetha et al. 2010 [48] | |
| Hemiptera | Nilaparvata lugens | CLO | 4.49 | 1.885 | −0.233 | 1.0 | 22 | 6 | 2 | Preetha et al. 2010 [48] | |
| Hymenoptera | Apis mellifera | CLO | 1.19 | 2.538 | −0.841 | 0.94 | 11 | 6 | 3 | Laurino et al. 2011 [46] | |
| Hymenoptera | Apis mellifera | CLO | 1.62 | 3.980 | −0.617 | 1.0 | 14 | 8 | 10 | Alkassab & Kirchner 2016 [49] | |
| Coleoptera | Strategus aloeus | Adults | IMI | 2.29 | 2.073 | −0.437 | 1.0 | Na | 7 | 3 | Martinez et al. 2014 [50] |
| Diptera | Drosophila melanogaster | Males | IMI | 1.42 | 8.654 | −0.703 | 1.0 | 29 | 10 | 8 | Charpentier et al. 2014 [51] |
| Diptera | Drosophila melanogaster | Females | IMI | 2.18 | 5.957 | −0.460 | 1.0 | 172 | 10 | 8 | Charpentier et al. 2014 [51] |
| Diptera | Drosophila melanogaster | Larvae | IMI | 1.67 | 6.052 | −0.598 | 1.0 | 52 | 10 | 8 | Charpentier et al. 2014 [51] |
| Hemiptera | Cyrtorhinus lividipennis | IMI | 1.50 | 4.811 | −0.665 | 1.0 | 3 | 6 | 2 | Preetha et al. 2010 [48] | |
| Hymenoptera | Aphidius colemani | Adults | IMI | 2.29 | 3.540 | −0.437 | 0.59 | na | 6 | 8 | D’Avila et al. 2018 [52] |
| Hymenoptera | Apis florea | IMI | 2.74 | 1.177 | −0.365 | 0.98 | na | 5 | 2 | Husain et al. 2014 [53] | |
| Hymenoptera | Apis dorsata | IMI | 2.60 | 1.454 | −0.384 | 0.99 | na | 5 | 2 | Husain et al. 2014 [53] | |
| Hymenoptera | Apis mellifera | IMI | 2.41 | 1.190 | −0.416 | 0.91 | na | 5 | 2 | Husain et al. 2014 [53] | |
| Hymenoptera | Apis mellifera | IMI | 5.83 | 5.190 | −0.170 | 0.85 | na | 5 | 10 | Suchail et al. 2001 [54] | |
| Hymenoptera | Apis mellifera | IMI | 2.67 | 4.836 | −0.375 | 0.94 | 46 | 5 | 10 | DEFRA 2007 [55] | |
| Hymenoptera | Bracon hebetor | Adults | IMI | 1.80 | 2.387 | −0.554 | 1.0 | 3 | 3 | 2 | Preetha et al. 2010 [56] |
| Hymenoptera | Chelonus blackburnii | Adults | IMI | 1.51 | 5.377 | −0.662 | 0.99 | 7 | 3 | 1 | Preetha et al. 2010 [56] |
| Hymenoptera | Haeckeliania sperata | IMI | 1.52 | −1.039 | −0.656 | 0.92 | na | 5 | 2 | Carrillo et al. 2009 [57] | |
| Hymenoptera | Linepithema humile | IMI | 3.47 | 0.476 | −0.288 | 0.69 | na | 4 | 14 | Rust et al. 2004 [58] | |
| Isoptera | Reticulitermes flavipes | Sand | IMI | 1.89 | 3.125 | −0.528 | 0.95 | 1167 | 5 | 21 | Ramakrishnan et al. 2000 [59] |
| Isoptera | Reticulitermes flavipes | Sandy loam | IMI | 2.65 | 3.773 | −0.378 | 0.89 | 14 | 7 | 21 | Ramakrishnan et al. 2000 [59] |
| Isoptera | Reticulitermes flavipes | Silty clay loam | IMI | 4.00 | 3.247 | −0.250 | 0.83 | 3126 | 7 | 21 | Ramakrishnan et al. 2000 [59] |
| Thysanoptera | Frankiniella occidentalis | Larvae | IMI | 1.97 | 0.495 | −0.508 | 0.92 | na | 5 | 8 | Niassy et al. 2012 [60] |
| Hymenoptera | Apis mellifera | THC | 2.10 | 1.838 | −0.477 | 0.44 | 23 | 3 | 3 | Laurino et al. 2011 [46] | |
| Hymenoptera | Apis mellifera | TMX | 2.21 | 4.040 | −0.452 | 0.95 | na | 3 | 18 | Oliveira et al. 2014 [61] | |
| Hymenoptera | Linepithema humile | TMX | 1.55 | −5.538 | −0.643 | 0.73 | na | 4 | 14 | Rust et al. 2004 [58] | |
| Thysanoptera | Frankiniella occidentalis | Larvae | TMX | 1.55 | −0.075 | −0.645 | 0.98 | na | 5 | 8 | Niassy et al. 2012 [60] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Bayo, F.; Tennekes, H.A. Time-Cumulative Toxicity of Neonicotinoids: Experimental Evidence and Implications for Environmental Risk Assessments. Int. J. Environ. Res. Public Health 2020, 17, 1629. https://doi.org/10.3390/ijerph17051629
Sánchez-Bayo F, Tennekes HA. Time-Cumulative Toxicity of Neonicotinoids: Experimental Evidence and Implications for Environmental Risk Assessments. International Journal of Environmental Research and Public Health. 2020; 17(5):1629. https://doi.org/10.3390/ijerph17051629
Chicago/Turabian StyleSánchez-Bayo, Francisco, and Henk A. Tennekes. 2020. "Time-Cumulative Toxicity of Neonicotinoids: Experimental Evidence and Implications for Environmental Risk Assessments" International Journal of Environmental Research and Public Health 17, no. 5: 1629. https://doi.org/10.3390/ijerph17051629
APA StyleSánchez-Bayo, F., & Tennekes, H. A. (2020). Time-Cumulative Toxicity of Neonicotinoids: Experimental Evidence and Implications for Environmental Risk Assessments. International Journal of Environmental Research and Public Health, 17(5), 1629. https://doi.org/10.3390/ijerph17051629




