Interannual Survey on Polycyclic Aromatic Hydrocarbons (PAHs) in Seawater of North Nanao Bay, Ishikawa, Japan, from 2015 to 2018: Sources, Pathways and Ecological Risk Assessment
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
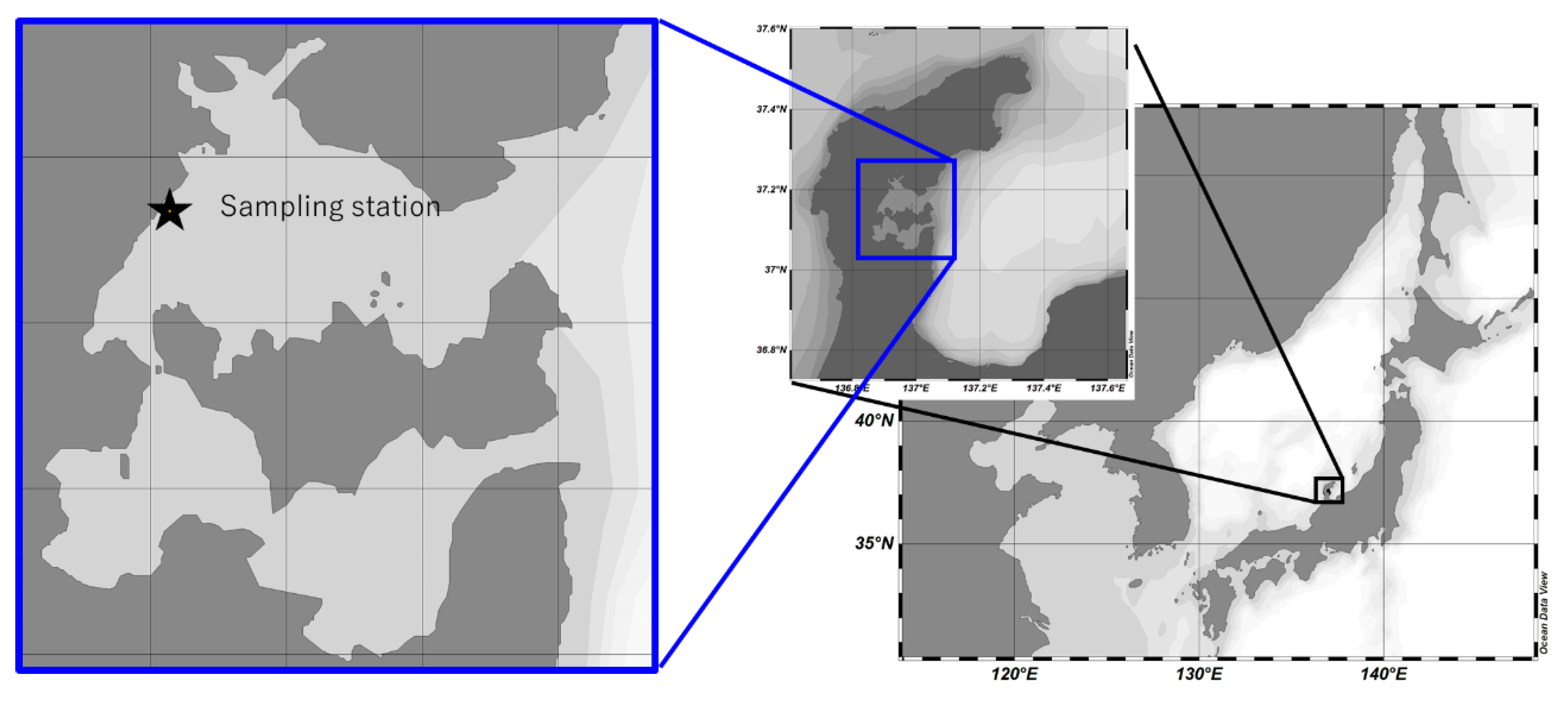
2.2. Seawater Sampling and Processing
2.3. Extraction
2.4. HPLC Analysis
2.5. Quality Assurance
2.6. PAH Emission Source Characterization
2.7. Atmospheric Mass Long Range Transportation
2.8. Ecological Risk Assessment
3. Results and Discussion
3.1. General Results
3.2. Comparison of PAH Values with Other Remote Areas Worldwide
3.3. PAHs Source Determination
3.4. Environmental Pathways
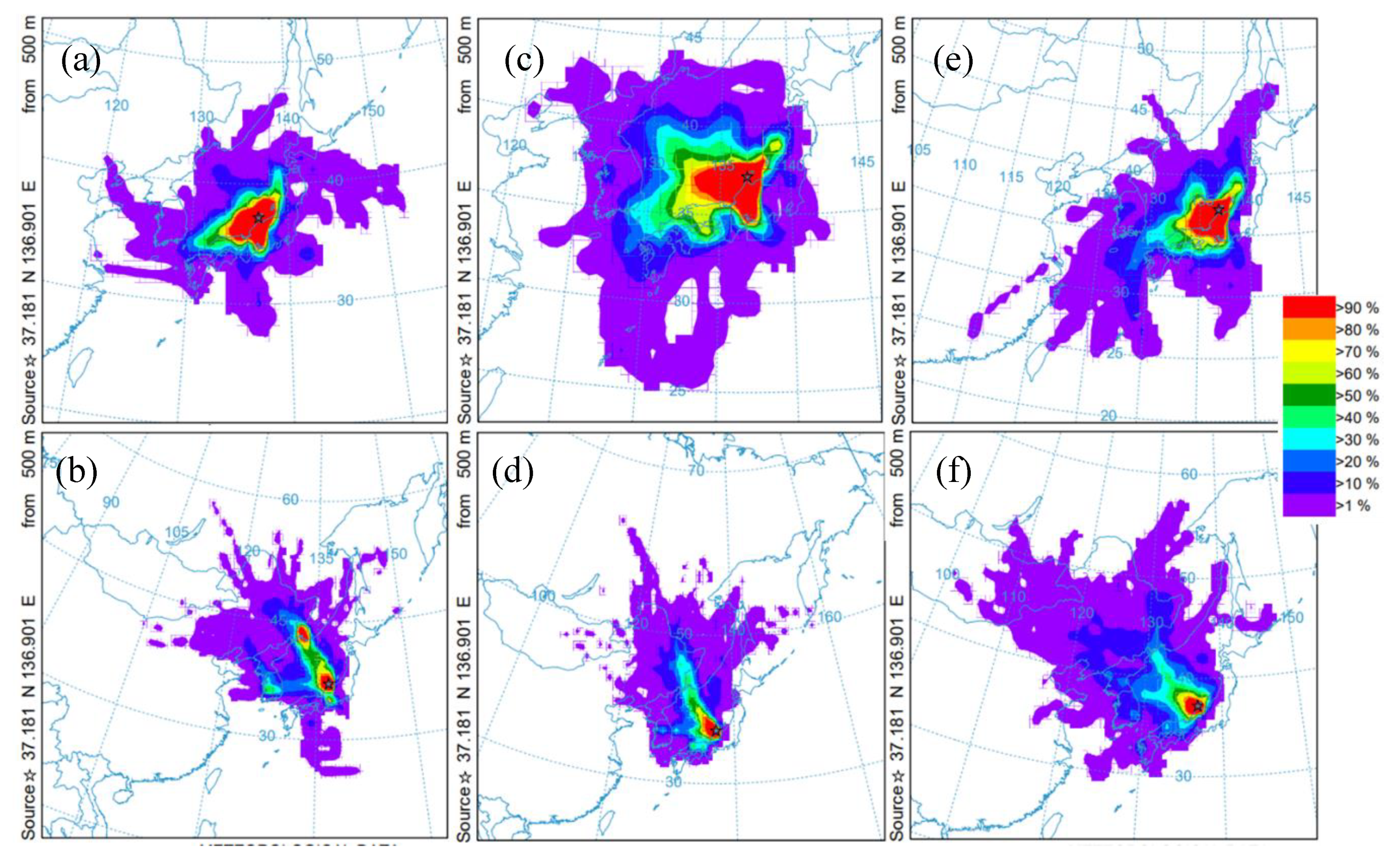
3.4.1. Long-Range Transportation of Pyrogenic PAHs
3.4.2. Petrogenic Sources as Local Inputs
3.5. Ecological Risk
4. Conclusions
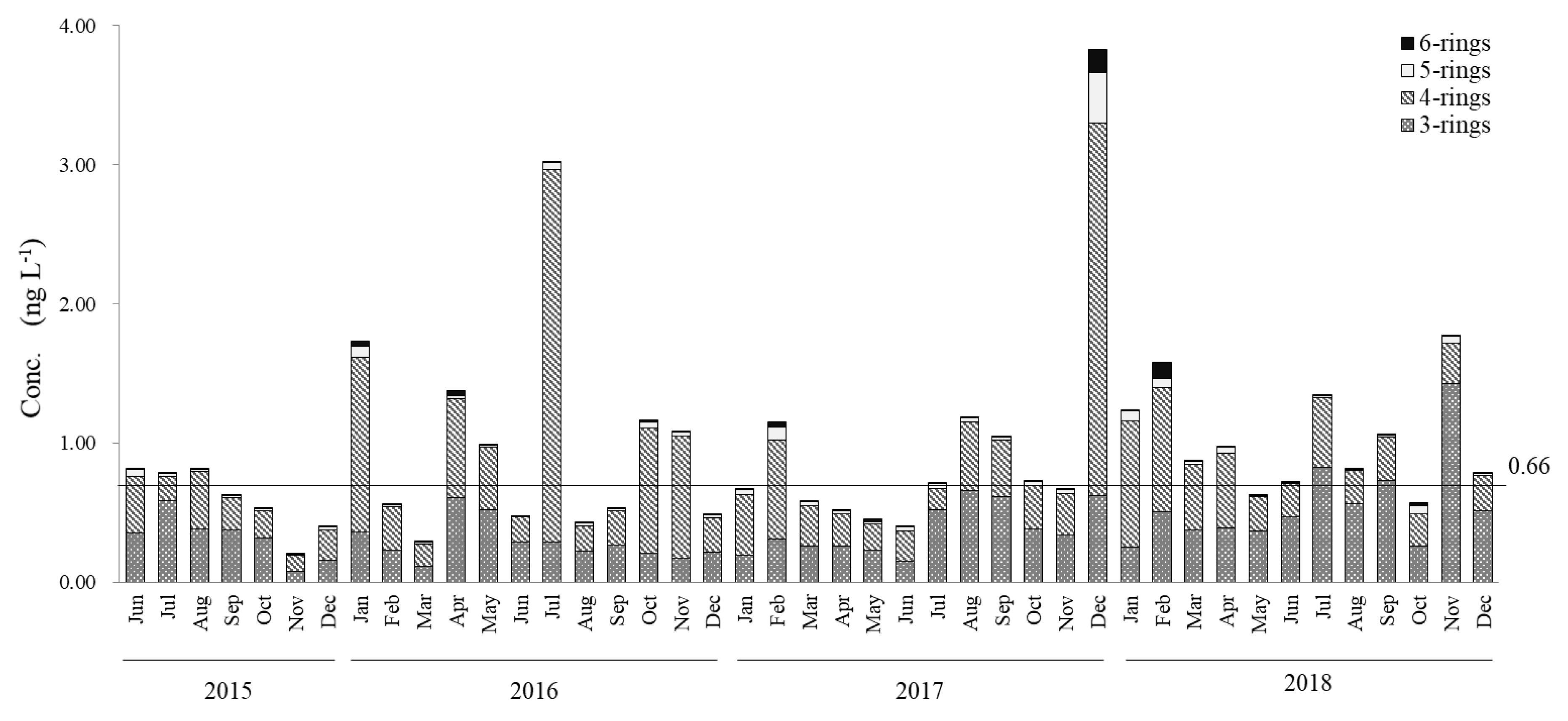
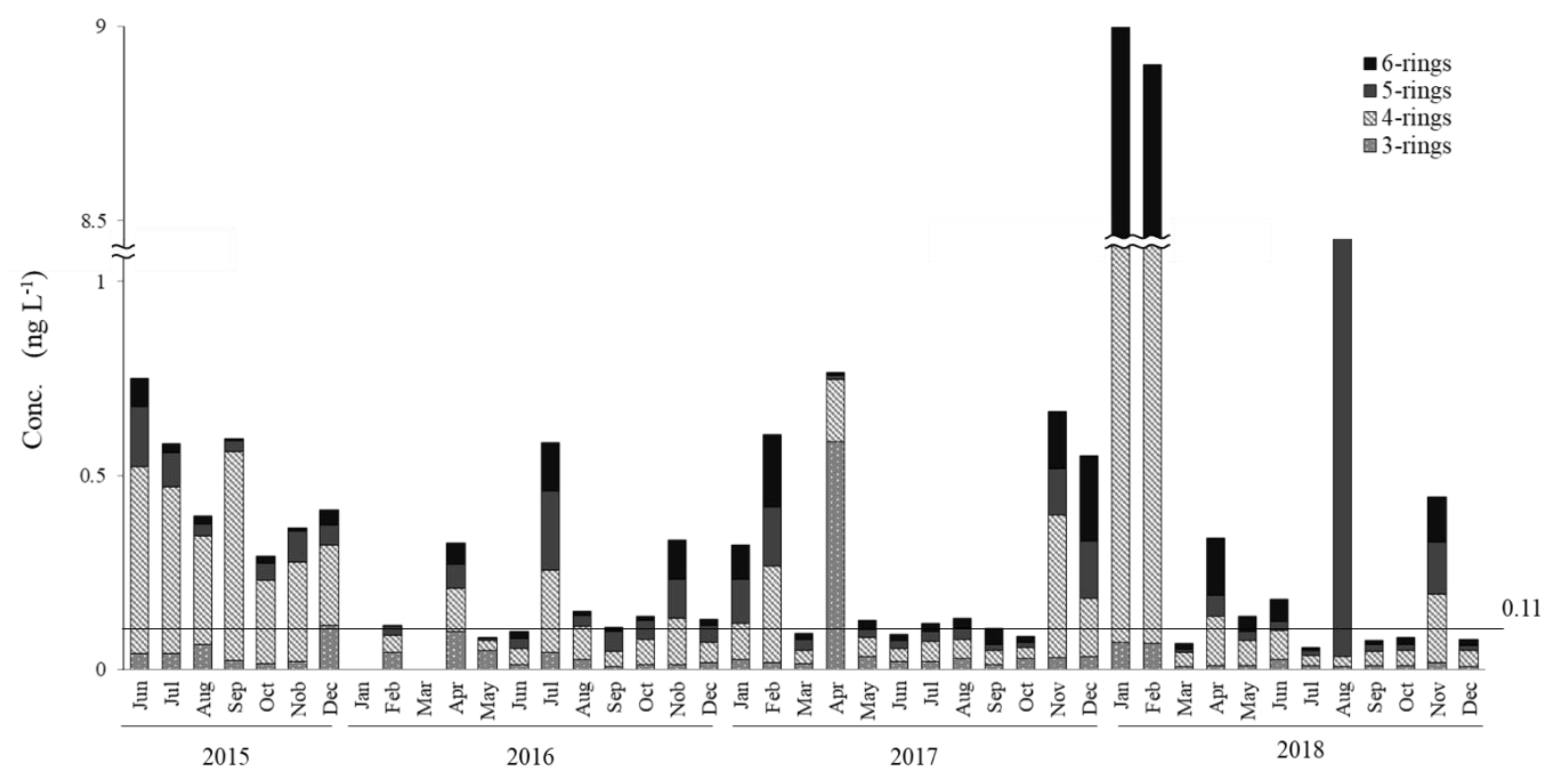
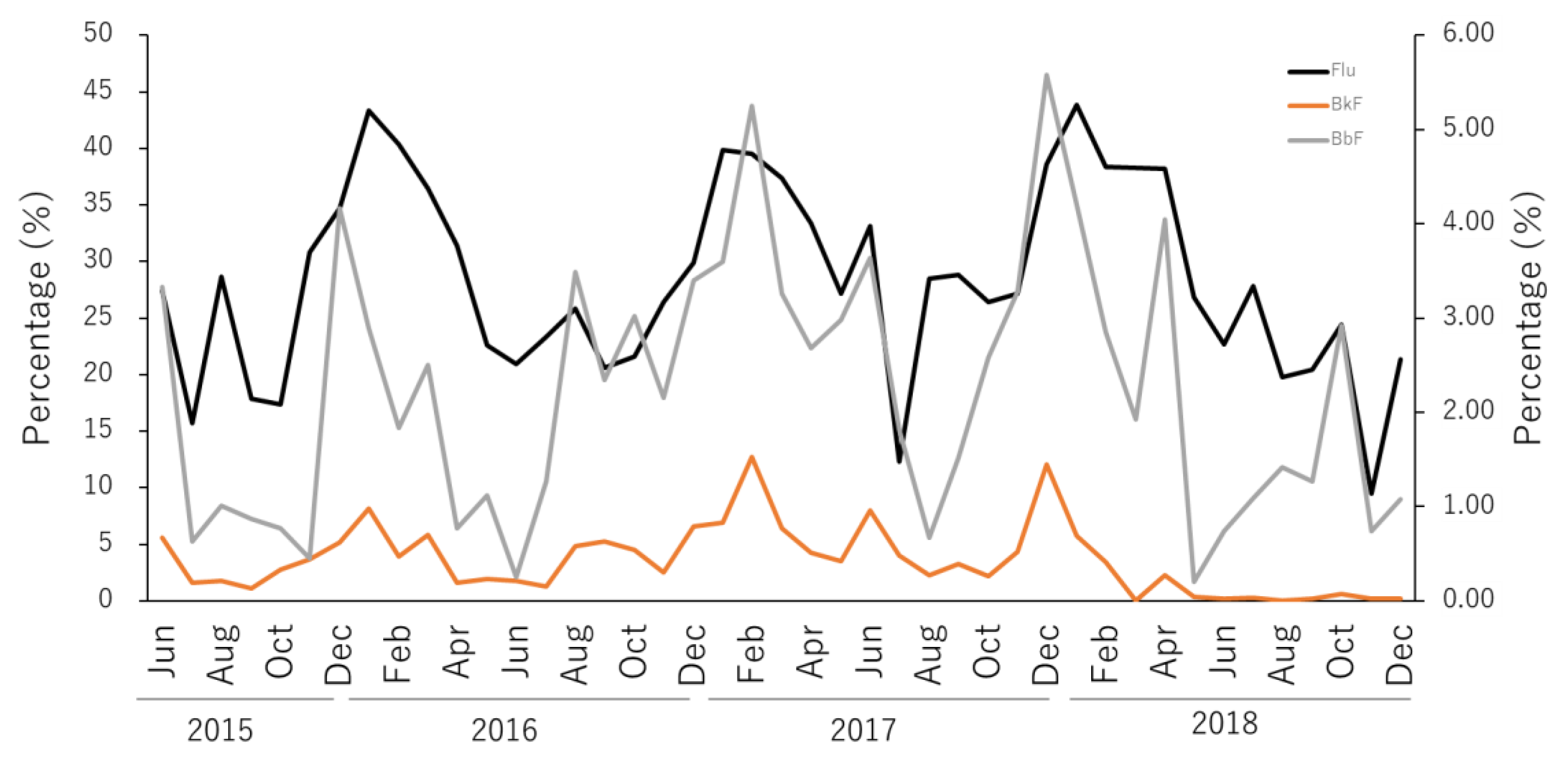
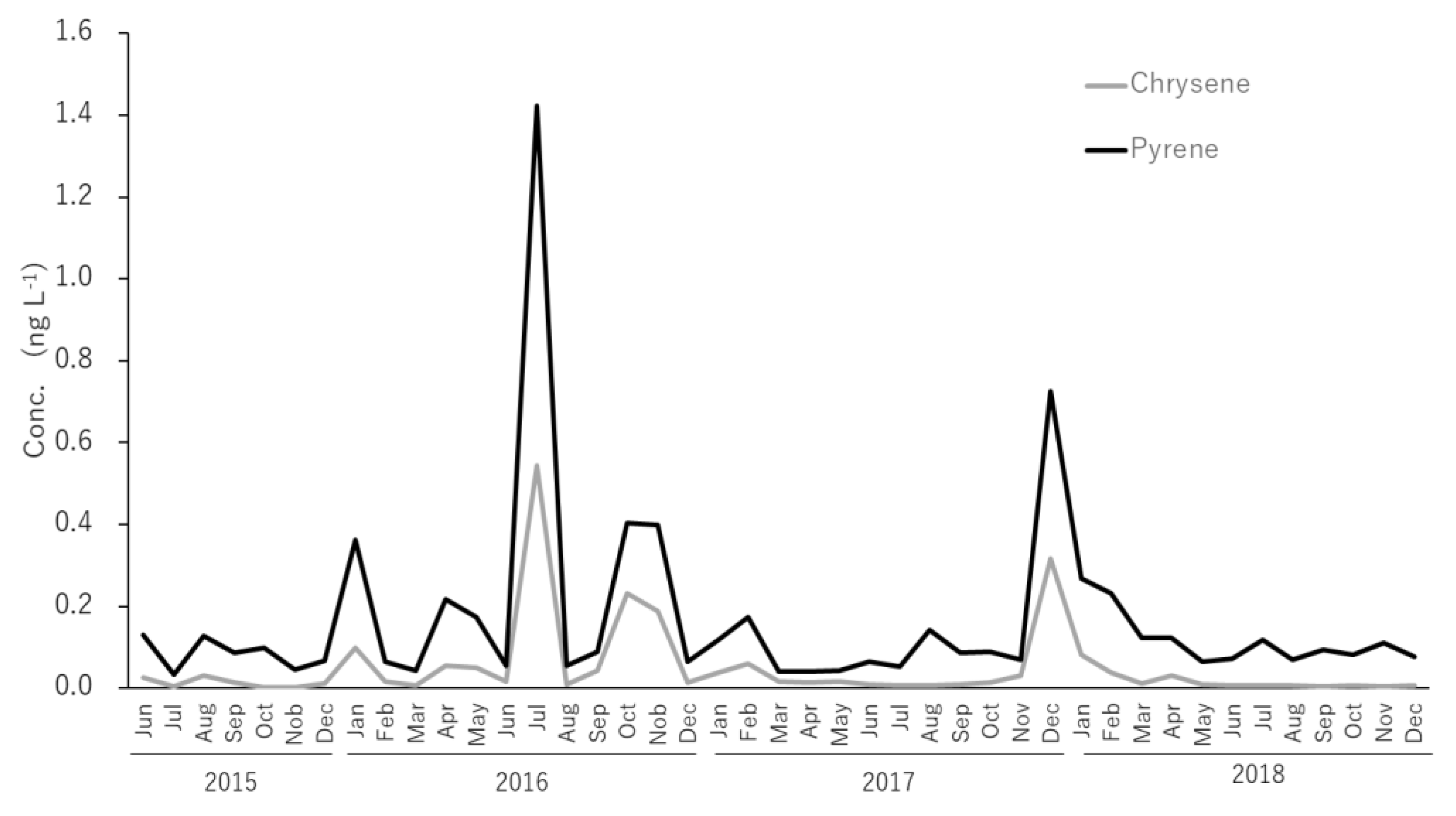
- Particulate and dissolved PAHs had average concentrations of 0.72 ng∙L−1 and 0.95 ng∙L−1, respectively. While most of the samples were Lower than 1 ng∙L−1, abnormally high levels up to ~10 ng∙L−1 were observed in the winter of 2017–2018 for particulate PAHs. In general, dissolved PAHs were 1.3 times greater than particulate PAHs, with the three-ring and four-ring PAHs being the dominant species in dissolved PAHs, in the range of 40%−50% and 45%−55%, while four-ring and five-ring PAH were the major components for particulate PAHs, with ranges of 45%−50% and 20%−30%, respectively. Total PAH concentrations were below 2.0 ng∙L−1 for most samples, showing isolated spikes in April, July and November 2016; and December 2017 and January, February 2018. The relative abundance in dissolved PAHs of Flu showed clear seasonal trends, presenting clear highs in winter (ca. 40%) and lows in summer (ca. 20%). Chr and Pyr, in dissolved PAHs, presented an overall high correlation (r2 = 0.95) for the 43 months of the survey.
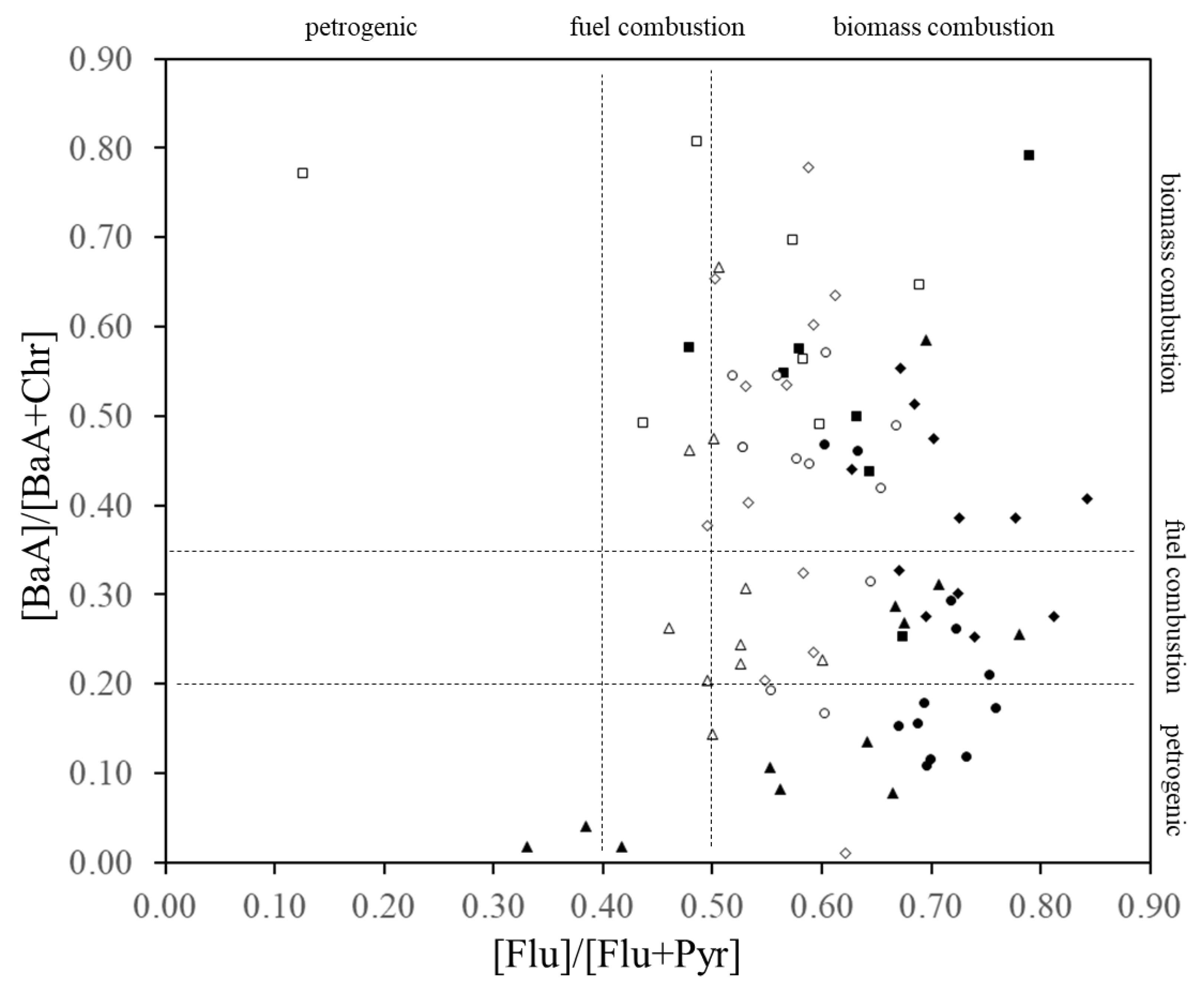
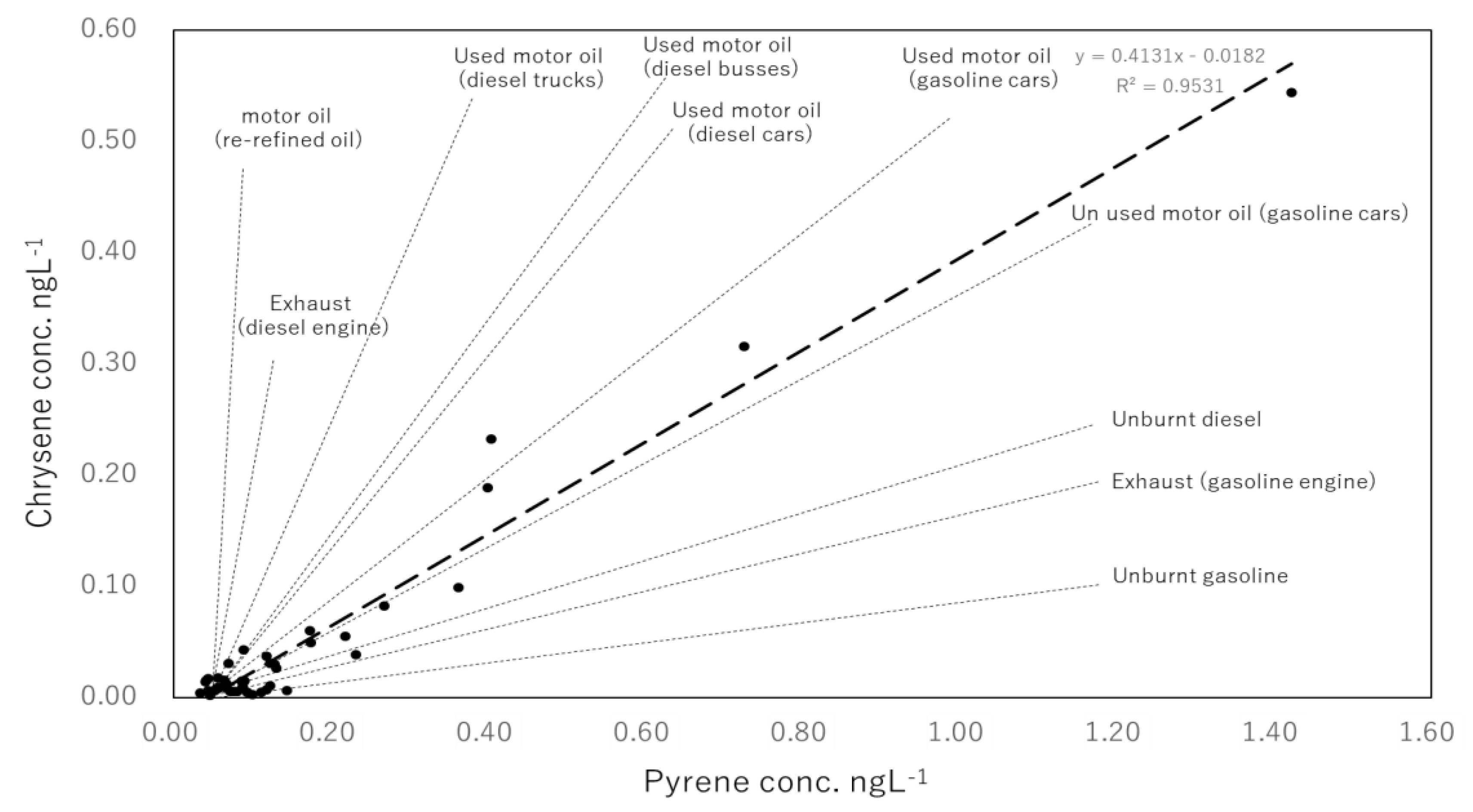
- Based on the isomer ratios of Flu to Flu plus Pyr, it was possible to determine that pyrogenic sources were greater than petrogenic sources in all but three samples (July, October and November 2016; dissolved PAHs). In addition, from the same isomer ratios of Flu to Flu plus Pyr, it was also visible that petrogenic sources were slightly more relevant in the particulate PAHs than in dissolved PAHs.
- The seasonal trends in Flu as a percentage of dissolved PAHs, as well as the abnormally high concentrations of dissolved PAHs in the winter of 2017–2018, indicate that a portion of the pyrogenic emissions from East Eurasia are transported long-range in the atmosphere to be finally deposited into the coastal aquatic environments of Japan. In addition, it was determined that the high PAHs in the dissolved phase in July, October and November 2016 and December 2017 have petrogenic origins. Such petrogenic sources were further identified as a mixture of clean and used lubricating oil for gasoline engine vehicles.
- Total PAH concentrations in surface seawater varied from 0.5 ng∙L−1 to 10 ng∙L−1 in the monthly sampling from 2015 to 2018, representing a very low risk to marine life during the 3 years of the interannual survey.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Manzetti, S. Polyclic aromatic hydrocarbons in the environment: Environmental fate and transformation. Polycycl. Aromat. Compd. 2013, 33, 311–330. [Google Scholar] [CrossRef]
- De Soysa, T.Y.; Ulrich, A.; Friedrich, T.; Pite, D.; Compton, S.L.; Ok, D.; Bernardos, R.L.; Downes, G.B.; Hsieh, S.; Stein, R.; et al. Macondo crude oil from the Deepwater Horizon oil spill disrupts specific developmental processes during zebrafish embryogenesis. BMC Biol. 2012, 10, 40. [Google Scholar] [CrossRef] [PubMed]
- Jafarabadi, A.R.; Bakhtiari, A.R.; Yaghoobi, Z.; Yap, C.K.; Maisano, M.; Cappello, T. Distributions and compositional patterns of Polycyclic Aromatic Hydrocarbons (PAHs) and their derivatives in three edible fishes from Kharg coral Island, Persian Gulf, Iran. Chemosphere 2019, 215, 835–845. [Google Scholar] [CrossRef] [PubMed]
- Fasulo, S.; Maisano, M.; Sperone, E.; Mauceri, A.; Bernab, I.; Cappello, T.; D’agata, A.; Tripepi, S.; Brunelli, E. Toxicity of Foroozan crude oil to ornate wrasse (Thalassoma pavo, Osteichthyes, Labridae): Ultrastructure and cellular biomarkers. Ital. J. Zool. 2012, 79, 182–199. [Google Scholar] [CrossRef]
- Gornatil, R.; Maisano, M.; Pirronel, C.; Cappello, T.; Rossil, F.; Borgessel, M.; Giannetto, A.; Cappello, S.; Mancini, G.; Bernardini, G.; et al. Mesocosm System to Evaluate BF-MBR Efficacy in Mitigating Oily Wastewater Discharges: An Integrated Study on Mytilus galloprovincialis. Mar. Biotechnol. 2019, 21, 773–790. [Google Scholar] [CrossRef]
- Cappello, T.; Maisano, M.; Mauceri, A.; Fasulo, S. 1H NMR-based metabolomics investigation on the effects of petrochemical contamination in posterior adductor muscles of caged mussel Mytilus galloprovincialis. Ecotoxicol. Environ. Saf. 2017, 142, 417–422. [Google Scholar] [CrossRef]
- Environmental Protection Agency. Appendix A to 40 CFR, Part 423–126 Priority Pollutants. 2012. Available online: https://www3.epa.gov/region1/npdes/permits/generic/prioritypollutants.pdf (accessed on 24 December 2019).
- Commission Regulation (EC) No 1881/2006 of 19 December 2006 (OJ L 364, 2006. p. 5) and Amending Commission Regulation (EC) No 835/2011 of 19 August 2011 (L 215/4, 20-08-2011, 5). Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:02006R1881-20160311&from=HU and https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2011:215:0004:0008:EN:PDF (accessed on 24 December 2019).
- Lima, A.L.C.; Eglinton, T.I.; Reddy, C.M. High-resolution record of pyrogenic polycyclic aromatic hydrocarbon deposition during the 20th century. Environ. Sci. Technol. 2003, 37, 53–61. [Google Scholar] [CrossRef]
- Hayakawa, K.; Nomura, M.; Nakagawa, T.; Oguri, S.; Kawanishi, T.; Toriba, A.; Kizu, R.; Sakaguchi, T.; Tamiya, E. Damage to and recovery of coastlines polluted with C-heavy oil spilled from the Nakhodka. Water Res. 2006, 40, 981–989. [Google Scholar] [CrossRef]
- Marinov, D.; Dueri, S.; Puillat, I.; Carafa, R.; Jurado, E.; Berrojalbiz, N.; Dachs, J.; Zaldívar, J.M. Integrated modelling of Polycyclic Aromatic Hydrocarbons in the marine environment: Coupling of hydrodynamic, fate and transport, bioaccumulation and planktonic food-web models. Mar. Pollut. Bull. 2009, 58, 1554–1561. [Google Scholar] [CrossRef]
- Meador, J.P.; Stein, J.E.; Reichert, W.L.; Varanasi, U. Bioaccumulation of polycyclic aromatic hydrocarbons by marine organisms. Rev. Environ. Contam. Toxicol. 1995, 143, 79–165. [Google Scholar]
- Hsieh, H.Y.; Huang, K.C.; Cheng, J.O.; Lo, W.T.; Meng, P.J.; Ko, F.C. Environmental effects on the bioaccumulation of PAHs in marine zooplankton in Gaoping coastal waters, Taiwan: Concentration, distribution, profile, and sources. Mar. Pollut. Bull. 2019, 144, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Chizhova, T.; Hayakawa, K.; Tishchenko, P.; Nakase, H.; Koudryashova, Y. Distribution of PAHs in the north western part of the Japan Sea. Deep Sea Res. Part II Top. Stud. Oceanogr. 2013, 86-87, 19–24. [Google Scholar] [CrossRef]
- Nagato, E.G.; Makino, F.; Nakase, H.; Yoshida, S.; Hayakawa, K. Improvements in polycyclic aromatic hydrocarbon contamination in the Japan Sea: An interannual survey from 2008 to 2014. Mar. Pollut. Bull. 2019, 138, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Full Report—BP Statistical Review of World Energy. BP p.l.c.: London, UK, 2018.
- Shen, H.; Huang, Y.; Wang, R.; Zhu, D.; Li, W.; Shen, G.; Wang, B.; Xhang, Y.; Chen, Y.; Lu, Y.; et al. Global atmospheric emissions of polycyclic aromatic hydrocarbons from 1960 to 2008 and future prediction. Environ. Sci. Technol. 2013, 47, 6415–6424. [Google Scholar] [CrossRef]
- Ishikawa Stadistical Information. Available online: http://toukei.pref.ishikawa.jp/search/detail.asp?d_id=3061 (accessed on 21 October 2019).
- Tang, N.; Hakamata, M.; Sato, K.; Okada, Y.; Yang, X.; Tatematsu, M.; Toriba, A.; Kameda, T.; Hayakawa, K. Atmospheric behaviors of polycyclic aromatic hydrocarbons at a Japanese remote background site, Noto peninsula, from 2004 to 2014. Atmos. Environ. 2015, 120, 144–151. [Google Scholar] [CrossRef]
- Yunker, M.B.; Macdonald, R.W.; Vingarzan, R.; Mitchell, R.H.; Goyette, D.; Sylvestre, S. PAHs in the Fraser River basin: A critical appraisal of PAH ratios as indicators of PAH source and composition. Org. Geochem. 2002, 33, 489–515. [Google Scholar] [CrossRef]
- Hayakawa, K.; Makino, F.; Yasuma, M.; Yoshida, S.; Chondo, Y.; Toriba, A.; Kameda, T.; Tang, N.; Kunugi, M.; Nakase, H.; et al. Polycyclic aromatic hydrocarbons in surface water of the south eastern Japan Sea. Chem. Pharm. Bull. 2016, 64, 625–631. [Google Scholar] [CrossRef]
- Tobiszewski, M.; Namiesnik, J. PAH diagnostic ratios for the identification of pollution emission sources. Environ. Pollut. 2012, 162, 110–119. [Google Scholar] [CrossRef]
- Draxlter, D.R.; Hess, G.D. An overview of the HYSPLIT_4 modelling system for trajectories, dispersion, and deposition. Aust. Meteorol. Mag. 1998, 47, 295–308. [Google Scholar]
- Miki, S.; Uno, S.; Ito, K.; Koyama, J.; Tanaka, H. Distributions of polycyclic aromatic hydrocarbons and alkylated polycyclic aromatic hydrocarbons in Osaka Bay, Japan. Mar. Pollut. Bull. 2014, 85, 558–565. [Google Scholar] [CrossRef]
- Kalf, D.F.; Crommentuijn, T.; Vandeplassche, E.J. Environment quality objectives for l0 Polycyclic aromatic hydrocarbons (PAHs). Ecotoxicol. Environ. 1997, 36, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, P.; Ma, W.; Song, Q.; Zhou, H.; Han, Q.; Diao, X. Spatial and temporal distribution and risk assessment of polycyclic aromatic hydrocarbons in surface seawater from the Haikou Bay, China. Mar. Pollut. Bull. 2015, 92, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Liu, J.; Luan, Y.; Li, Y.; Ma, M.; Xu, J.; Han, S. Distribution and ecosystem risk assessment of polycyclic aromatic hydrocarbons in the Luan River, China. Ecotoxicology 2010, 19, 827–837. [Google Scholar] [CrossRef] [PubMed]
- Office of Environmental Health Hazard Assessment (OEHHA). Chemical-Specific Summaries of the Information Used to Derive Unit Risk and Cancer Potency Values; (Appendix B); OEHHA: San Francisco, CA, USA, 2011. [Google Scholar]
- Haitzer, M.; Abbt-Braun, G.; Traunspurger, W.; Steinberg, C.E.W. Effects of humic substances on the bioconcentration of polycyclic aromatic hydrocarbons: Correlations with spectroscopic and chemical properties of humic substances. Environ. Toxicol. Chem. 1999, 18, 2782–2788. [Google Scholar] [CrossRef]
- Vecchiato, M.; Turetta, C.; Pattic, B.; Barbante, C.; Piazza, R.; Bonato, T.; Gambaroa, A. Distribution of fragrances and PAHs in the surface seawater of the Sicily Channel, Central Mediterranean. Sci. Total Environ. 2018, 634, 983–989. [Google Scholar] [CrossRef]
- Berrojalbiz, N.; Dachs, J.; Ojeda, M.J.; Valle, M.C.; Jiménez, J.C.; Wollgast, J.; Ghiani, M.; Hanke, G.; Zaldivar, J.M. Biogeochemical and physical controls on concentrations of polycyclic aromatic hydrocarbons in water and plankton of the Mediterranean and Black Seas. Glob. Biogeochem. Cycles 2011, 25, GB4003. [Google Scholar] [CrossRef]
- Valavanidis, A.; Vlachogianni, T.; Triantafillaki, S.; Dassenakis, M.; Androutsos, F.; Scoullos, M. Polycyclic aromatic hydrocarbons in surface seawater and in indigenous mussels (Mytilus galloprovincialis) from coastal areas of the Saronikos Gulf (Greece). Estuar. Coast. Shelf Sci. 2008, 79, 733–739. [Google Scholar] [CrossRef]
- Hong, W.J.; Jia, H.; Li, Y.F.; Sun, Y.; Liu, X.; Wang, L. Polycyclic aromatic hydrocarbons (PAHs) and alkylated PAHs in the coastal seawater, surface sediment and oyster from Dalian, Northeast China. Ecotoxicol. Environ. Saf. 2016, 128, 11–20. [Google Scholar] [CrossRef]
- Lim, L.; Wurl, O.; Karuppiah, S.; Obbar, J.P. Atmospheric wet deposition of PAHs to the sea-surface microlayer. Mar. Pollut. Bull. 2007, 54, 1212–1219. [Google Scholar] [CrossRef]
- Richter, H.; Howard, J.B. Formation of polycyclic aromatic hydrocarbons and their growth to soot—A review of chemical reaction pathways. Prog. Energy Combust. Sci. 2000, 26, 565–608. [Google Scholar] [CrossRef]
- Shukla, B.; Koshi, M. Comparative study on the growth mechanisms of PAHs. Combust. Flame 2011, 158, 369–375. [Google Scholar] [CrossRef]
- Japan Metereological Agency. Past Weather Data Search; Monthly Values. Available online: http://www.jma.go.jp/jma/index.html (accessed on 21 October 2019).
- Neroda, A.S.; Goncharova, A.A.; Mishukov, V.F. PAHs in the atmospheric aerosols and seawater in the North–West Pacific Ocean and Sea of Japan. Atmos. Environ. 2020, 222, 117. [Google Scholar] [CrossRef]
- Liu, W.X.; Zhi, H.D.; Biao, C.W.; Wei, C.; Yuan, X.Q.; Tao, L.S. Emission characteristics of polycyclic aromatic hydrocarbons from combustion of different residential coals in North China. Sci. Total Environ. 2009, 407, 1436–1446. [Google Scholar] [CrossRef] [PubMed]
- Byambaa, B.; Yang, L.; Matsuki, A.; Nagato, E.G.; Gankhuyag, K.; Chuluunpurev, B.; Banzragch, L.; Chonokhuu, S.; Tang, N.; Hayakawa, K. Sources and Characteristics of Polycyclic Aromatic Hydrocarbons in Ambient Total Suspended Particles in Ulaanbaatar City, Mongolia. Int. J. Environ. Res. Public Health 2019, 16, 442. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.I.; Son, S.W.; Kim, H.C.; Kim, S.W.; Park, R.J.; Chen, D. Contrasting synoptic weather patterns between non-dust high particulate matter events and Asian dust events in Seoul, South Korea. Atmos. Environ. 2019, 214, 116864. [Google Scholar] [CrossRef]
- Grimmer, G.; Jacob, J.; Naujack, K.W.; Dettbarn, G. Profile of the Polycyclic Aromatic Hydrocarbons from Used Engine Oil-Inventory by GCGC/MS-PAH in Environmental Materials, Part 2. Fresenius Z. Anal. Chem. 1981, 309, 13–19. [Google Scholar] [CrossRef]
- Khaili, N.R.; Scheff, P.A.; Holsen, T.M. PAH source fingerprints for coke ovens, diesel and gasoline engines, highway tunnels, and wood combustion emissions. Atmos. Environ. 1995, 29, 533–542. [Google Scholar] [CrossRef]
- Westerholm, R.; Li, H. A multivariate statistical analysis of fuel-related polycyclic aromatic hydrocarbons emissions from heavy-duty diesel vehicles. Environ. Sci. Technol. 1994, 28, 965–972. [Google Scholar] [CrossRef]
- Perez, J.L.; Sanchez, M.N.; Fernandez, M.E.; Cordero, B.M. Determination of aromatic and polycyclic aromatic hydrocarbons in gasoline using programmed temperature vaporization-gas chromatography-mass spectrometry. J. Chromatogr. A 2008, 1201, 196–202. [Google Scholar] [CrossRef]
| Location | Survey Date | Concentrations (ng∙L−1) | References |
|---|---|---|---|
| Black Sea and Marmara Sea | 2006–2007 | 605.3 | [31] |
| Mediterranean Sea | 2006–2007 | 306.1 | [31] |
| Saronikos Gulf, Greece | 2005–2006 | 245 | [32] |
| Dalian, Northeast China | 2010 | 79.57 | [33] |
| Singapore | 2005 | 10.84 | [34] |
| Northwestern Japan Sea | 2010 | 7.9 | [14] |
| North Nanao Bay, Japan | 2015–2018 | 1.67 | This study |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mundo, R.; Matsunaka, T.; Iwai, H.; Ogiso, S.; Suzuki, N.; Tang, N.; Hayakawa, K.; Nagao, S. Interannual Survey on Polycyclic Aromatic Hydrocarbons (PAHs) in Seawater of North Nanao Bay, Ishikawa, Japan, from 2015 to 2018: Sources, Pathways and Ecological Risk Assessment. Int. J. Environ. Res. Public Health 2020, 17, 904. https://doi.org/10.3390/ijerph17030904
Mundo R, Matsunaka T, Iwai H, Ogiso S, Suzuki N, Tang N, Hayakawa K, Nagao S. Interannual Survey on Polycyclic Aromatic Hydrocarbons (PAHs) in Seawater of North Nanao Bay, Ishikawa, Japan, from 2015 to 2018: Sources, Pathways and Ecological Risk Assessment. International Journal of Environmental Research and Public Health. 2020; 17(3):904. https://doi.org/10.3390/ijerph17030904
Chicago/Turabian StyleMundo, Rodrigo, Tetsuya Matsunaka, Hisanori Iwai, Shouzo Ogiso, Nobuo Suzuki, Ning Tang, Kazuichi Hayakawa, and Seiya Nagao. 2020. "Interannual Survey on Polycyclic Aromatic Hydrocarbons (PAHs) in Seawater of North Nanao Bay, Ishikawa, Japan, from 2015 to 2018: Sources, Pathways and Ecological Risk Assessment" International Journal of Environmental Research and Public Health 17, no. 3: 904. https://doi.org/10.3390/ijerph17030904
APA StyleMundo, R., Matsunaka, T., Iwai, H., Ogiso, S., Suzuki, N., Tang, N., Hayakawa, K., & Nagao, S. (2020). Interannual Survey on Polycyclic Aromatic Hydrocarbons (PAHs) in Seawater of North Nanao Bay, Ishikawa, Japan, from 2015 to 2018: Sources, Pathways and Ecological Risk Assessment. International Journal of Environmental Research and Public Health, 17(3), 904. https://doi.org/10.3390/ijerph17030904





