PTP4A3, A Novel Target Gene of HIF-1alpha, Participates in Benzene-Induced Cell Proliferation Inhibition and Apoptosis through PI3K/AKT Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Establishment of Benzene Poisoning Mice Model
2.2. Cell Culture
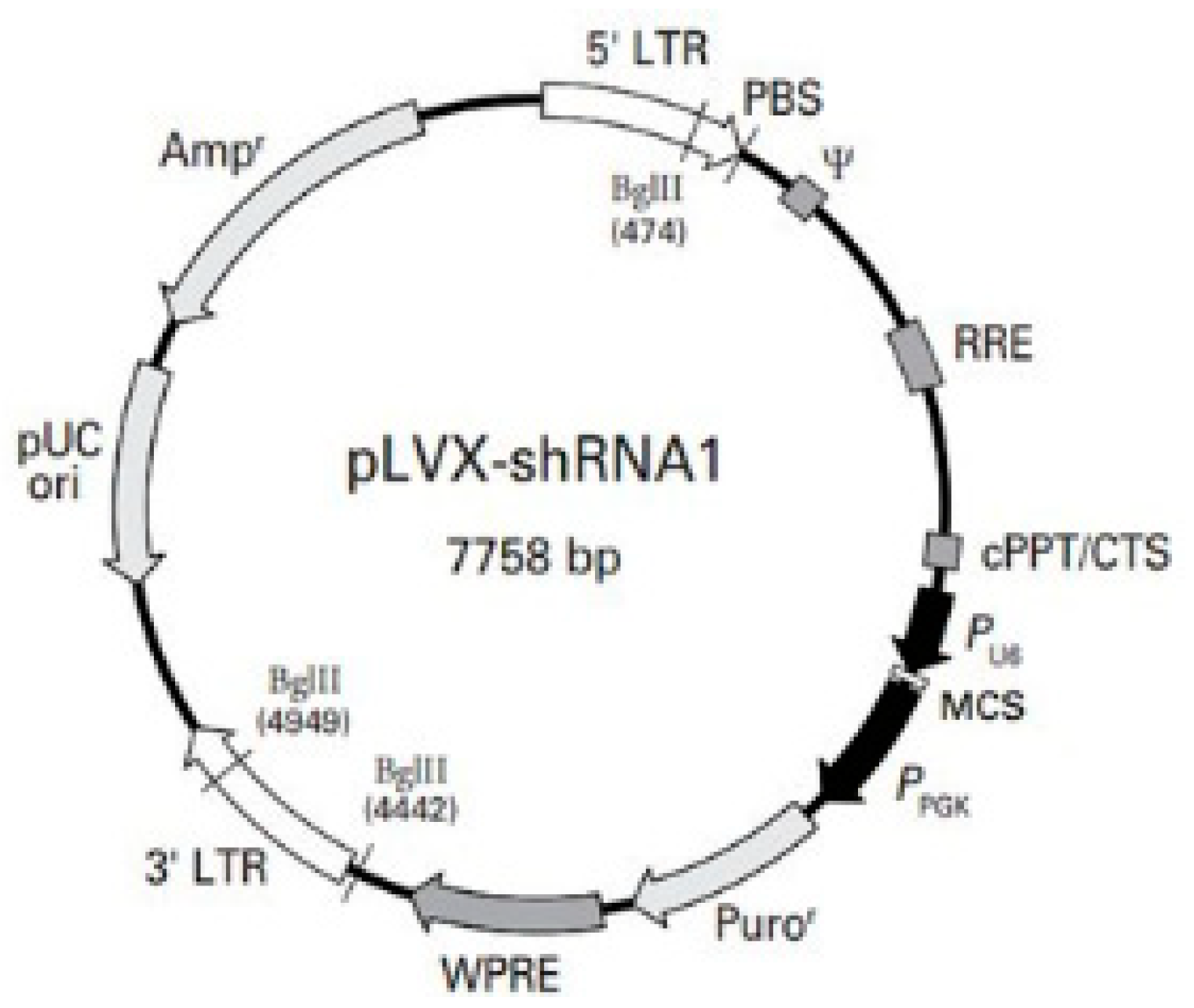
2.3. Lentiviral Transduction for PTP4A3 Knockdown
2.4. Cell Proliferation Assay
2.5. 5-Ethynyl-2’-deoxyuridine(EdU) Assay
2.6. Apoptosis
2.7. Real-Time Quantitative PCR (qPCR) Detecting System
2.8. Protein Extraction and Western Blotting
2.9. Chromatin Immunoprecipitation and Quantitative PCR (ChIP-qPCR)
2.10. Comet Assay
2.11. ROS Detection
2.12. Statistics
3. Results
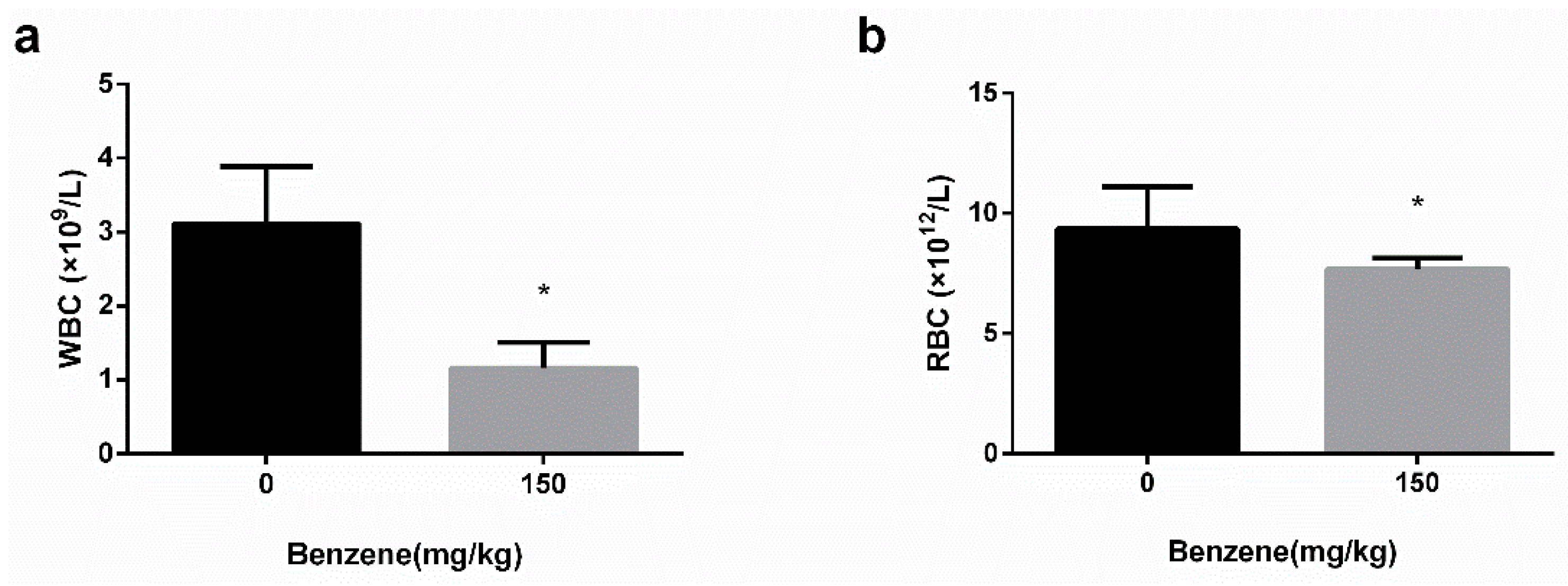
3.1. Mice Model of Benzene Poisoning
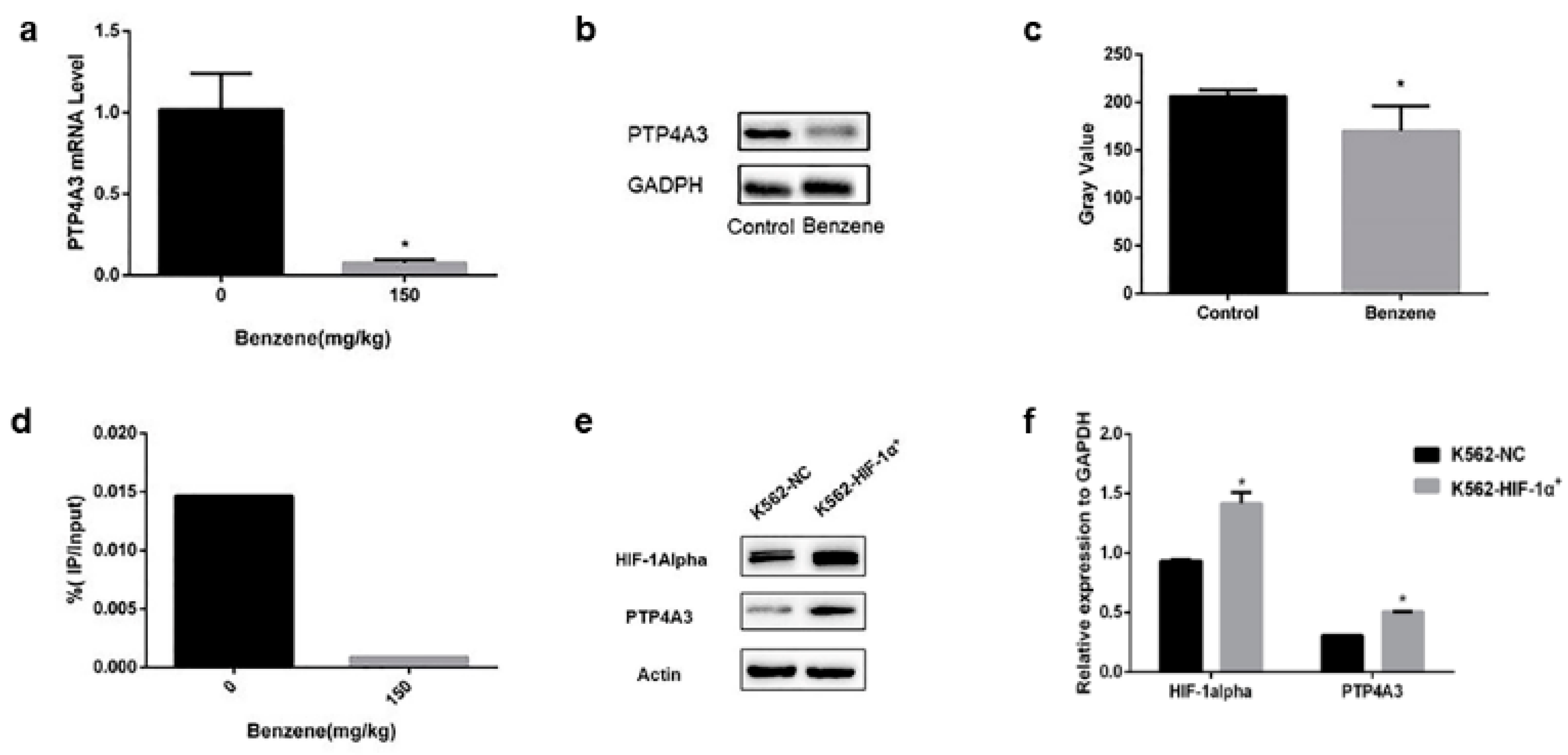
3.2. PTP4A3 Participated in Benzene Toxicity via HIF-1alpha Regulation
3.3. Establishment of Down-Regulated PTP4A3 Cell Line
3.4. Down-Regulation of PTP4A3 Aggravated 1,4-BQ-induced Reduction in Cell Viability and Proliferation
3.5. The effects of Down-Regulation of PTP4A3 on ROS Production, DNA Damage and Apoptosis after 1,4-BQ Exposure
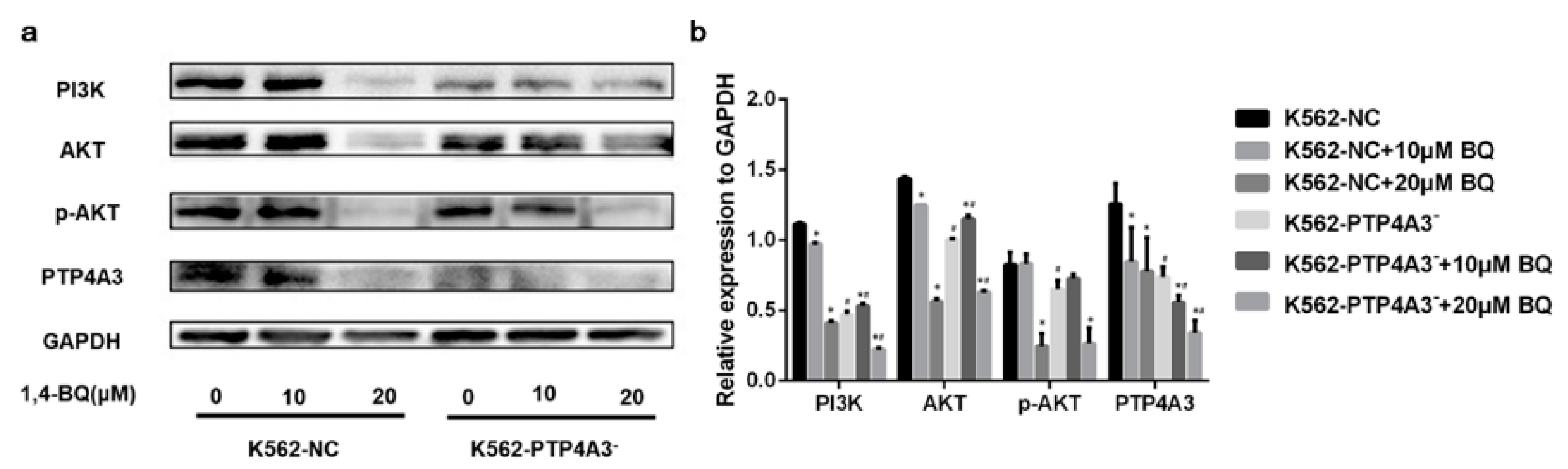
3.6. PTP4A3 Participated in 1,4-BQ-induced Cytotoxicity through PI3K/AKT Pathway
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Stenehjem, J.S.; Kjærheim, K.; Bråtveit, M.; O Samuelsen, S.; Barone-Adesi, F.; Rothman, N.; Lan, Q.; Grimsrud, T.K. Benzene exposure and risk of lymphohaematopoietic cancers in 25 000 offshore oil industry workers. Br. J. Cancer 2015, 112, 1603–1612. [Google Scholar] [CrossRef] [PubMed]
- Khalade, A.; Jaakkola, M.S.; Pukkala, E.; Jaakkola, J.J. Exposure to benzene at work and the risk of leukemia: a systematic review and meta-analysis. Environ. Health 2010, 9, 31. [Google Scholar] [CrossRef] [PubMed]
- Vlaanderen, J.; Lan, Q.; Kromhout, H.; Rothman, N.; Vermeulen, R. Occupational Benzene Exposure and the Risk of Lymphoma Subtypes: A Meta-analysis of Cohort Studies Incorporating Three Study Quality Dimensions. Environ. Health Perspect. 2011, 119, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; He, X.; Bi, Y.; Ma, Q. Stem Cell and Benzene-Induced Malignancy and Hematotoxicity. Chem. Res. Toxicol. 2012, 25, 1303–1315. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Lai, Y.; Hu, K.; Wei, Q.; Liu, Y. Human CYP2E1-dependent and human sulfotransferase 1A1-modulated induction of micronuclei by benzene and its hydroxylated metabolites in Chinese hamster V79-derived cells. Mutat. Res. Mol. Mech. Mutagen. 2014, 770, 37–44. [Google Scholar] [CrossRef]
- McHale, C.M.; Zhang, L.; Smith, M.T. Current understanding of the mechanism of benzene-induced leukemia in humans: implications for risk assessment. Carcinogenesis 2012, 33, 240–252. [Google Scholar] [CrossRef]
- Ross, D. The Role of Metabolism and Specific Metabolites In Benzene-Induced Toxicity: Evidence and Issues. J. Toxicol. Environ. Health Part A 2000, 61, 357–372. [Google Scholar] [CrossRef]
- Ruiz-Ramos, R.; Cebrian, M.E.; Garrido, E. Benzoquinone activates the ERK/MAPK signaling pathway via ROS production in HL-60 cells. Toxicol. 2005, 209, 279–287. [Google Scholar] [CrossRef]
- Chowdhury, A.R.; Long, A.; Fuchs, S.Y.; Rustgi, A.; Avadhani, N.G. Mitochondrial stress-induced p53 attenuates HIF-1 alpha activity by physical association and enhanced ubiquitination. Oncogene 2017, 36, 397–409. [Google Scholar] [CrossRef]
- Hu, C.-J.; Iyer, S.; Sataur, A.; Covello, K.L.; Chodosh, L.A.; Simon, M.C. Differential Regulation of the Transcriptional Activities of Hypoxia-Inducible Factor 1 Alpha (HIF-1α) and HIF-2α in Stem Cells†. Mol. Cell. Boil. 2006, 26, 3514–3526. [Google Scholar] [CrossRef]
- Semenza, G.L.; Shimoda, L.A.; Prabhakar, N.R. Regulation of gene expression by HIF-1. Ciba Found. Symp. Bilharziasis 2006, 272, 2–14. [Google Scholar]
- Testa, U.; Labbaye, C.; Castelli, G.; Pelosi, E. Oxidative stress and hypoxia in normal and leukemic stem cells. Exp. Hematol. 2016, 44, 540–560. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.C.; Sadek, H.A. Hypoxia and Metabolic Properties of Hematopoietic Stem Cells. Antioxid. Redox Signal. 2014, 20, 1891–1901. [Google Scholar] [CrossRef] [PubMed]
- Kocabas, F.; Zheng, J.; Thet, S.; Copeland, N.G.; Jenkins, N.A.; DeBerardinis, R.J.; Zhang, C.; Sadek, H.A. Meis1 regulates the metabolic phenotype and oxidant defense of hematopoietic stem cells. Blood 2012, 120, 4963–4972. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Zhang, J.; Yin, L.; Pu, Y. Involvement of hypoxia-inducible factor-1 α (HIF-1α) in inhibition of benzene on mouse hematopoietic system. J. Toxicol. Environ. Health Part A 2016, 79, 402–406. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Meng, X.; Pu, Y.; Sun, F.; Man, Z.; Zhang, J.; Yin, L.; Pu, Y. Overexpression of HIF-1a could partially protect K562 cells from 1,4-benzoquinone induced toxicity by inhibiting ROS, apoptosis and enhancing glycolysis. Toxicol. In Vitro 2019, 55, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Tian, W.; Meng, L.; Qu, L.; Shou, C. PRL-3 promotes gastric cancer migration and invasion through a NF-kappa B-HIF-1 alpha-miR-210 axis. J. Mol. Med. 2016, 94, 401–415. [Google Scholar] [CrossRef]
- Bessette, D.C.; Qiu, D.; Pallen, C.J. PRL PTPs: mediators and markers of cancer progression. Cancer Metastasis Rev. 2008, 27, 231–252. [Google Scholar] [CrossRef]
- Al-Aidaroos, A.Q.O.; Zeng, Q. PRL-3 phosphatase and cancer metastasis. J. Cell. Biochem. 2010, 111, 1087–1098. [Google Scholar] [CrossRef]
- Rios, P.; Li, X.; Koehn, M. Molecular mechanisms of the PRL phosphatases. FEBS J. 2013, 280, 505–524. [Google Scholar] [CrossRef]
- Broyl, A.; Hose, D.; Lokhorst, H.; De Knegt, Y.; Peeters, J.; Jauch, A.; Bertsch, U.; Buijs, A.; Stevens-Kroef, M.; Beverloo, H.B.; et al. Gene expression profiling for molecular classification of multiple myeloma in newly diagnosed patients. Blood 2010, 116, 2543–2553. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Cheong, L.-L.; Liu, S.-C.; Chong, P.S.; Mahara, S.; Bi, C.; Ong, K.O.; Zeng, Q.; Chng, W.J. The pro-metastasis tyrosine phosphatase, PRL-3 (PTP4A3), is a novel mediator of oncogenic function of BCR-ABL in human chronic myeloid leukemia. Mol. Cancer 2012, 11, 72. [Google Scholar] [CrossRef] [PubMed]
- Park, J.E.; Yuen, H.F.; Zhou, J.B.; Al-aidaroos, A.Q.O.; Guo, K.; Valk, P.J.; Zhang, S.D.; Chng, W.J.; Hong, C.W.; Mills, K.; et al. Oncogenic roles of PRL-3 in FLT3-ITD induced acute myeloid leukaemia. EMBO Mol. Med. 2013, 5, 1351–1366. [Google Scholar] [CrossRef] [PubMed]
- Hjort, M.A.; Abdollahi, P.; Vandsemb, E.N.; Fenstad, M.H.; Lund, B.; Slordahl, T.S.; Borset, M.; Ro, T.B. Phosphatase of regenerating liver-3 is expressed in acute lymphoblastic leukemia and mediates leukemic cell adhesion, migration and drug resistance. Oncotarget 2018, 9, 3549–3561. [Google Scholar] [CrossRef]
- Zhou, J.; Chan, Z.L.; Bi, C.; Lu, X.; Chong, P.S.; Chooi, J.Y.; Cheong, L.L.; Liu, S.C.; Ching, Y.Q.; Zhou, Y.; et al. LIN28B Activation by PRL-3 Promotes Leukemogenesis and a Stem Cell-like Transcriptional Program in AML. Mol. Cancer Res. 2017, 15, 294–303. [Google Scholar] [CrossRef]
- Liang, F.; Liang, J.; Wang, W.-Q.; Sun, A.-P.; Udho, E.; Zhang, Z.-Y. PRL3 promotes cell invasion and proliferation by down-regulation of Csk leading to Src activation. J. Biol. Chem. 2007, 282, 5413–5419. [Google Scholar] [CrossRef]
- Vandsemb, E.N.; Bertilsson, H.; Abdollahi, P.; Størkersen, Ø.; Våtsveen, T.K.; Rye, M.B.; Rø, T.B.; Børset, M.; Slørdahl, T.S. Phosphatase of regenerating liver 3 (PRL-3) is overexpressed in human prostate cancer tissue and promotes growth and migration. J. Transl. Med. 2016, 14, 71. [Google Scholar] [CrossRef]
- Xu, H.; Zeng, Y.; Liu, L.; Gao, Q.; Jin, S.; Lan, Q.; Lai, W.; Luo, X.; Wu, H.; Huang, Y.; et al. PRL-3 improves colorectal cancer cell proliferation and invasion through IL-8 mediated glycolysis metabolism. Int. J. Oncol. 2017, 51, 1271–1279. [Google Scholar] [CrossRef]
- Radke, I.; Götte, M.; Smollich, M.; Scharle, N.; Kiesel, L.; Wülfing, P. Expression of PRL-3 regulates proliferation and invasion of breast cancer cells in vitro. Arch. Gynecol. Obstet. 2017, 296, 1153–1160. [Google Scholar] [CrossRef]
- Lee, S.K.; Han, Y.M.; Yun, J.; Lee, C.W.; Shin, D.S.; Ha, Y.R.; Kim, J.; Koh, J.S.; Hong, S.H.; Han, D.C.; et al. Phosphatase of regenerating liver-3 promotes migration and invasion by upregulating matrix metalloproteinases-7 in human colorectal cancer cells. Int. J. Cancer 2012, 131, E190–E203. [Google Scholar] [CrossRef]
- Smith, M.T. Advances in understanding benzene health effects and susceptibility. Annu. Rev. Public Health 2010, 31, 133–148. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; McHale, C.M.; Rothman, N.; Li, G.; Ji, Z.; Vermeulen, R.; Hubbard, A.E.; Ren, X.; Shen, M.; Rappaport, S.M.; et al. Systems biology of human benzene exposure. Chem.-Biol. Interact. 2010, 184, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Zhang, J.; Wei, H.; Meng, X.; Ding, Q.; Sun, F.; Cao, M.; Yin, L.; Pu, Y. Acetyl- l -carnitine partially prevents benzene-induced hematotoxicity and oxidative stress in C3H/He mice. Environ. Toxicol. Pharmacol. 2017, 51, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Man, Z.; Meng, X.; Sun, F.; Pu, Y.; Xu, K.; Sun, R.; Zhang, J.; Yin, L.; Pu, Y. Global Identification of HIF-1α Target Genes in Benzene Poisoning Mouse Bone Marrow Cells. Int. J. Environ. Res. Public Health 2018, 15, 2531. [Google Scholar] [CrossRef]
- Snyder, R. Leukemia and Benzene. Int. J. Environ. Res. Public Health 2012, 9, 2875–2893. [Google Scholar] [CrossRef]
- Lamm, S.H.; Engel, A.; Joshi, K.P.; Byrd, D.M.; Chen, R.; Iii, D.M.B. Chronic myelogenous leukemia and benzene exposure: A systematic review and meta-analysis of the case–control literature. Chem. Interact. 2009, 182, 93–97. [Google Scholar] [CrossRef]
- Polato, F.; Codegoni, A.; Fruscio, R.; Perego, P.; Mangioni, C.; Saha, S.; Bardelli, A.; Broggini, M. PRL-3 Phosphatase Is Implicated in Ovarian Cancer Growth. Clin. Cancer Res. 2005, 11, 6835–6839. [Google Scholar] [CrossRef]
- Li, B.-H.; Wang, Y.; Wang, C.-Y.; Zhao, M.-J.; Deng, T.; Ren, X.-Q. Up-Regulation of Phosphatase in Regenerating Liver-3 (PRL-3) Contributes to Malignant Progression of Hepatocellular Carcinoma by Activating Phosphatase and Tensin Homolog Deleted on Chromosome Ten (PTEN)/Phosphoinositide 3-Kinase (PI3K)/AKT Signaling Pathway. Med Sci. Monit. 2018, 24, 8105–8114. [Google Scholar]
- Sun, P.; Wang, J.; Guo, X.; Chen, Y.; Xing, C.; Gao, A. Benzene and its metabolite decreases cell proliferation via LncRNA-OBFC2A-mediated anti-proliferation effect involving NOTCH1 and KLF15. Oncotarget 2017, 8, 40857–40871. [Google Scholar] [CrossRef]
- Sun, P.; Guo, X.; Chen, Y.; Zhang, W.; Duan, H.; Gao, A. VNN3, a potential novel biomarker for benzene toxicity, is involved in 1, 4-benzoquinone induced cell proliferation. Environ. Pollut. 2018, 233, 323–330. [Google Scholar] [CrossRef]
- Son, M.Y.; Deng, C.-X.; Hoeijmarkers, J.H.; Rebel, V.I.; Hasty, P. A mechanism for 1,4-Benzoquinone-induced genotoxicity. Oncotarget 2016, 7, 46433–46447. [Google Scholar] [CrossRef] [PubMed]
- Lian, S.; Meng, L.; Yang, Y.; Ma, T.; Xing, X.; Feng, Q.; Song, Q.; Liu, C.; Tian, Z.; Qu, L.; et al. PRL-3 promotes telomere deprotection and chromosomal instability. Nucleic Acids Res. 2017, 45, 6546–6571. [Google Scholar] [CrossRef] [PubMed]
- Movafagh, S.; Crook, S.; Vo, K. Regulation of Hypoxia-Inducible Factor-1a by Reactive Oxygen Species: New Developments in an Old Debate. J. Cell. Biochem. 2015, 116, 696–703. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; An, J.; Li, H.; Qiu, X.; Wei, Y.; Shang, Y. The methyl-triclosan induced caspase-dependent mitochondrial apoptosis in HepG2 cells mediated through oxidative stress. Ecotoxicol. Environ. Saf. 2019, 182, 109391. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yu, X.; Gao, J.; Zhang, Q.; Sun, S.; Zhu, H.; Yang, X.; Yan, H. PINK1/Parkin-mediated mitophagy was activated against 1,4-Benzoquinone-induced apoptosis in HL-60 cells. Toxicol. In Vitro 2018, 50, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Hollander, P.D.; Rawls, K.; Tsimelzon, A.; Shepherd, J.; Mazumdar, A.; Hill, J.; Fuqua, S.A.W.; Chang, J.C.; Osborne, C.K.; Hilsenbeck, S.G.; et al. Phosphatase PTP4A3 Promotes Triple-Negative Breast Cancer Growth and Predicts Poor Patient Survival. Cancer Res. 2016, 76, 1942–1953. [Google Scholar] [CrossRef] [PubMed]
- Ooki, A.; Yamashita, K.; Kikuchi, S.; Sakuramoto, S.; Katada, N.; Waraya, M.; Kawamata, H.; Nishimiya, H.; Nakamura, K.; Watanabe, M. Therapeutic potential of PRL-3 targeting and clinical significance of PRL-3 genomic amplification in gastric cancer. BMC Cancer 2011, 11, 122. [Google Scholar] [CrossRef]
- Nepstad, I.; Hatfield, K.J.; Tvedt, T.H.A.; Reikvam, H.; Øystein, B. Clonal Heterogeneity Reflected by PI3K-AKT-mTOR Signaling in Human Acute Myeloid Leukemia Cells and Its Association with Adverse Prognosis. Cancers 2018, 10, 332. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Z.; Fan, X.; Xiong, J.; Zhang, G.; Luo, X.; Li, K.; Jie, Z.; Cao, Y.; Huang, Z.; et al. PRL-3 promotes gastric cancer peritoneal metastasis via the PI3K/AKT signaling pathway in vitro and in vivo. Oncol. Lett. 2018, 15, 9069–9074. [Google Scholar] [CrossRef]
- Xiong, J.; Li, Z.; Zhang, Y.; Li, D.; Zhang, G.; Luo, X.; Jie, Z.; Liu, Y.; Cao, Y.; Le, Z.; et al. PRL-3 promotes the peritoneal metastasis of gastric cancer through the PI3K/Akt signaling pathway by regulating PTEN. Oncol. Rep. 2016, 36, 1819–1828. [Google Scholar] [CrossRef]
- Jia, L.; Zhu, Z.; Li, H.; Li, Y. Shikonin inhibits proliferation, migration, invasion and promotes apoptosis in NCI-N87 cells via inhibition of PI3K/AKT signal pathway. Artif. Cells Nanomed. Biotechnol. 2019, 47, 2662–2669. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Yi, Z.; Guo, K.; Long, X. Long noncoding RNA BCAR4 promotes glioma cell proliferation via EGFR/PI3K/AKT signaling pathway. J. Cell. Physiol. 2019, 234, 23608–23617. [Google Scholar] [CrossRef] [PubMed]


| Species | Genes | Primers(5′-3′) |
|---|---|---|
| Mouse | β-actin | F:CTATGCTCTCCCTCACGCCA |
| R: TCACGCACGATTTCCCTCTC | ||
| Mouse | PTP4A3 | F: CCTGTAAGGCAGCCCCAACTA |
| R: GTGTCTTAGCCAGGGTTTTATG | ||
| Human | β-actin | F: ATCCGCAAAGACCTGT |
| R: GGGTGTAACGCAACTAAG | ||
| Human | PTP4A3 | F: CAGCCAGTCTTCCACTACCTT |
| R: GCTTCCTCATCACCCACAACC |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pu, Y.; Sun, F.; Sun, R.; Man, Z.; Ji, S.; Xu, K.; Yin, L.; Zhang, J.; Pu, Y. PTP4A3, A Novel Target Gene of HIF-1alpha, Participates in Benzene-Induced Cell Proliferation Inhibition and Apoptosis through PI3K/AKT Pathway. Int. J. Environ. Res. Public Health 2020, 17, 910. https://doi.org/10.3390/ijerph17030910
Pu Y, Sun F, Sun R, Man Z, Ji S, Xu K, Yin L, Zhang J, Pu Y. PTP4A3, A Novel Target Gene of HIF-1alpha, Participates in Benzene-Induced Cell Proliferation Inhibition and Apoptosis through PI3K/AKT Pathway. International Journal of Environmental Research and Public Health. 2020; 17(3):910. https://doi.org/10.3390/ijerph17030910
Chicago/Turabian StylePu, Yunqiu, Fengxia Sun, Rongli Sun, Zhaodi Man, Shuangbin Ji, Kai Xu, Lihong Yin, Juan Zhang, and Yuepu Pu. 2020. "PTP4A3, A Novel Target Gene of HIF-1alpha, Participates in Benzene-Induced Cell Proliferation Inhibition and Apoptosis through PI3K/AKT Pathway" International Journal of Environmental Research and Public Health 17, no. 3: 910. https://doi.org/10.3390/ijerph17030910
APA StylePu, Y., Sun, F., Sun, R., Man, Z., Ji, S., Xu, K., Yin, L., Zhang, J., & Pu, Y. (2020). PTP4A3, A Novel Target Gene of HIF-1alpha, Participates in Benzene-Induced Cell Proliferation Inhibition and Apoptosis through PI3K/AKT Pathway. International Journal of Environmental Research and Public Health, 17(3), 910. https://doi.org/10.3390/ijerph17030910






