Human Activity and Hydrogeochemical Processes Relating to Groundwater Quality Degradation in the Yuncheng Basin, Northern China
Abstract
1. Introduction
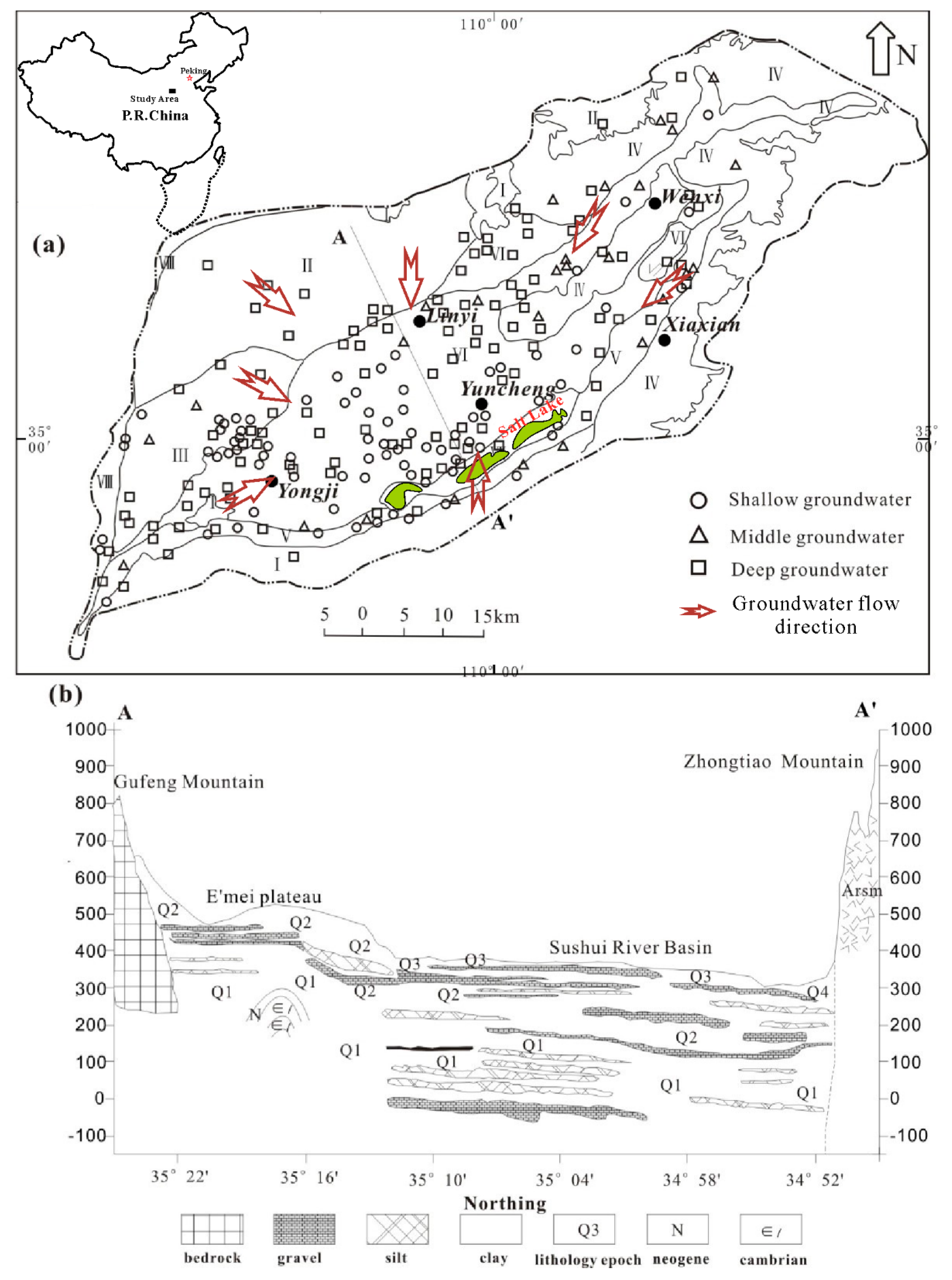
2. Study Area
3. Materials and Methods
4. Results
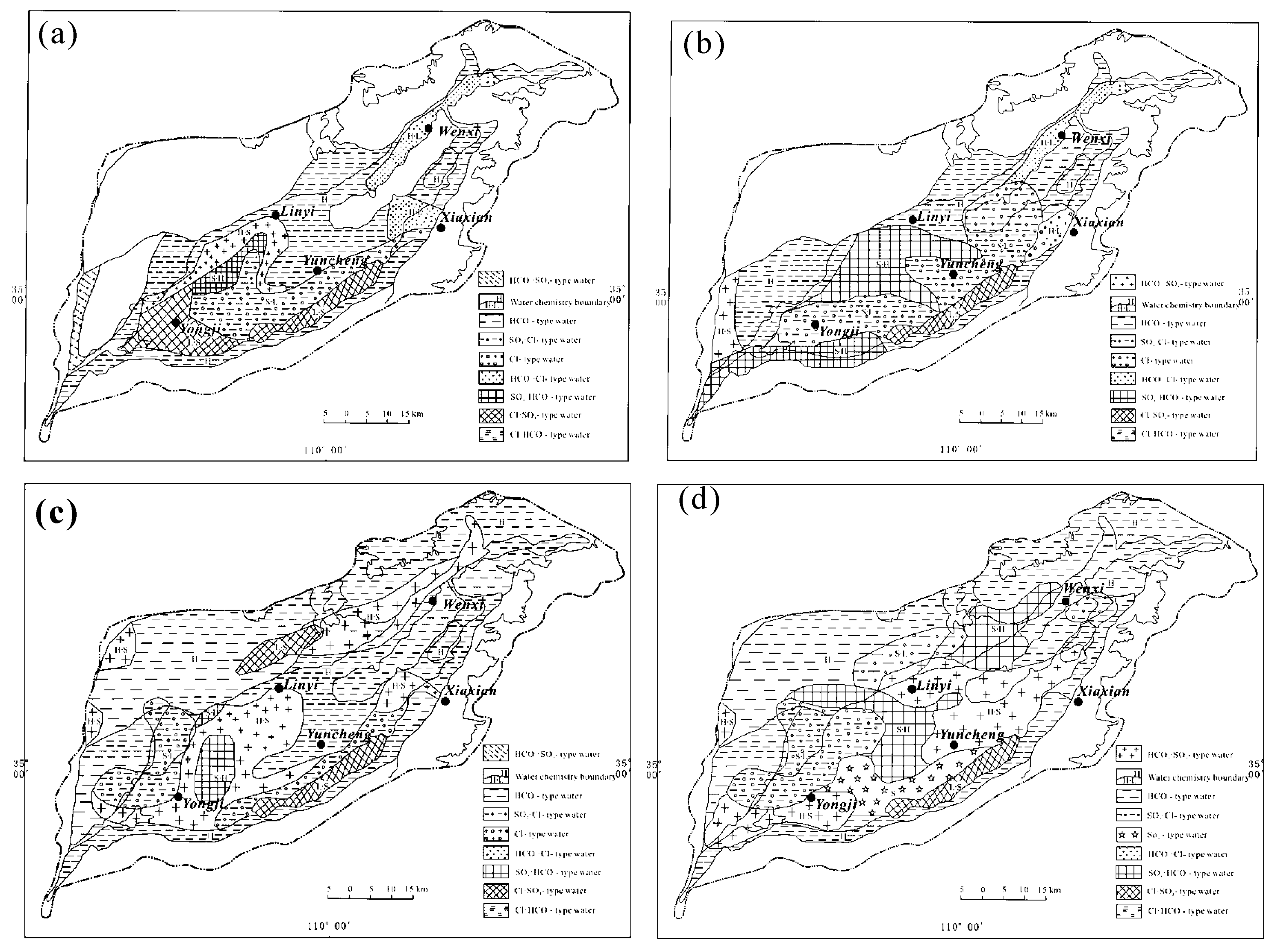
4.1. General Hydrogeochemistry
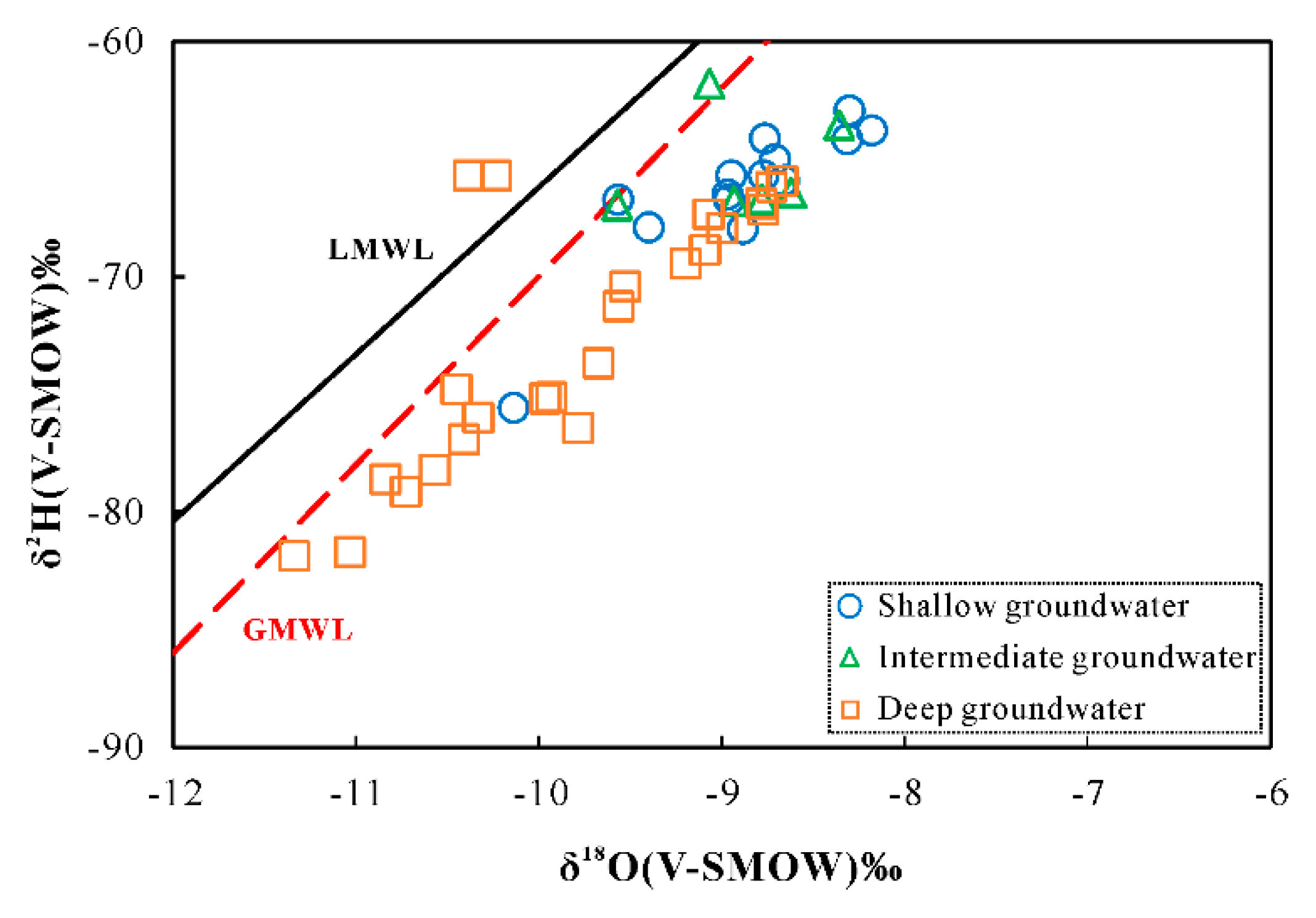
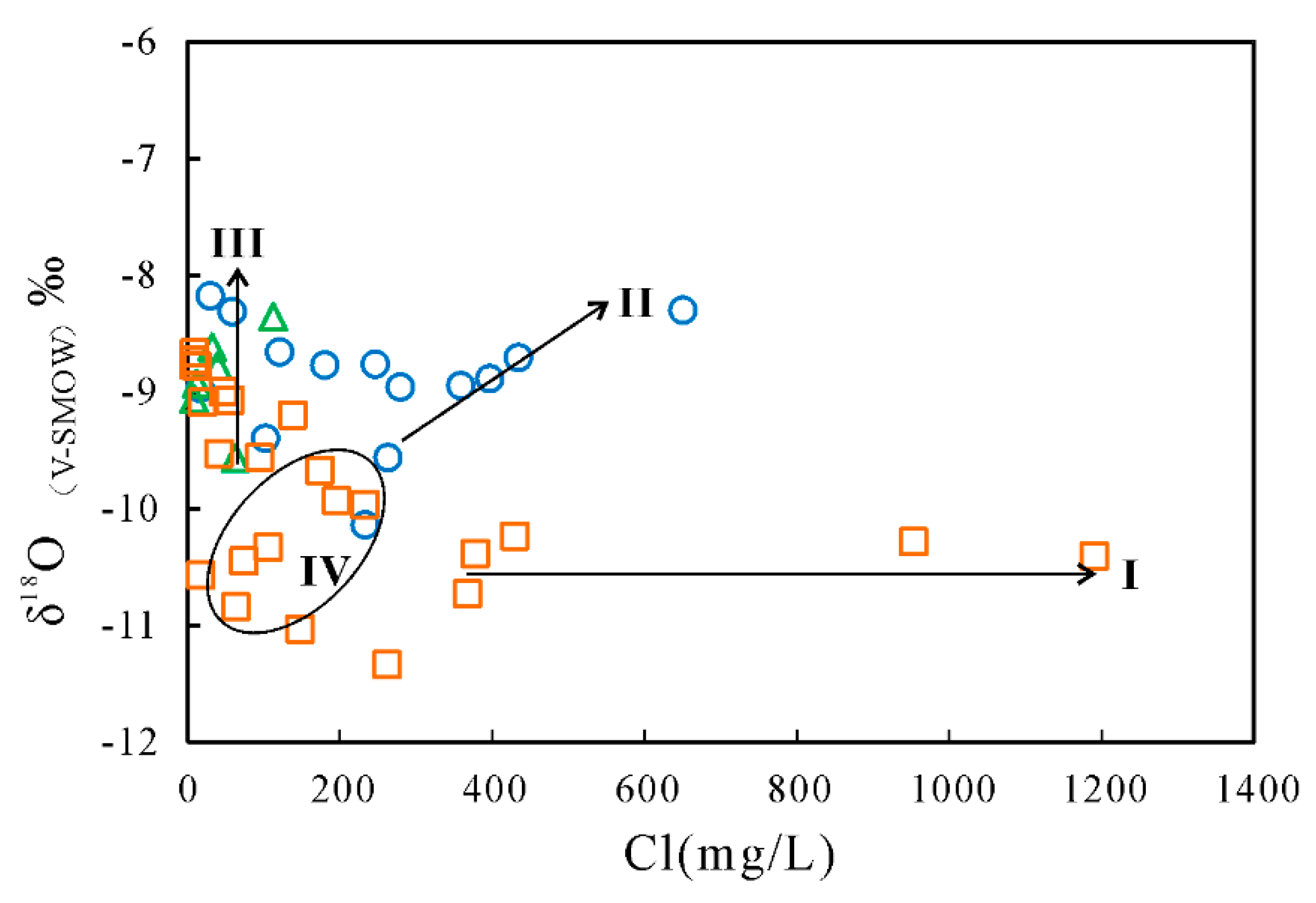
4.2. Stable Isotopes Oxygen and Hydrogen
5. Discussion
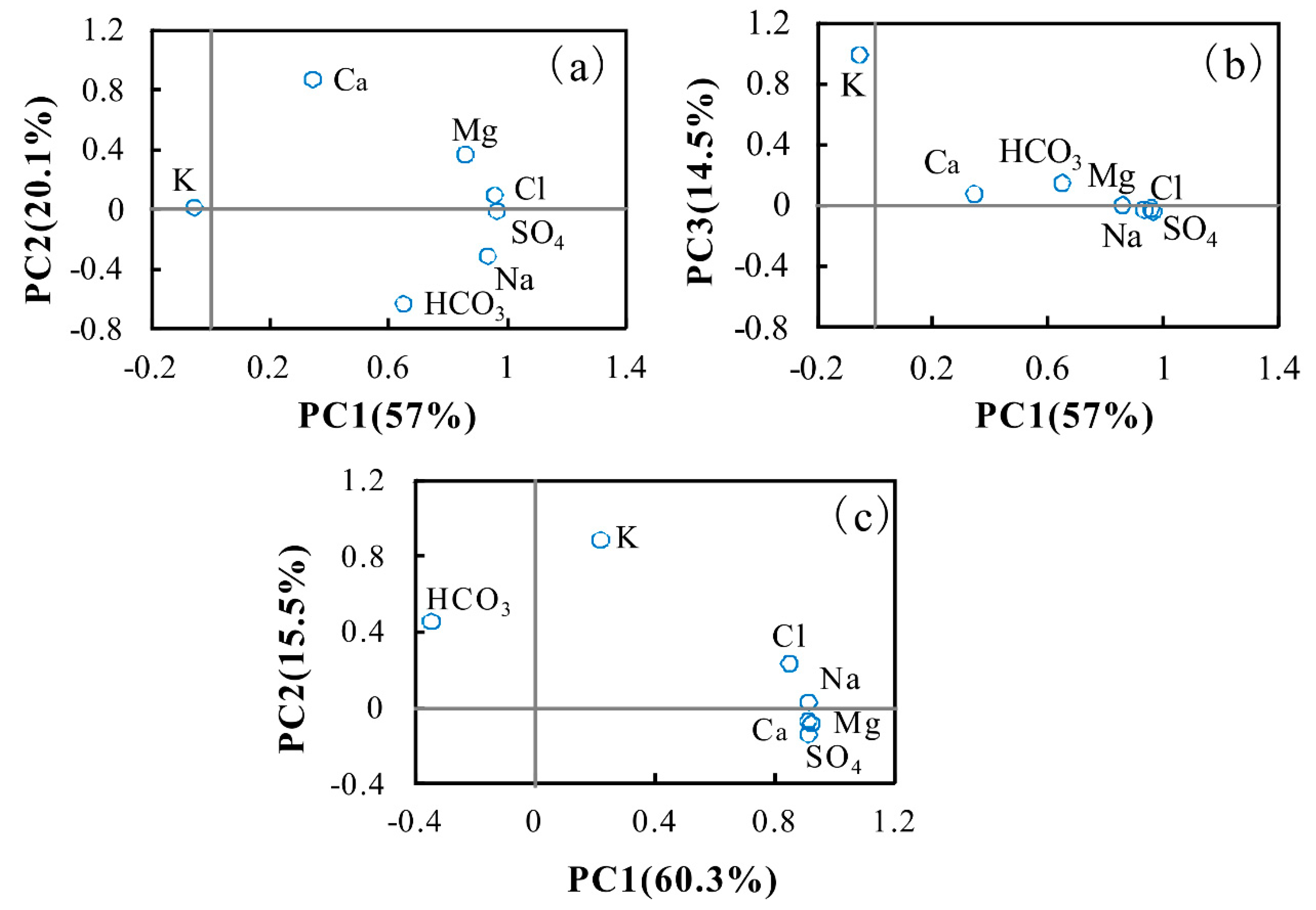
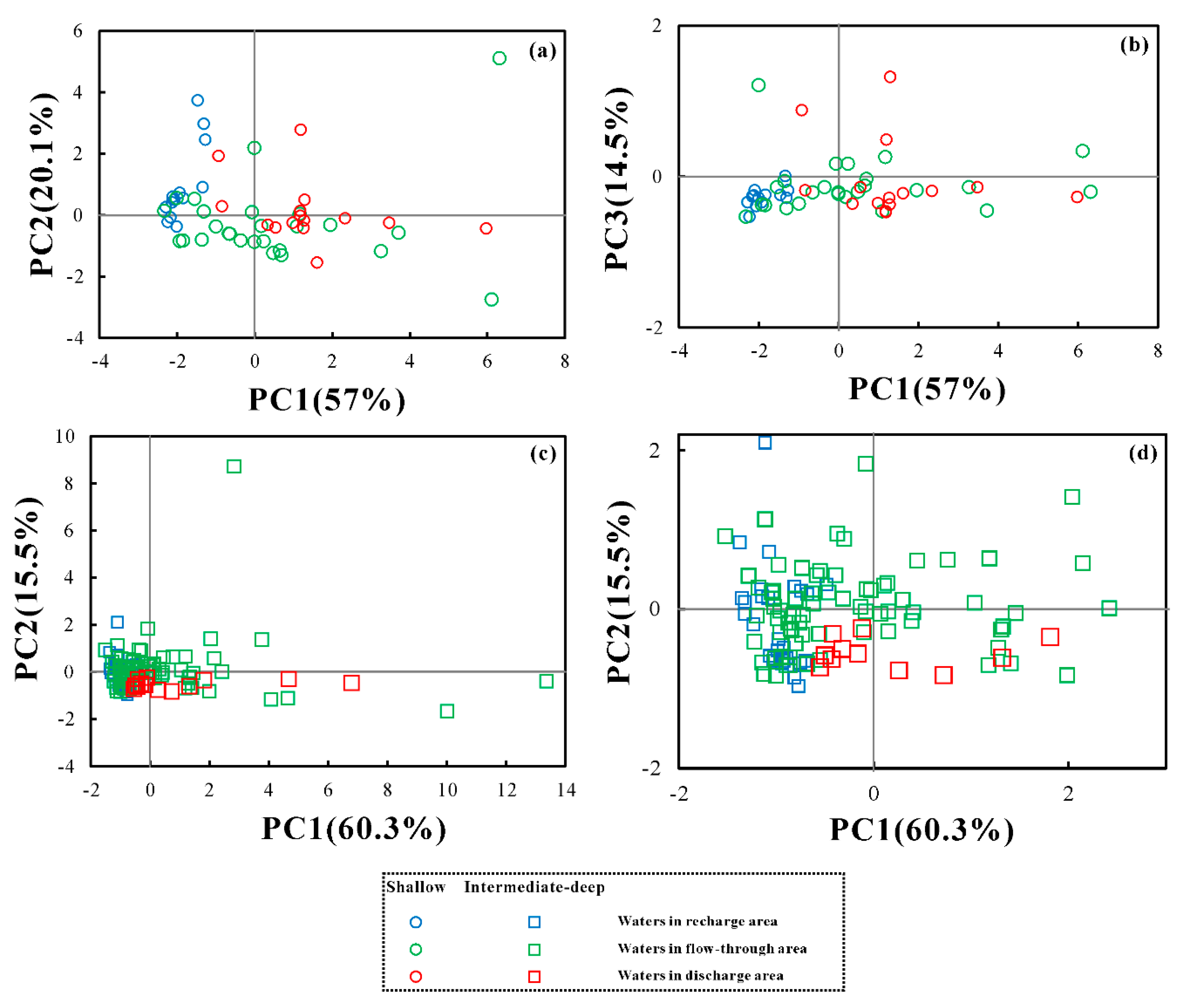
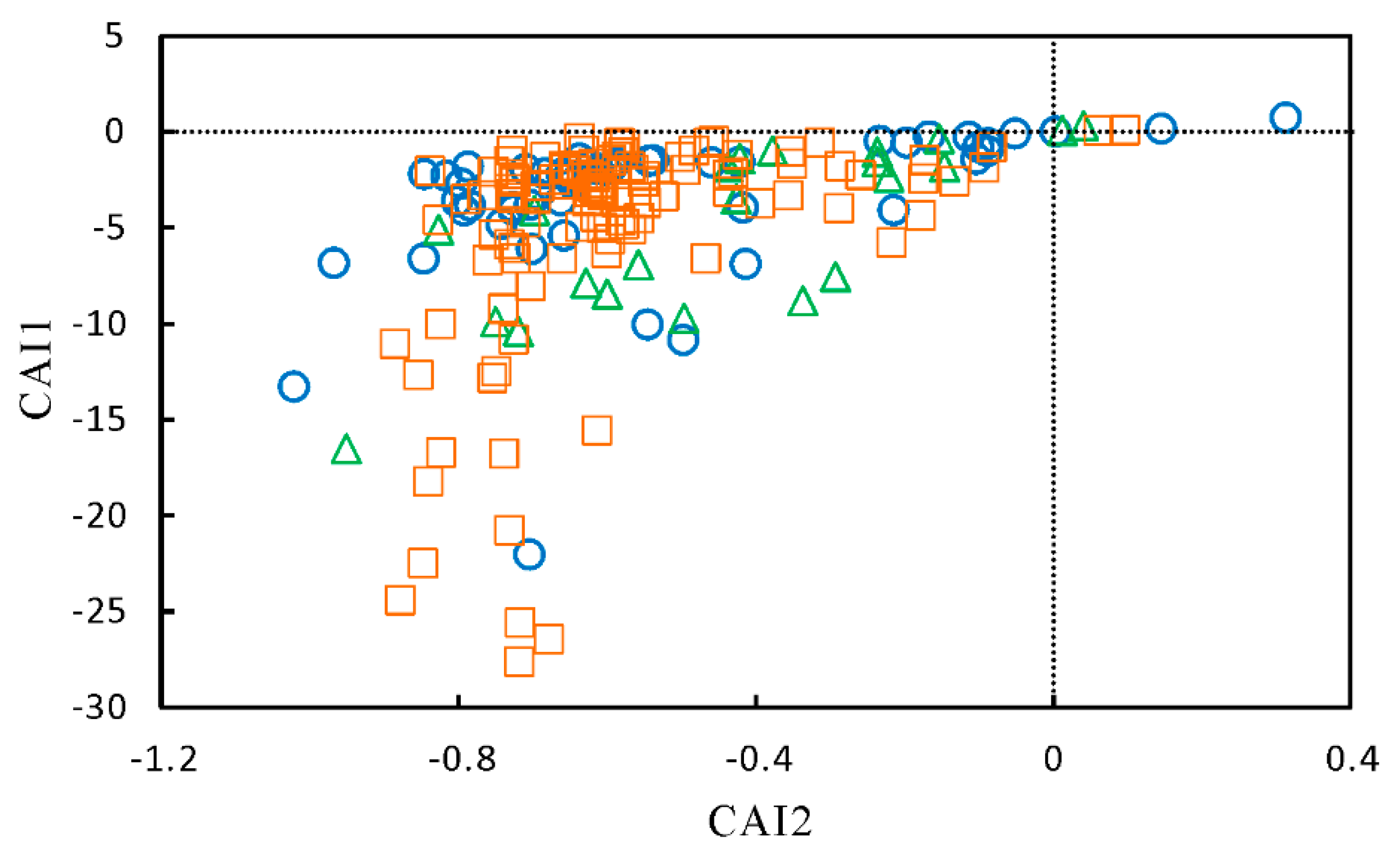
5.1. Extraction of Principal Components by PCA
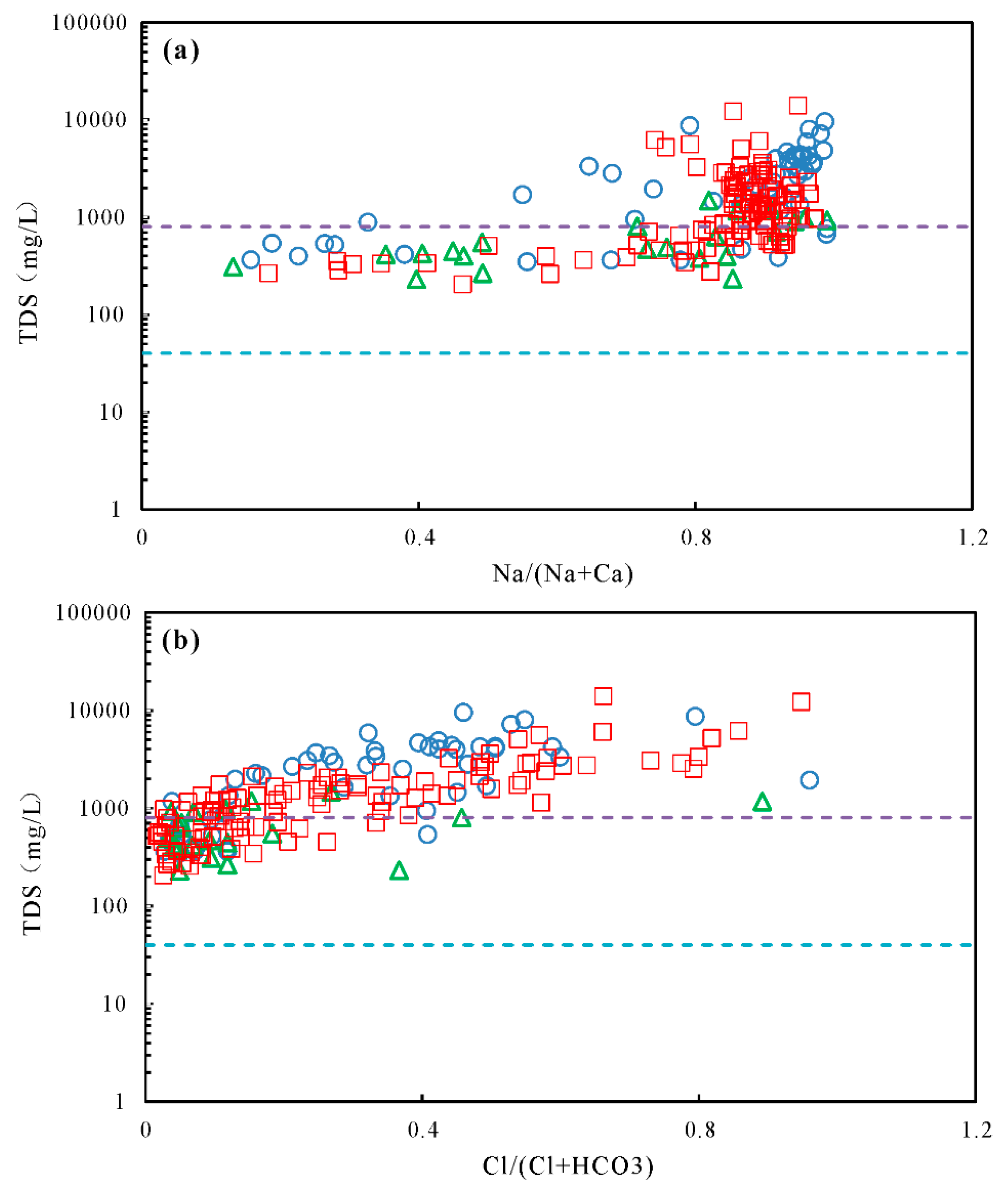
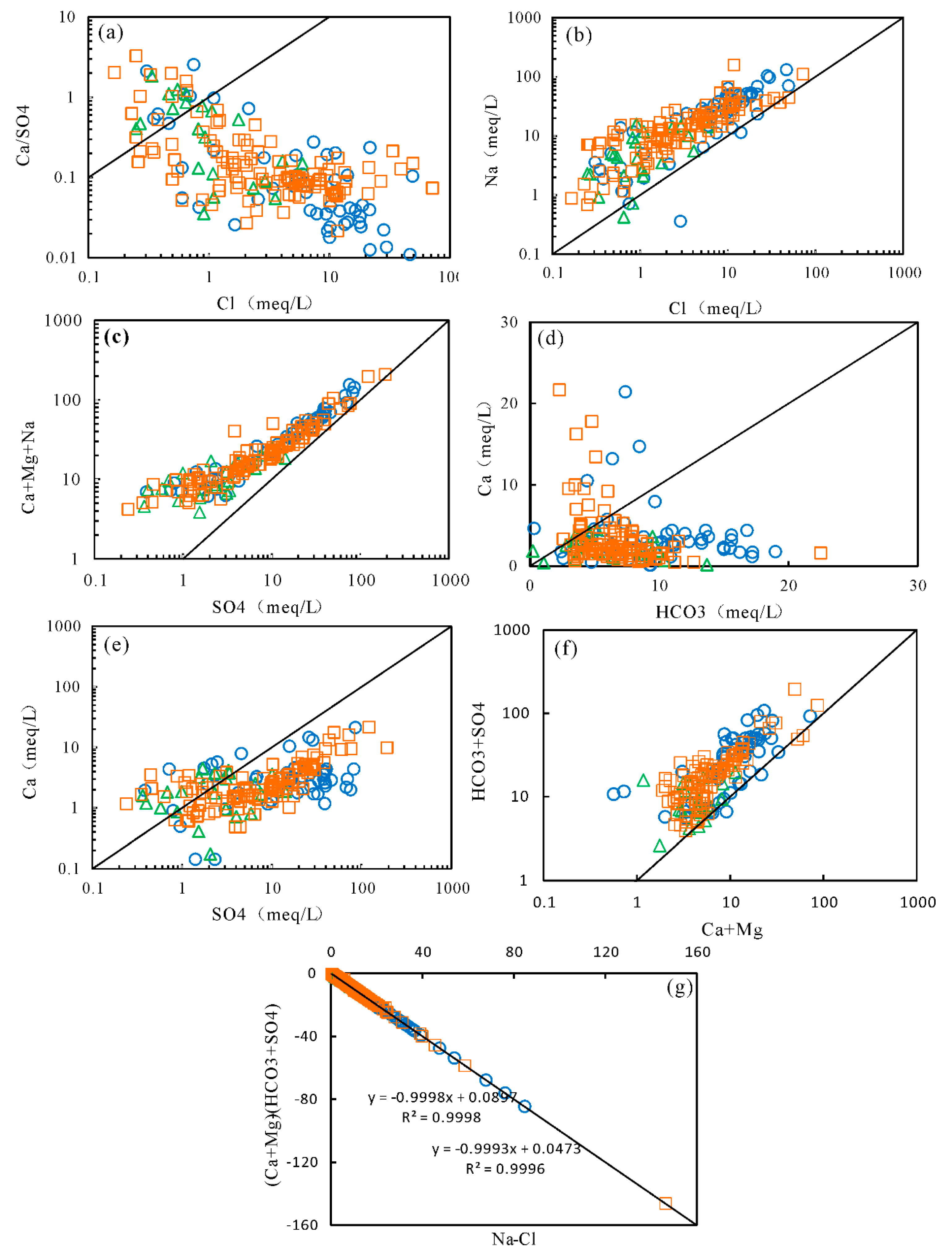
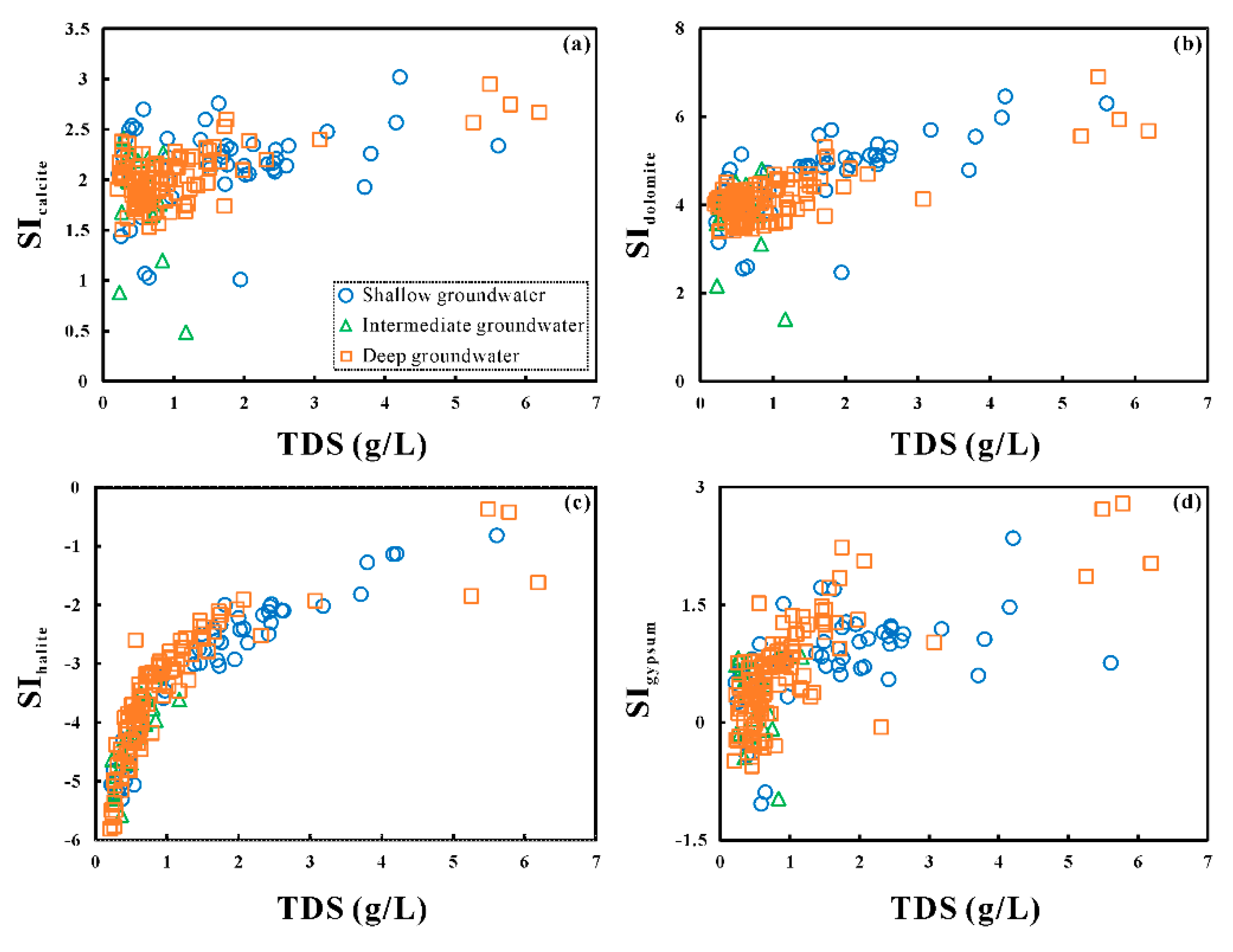
5.2. Mechanisms of Natural Processes Controlling Groundwater Chemistry
5.3. Anthropogenic Factors Affecting Groundwater Chemistry
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Edmunds, W.M.; Guendouz, A.H.; Mamou, A.; Moulla, A.; Shand, P.; Zouari, K. Groundwater evolution in the Continental Intercalaire aquifer of southern Algeria and Tunisia: Trace element and isotopic indicators. Appl. Geochem. 2003, 18, 805–822. [Google Scholar] [CrossRef]
- Gibson, J.J.; Edwards, T.W.D.; Birks, S.J.; St Amour, N.A.; Buhay, W.M.; McEachern, P.; Wolfe, B.B.; Peters, D.L. Progress in isotope tracer hydrology in Canada. Hydrogeol. J. 2005, 19, 303–327. [Google Scholar] [CrossRef]
- Qu, H.L. Assessment of Groundwater Resources in the Arid and Semiarid Land of China; Science Press: Beijing, China, 1991. [Google Scholar]
- Li, C.; Liu, T.; Xu, S.; Gao, X.; Wang, Y. Groundwater salinization in shallow aquifers adjacent to a low-altitude inland salt lake: A case study at Yuncheng Basin, northern China. Environ. Earth Sci. 2016, 75, 370. [Google Scholar] [CrossRef]
- Li, C.C.; Gao, X.B. Assessment of groundwater quality at Yuncheng Basin: Denotation for the water management in China. Groundwater 2018. [Google Scholar] [CrossRef] [PubMed]
- Currell, M.J.; Cartwright, I.; Bradley, D.C.; Han, D. Recharge history and controls on groundwater quality in the Yuncheng Basin, north China. J. Hydrol. 2010, 385, 216–229. [Google Scholar] [CrossRef]
- Li, P.; Qian, H.; Wu, J.; Zhang, Y.; Zhang, H. Major ion chemistry of shallow groundwater in the Dongsheng Coalfield, Ordos Basin, China. Mine Water Environ. 2013, 32, 195–206. [Google Scholar] [CrossRef]
- Abid, K.; Trabelsi, R.; Zouari, K.; Abidi, B. Caractérisation hydrogéochimique de la nappe du Continental Intercalaire (Sud Tunisien)/Hydrogeochemical characterization of the Continental Intercalaire aquifer (southern Tunisia). Hydrol. Sci. J. 2009, 54, 526–537. [Google Scholar] [CrossRef]
- Li, P.; Wu, J.; Qian, H. Groundwater quality assessment based on rough sets attribute reduction and TOPSIS method in a semi-arid area, China. Environ. Monit. Assess. 2012, 184, 4841–4854. [Google Scholar] [CrossRef]
- Wu, J.; Li, P.; Qian, H.; Duan, Z.; Zhang, X. Using correlation and multivariate statistical analysis to identify hydrogeochemical processes affecting the major ion chemistry of waters: A case study in Laoheba phosphorite mine in Sichuan, China. Arab. J. Geosci. 2014, 7, 3973–3982. [Google Scholar] [CrossRef]
- Yangui, H.; Zouari, K.; Rozanski, K. Hydrochemical and isotopic study of groundwater in Wadi El Hechim–Garaa Hamra basin, Central Tunisia. Environ. Earth Sci. 2012, 66, 1359–1370. [Google Scholar] [CrossRef]
- Azaza, F.H.; Ameur, M.; Bouhlila, R.; Gueddari, M. Geochemical characterization of groundwater in a Miocene Aquifer, Southeastern Tunisia. Environ. Eng. Geosci. 2012, 18, 159–174. [Google Scholar] [CrossRef]
- Hamzaoui-Azaza, F.; Ketata, M.; Bouhlila, R.; Gueddari, M.; Ribeiro, L. Hydrochemical evolution and evaluation of drinking water quality in Zeuss-Koutine Aquifer, South-Eastern of Tunisia. J. Environ. Monit. Asses. 2011, 174, 283–298. [Google Scholar] [CrossRef] [PubMed]
- Hamzaoui-Azaza, F.; Tlili-Zrelli, B.; Bouhlila, R.; Gueddari, M. An integrated statistical methods and modelling mineral—Water interaction to identifying hydrogeochemical processes in groundwater in Southern Tunisia. Chem. Spec. Bioavailab. 2013, 25, 165–178. [Google Scholar] [CrossRef]
- Barbieri, M. Isotopes in Hydrology and Hydrogeology. Water 2019, 11, 291. [Google Scholar] [CrossRef]
- Li, F.; Pan, G.; Tang, C.; Zhang, Q.; Yu, J. Recharge source and hydrogeochemical evolution of shallow groundwater in a complex alluvial fan system, southwest of North China Plain. Environ. Geol. 2008, 55, 1109–1122. [Google Scholar] [CrossRef]
- Neal, C.; Neal, M.; Warrington, A.; Ávila, A.; Piñol, J.; Rodà, F. Stable hydrogen and oxygen isotope studies of rainfall and streamwaters for two contrasting holm oak areas of Catalonia, northeastern Spain. J. Hydrol. 1992, 140, 163–178. [Google Scholar] [CrossRef]
- Palmer, P.C.; Gannett, M.W.; Hinkle, S.R. Isotopic characterization of three groundwater recharge sources and inferences for selected aquifers in the upper Klamath Basin of Oregon and California, USA. J. Hydrol. 2007, 336, 17–29. [Google Scholar] [CrossRef]
- Bencer, S.; Boudoukha, A.; Mouni, L. Multivariate statistical analysis of the groundwater of Ain Djacer area (Eastern of Algeria). Arab. J. Geosci. 2016, 9, 248. [Google Scholar] [CrossRef]
- Cloutier, V.; Lefebvre, R.; Therrien, R.; Savard, M.M. Multivariate statistical analysis of geochemical data as indicative of the hydrogeochemical evolution of groundwater in a sedimentary rock aquifer system. J. Hydrol. 2008, 353, 294–313. [Google Scholar] [CrossRef]
- Matiatos, I.; Alexopoulos, A.; Godelitsas, A. Multivariate statistical analysis of the hydrogeochemical and isotopic composition of the groundwater resources in northeastern Peloponnesus (Greece). Sci. Total Environ. 2014, 476, 577–590. [Google Scholar] [CrossRef]
- Moya, C.E.; Raiber, M.; Taulis, M.; Cox, M.E. Hydrochemical evolution and groundwater flow processes in the Galilee and Eromanga basins, Great Artesian Basin, Australia: A multivariate statistical approach. Sci. Total Environ. 2015, 508, 411–426. [Google Scholar] [CrossRef] [PubMed]
- Jolliffe, I. Principal Component Analysis; Springer: Berlin/Heidelberg, Germany, 2002. [Google Scholar]
- Kim, J.H.; Kim, R.H.; Lee, J.; Cheong, T.J.; Yum, B.W.; Chang, H.W. Multivariate statistical analysis to identify the major factors governing groundwater quality in the coastal area of Kimje, South Korea. Hydrogeol. J. 2005, 19, 1261–1276. [Google Scholar] [CrossRef]
- Liu, F.; Conklin, M.H.; Shaw, G.D. Insights into hydrologic and hydrochemical processes based on concentration-discharge and end-member mixing analyses in the mid-Merced River Basin, Sierra Nevada, California. Water Resour. Res. 2017, 53, 832–850. [Google Scholar] [CrossRef]
- Gao, X.; Wang, Y.; Li, Y.; Guo, Q. Enrichment of fluoride in groundwater under the impact of saline water intrusion at the salt lake area of Yuncheng basin, northern China. Environ. Geol. 2007, 53, 795–803. [Google Scholar] [CrossRef]
- Abou Zakhem, B.; Al-Charideh, A.; Kattaa, B. Using principal component analysis in the investigation of groundwater hydrochemistry of Upper Jezireh Basin, Syria. Hydrol. Sci. J. 2017, 62, 2266–2279. [Google Scholar] [CrossRef]
- Boschetti, T.; Cortecci, G.; Bolognesi, L. Chemical and isotopic study of the shallow groundwater system of Vulcano Island, Aeolian Archipelago, Italy: An update. GeoActa 2003, 2, 1–34. [Google Scholar]
- Li, C.; Gao, X.; Liu, Y.; Wang, Y. Impact of anthropogenic activities on the enrichment of fluoride and salinity in groundwater in the Yuncheng Basin constrained by Cl/Br ratio, δ18O, δ2H, δ13C and δ7Li isotopes. J. Hydrol. 2019, 579, 124211. [Google Scholar] [CrossRef]
- Freeze, R.A.; Cherry, J.A. Groundwater; Prentice-Hall Inc.: Upper Saddle River, NJ, USA, 1979. [Google Scholar]
- Li, C.; Gao, X.; Wang, Y. Hydrogeochemistry of high-fluoride groundwater at Yuncheng Basin, northern China. Sci. Total Environ. 2015, 508, 155–165. [Google Scholar] [CrossRef]
- Khair, A.M.; Li, C.; Hu, Q.; Gao, X.; Wang, Y. Fluoride and arsenic hydrogeochemistry of groundwater at Yuncheng basin, Northern China. Geochem. Int. 2014, 52, 868–881. [Google Scholar] [CrossRef]
- Liu, F.; Song, X.; Yang, L.; Han, D.; Zhang, Y.; Ma, Y.; Bu, H. The role of anthropogenic and natural factors in shaping the geochemical evolution of groundwater in the Subei Lake basin, Ordos energy base, Northwestern China. Sci. Total Environ. 2015, 538, 327–340. [Google Scholar] [CrossRef]
- Han, Y.; Yan, S.L.; Ma, H.T.; Zhang, H.M.; Wu, J.Z.; Wang, Y.X.; Liang, X.; Xu, H.L.; Ma, T.; Tang, Z.H. Groundwater Resources and Environmental Issues Assessment in the Six Major Basins of Shanxi Province; Geological Publishing House: Beijing, China, 2006. [Google Scholar]
- Craig, H. Isotopic variations in meteoric waters. Science 1961, 133, 1702–1703. [Google Scholar] [CrossRef] [PubMed]
- Gourcy, L.L.; Groening, M.; Aggarwal, P.K. Stable oxygen and hydrogen isotopes in precipitation. In Isotopes in the Water Cycle: Past, Present and Future of Developing Science; Aggarwal, P.K., Gat, J.R., Froehlich, K.F.O., Eds.; Springer: Dordrecht, The Netherlands, 2005; pp. 39–51. [Google Scholar]
- IAEA/WMO. Global network of isotopes in precipitation. The GNIP Database. 2007. Available online: http://isohis.iaea.org> (accessed on 29 January 2020).
- Gibbs, R.J. Mechanisms controlling world water chemistry. Science 1970, 170, 1088–1090. [Google Scholar] [CrossRef] [PubMed]
- Feth, J.H.; Gibbs, R.J. Mechanisms controlling world water chemistry: Evaporation-crystallization process. Science 1971, 172, 870–872. [Google Scholar] [CrossRef] [PubMed]
- Mamatha, P.; Rao, S.M. Geochemistry of fluoride rich groundwater in Kolar and Tumkur Districts of Karnataka. Environ. Earth Sci. 2010, 61, 131–142. [Google Scholar] [CrossRef]
- Taheri, M.; Gharaie, M.H.M.; Mehrzad, J.; Afshari, R.; Datta, S. Hydrogeochemical and isotopic evaluation of arsenic contaminated waters in an argillic alteration zone. J. Geochem. Explor. 2017, 175, 1–10. [Google Scholar] [CrossRef]
- Sahib, L.Y.; Marandi, A.; Schuth, C. Strotium isotopes as an indicator for groundwater salinity sources in the Kirkuk region, Iraq. Sci. Total Environ. 2016, 562, 935–945. [Google Scholar] [CrossRef]
- Marandi, A.; Shand, P. Groundwater chemistry and the Gibbs Diagram. Appl. Geochem. 2018, 97, 209–212. [Google Scholar] [CrossRef]
- Zhou, H.; Tang, J.; Guo, J.; Dai, Y.; Li, G.; Yan, B. Integrated system of comprehensive utilizing the concentrated brine of Yuncheng salt-lake basing on salt-forming diagram. Chin. J. Chem. Eng. 2019, 27, 182–190. [Google Scholar] [CrossRef]
- Wang, Y.; Su, C.; Xie, X.; Xie, Z. The genesis of high arsenic groundwater: A case study in Datong basin. Geol. China 2010, 3, 034. [Google Scholar]
- Datta, P.S.; Tyagi, S.K. Major ion chemistry of groundwater in Delhi area: Chemical weathering processes and groundwater flow regime. J. Geol. Soc. India 1996, 47, 179–188. [Google Scholar]
- Jalali, M. Hydrochemical identification of groundwater resources and their changes under the impacts of human activity in the Chah basin in western Iran. Environ. Monit. Assess. 2007, 130, 347–364. [Google Scholar] [CrossRef]
- Schoeller, H. Hydrodynamique dans le karst. Chronique d’Hydrogéologie 1967, 10, 7–21. [Google Scholar]
- Gat, J.R. Oxygen and hydrogen isotopes in the hydrologic cycle. Annu. Rev. Earth Planet. Sci. 1996, 24, 225–262. [Google Scholar] [CrossRef]
- Bortolotti, L.E.; Clark, R.G.; Wassenaar, L.I. Hydrogen isotope variability in prairie wetland systems: Implications for studies of migratory connectivity. Ecol. Appl. 2013, 23, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, R.D.; Bhattacharya, S.K.; Jani, R.A.; Gupta, S.K. Distribution of oxygen and hydrogen isotopes in shallow groundwaters from Southern India: Influence of a dual monsoon system. J. Hydrol. 2003, 271, 226–239. [Google Scholar] [CrossRef]
- Gammons, C.H.; Poulson, S.R.; Pellicori, D.A.; Reed, P.J.; Roesler, A.J.; Petrescu, E.M. The hydrogen and oxygen isotopic composition of precipitation, evaporated mine water, and river water in Montana, USA. J. Hydrol. 2006, 328, 319–330. [Google Scholar] [CrossRef]
- WHO. Guidelines for Drinking-Water Quality, 4th ed.; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
| EC (μs/cm) | T (°C) | pH | ORP (mV) | K+ (mg/L) | Na+ (mg/L) | Ca2+ (mg/L) | Mg2+ (mg/L) | Cl− (mg/L) | SO42+ (mg/L) | HCO3− (mg/L) | NO3− (mg/L) | TDS (mg/L) | δ18O (‰) | δD (‰) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Shallow groundwater (n = 51) | |||||||||||||||
| Min | 308 | 15.8 | 6.95 | −290 | 0.28 | 8.28 | 2.89 | 5.06 | 10.86 | 18.51 | 20.98 | 1.94 | 349 | −10.14 | −75.57 |
| Max | 8630 | 22.1 | 8.83 | 774 | 51.26 | 3024 | 429 | 605 | 1746 | 4108 | 1953 | 67.88 | 9590 | −8.18 | −62.93 |
| Mean | 2491 | 19 | 7.77 | 100 | 3.23 | 732 | 76.11 | 117 | 376 | 1168 | 631 | 18.17 | 2792 | −8.9 | −66.33 |
| Intermediate groundwater (n = 20) | |||||||||||||||
| Min | 324 | 16.5 | 7.55 | −30 | 0.52 | 9.75 | 3.53 | 10.89 | 8.86 | 17.51 | 15.39 | 1.16 | 229 | −9.57 | −66.96 |
| Max | 2190 | 22.2 | 8.63 | 734 | 5.59 | 365 | 89.62 | 92.61 | 213 | 680 | 836 | 26.56 | 1482 | −8.36 | −61.8 |
| Mean | 844 | 18.7 | 8.03 | 242 | 2.02 | 145 | 41.25 | 32.36 | 51.58 | 167 | 370 | 7.76 | 628 | −8.89 | −65.38 |
| Deep groundwater (n = 112) | |||||||||||||||
| Min | 321 | 16.5 | 6.80 | −95 | 0.26 | 15.73 | 9.78 | 11.67 | 5.85 | 11.51 | 140 | 1.31 | 205 | −11.34 | −81.87 |
| Max | 8860 | 39 | 9.02 | 873 | 37.69 | 3638 | 434 | 773 | 2552 | 9214 | 1373 | 135 | 14051 | −8.67 | −65.69 |
| Mean | 1457 | 20.4 | 7.95 | 157 | 2.36 | 440 | 64.89 | 64.99 | 221 | 717 | 401 | 11.91 | 1715 | −9.84 | −72.51 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, X.; Li, X.; Wang, W.; Li, C. Human Activity and Hydrogeochemical Processes Relating to Groundwater Quality Degradation in the Yuncheng Basin, Northern China. Int. J. Environ. Res. Public Health 2020, 17, 867. https://doi.org/10.3390/ijerph17030867
Gao X, Li X, Wang W, Li C. Human Activity and Hydrogeochemical Processes Relating to Groundwater Quality Degradation in the Yuncheng Basin, Northern China. International Journal of Environmental Research and Public Health. 2020; 17(3):867. https://doi.org/10.3390/ijerph17030867
Chicago/Turabian StyleGao, Xubo, Xue Li, Wanzhou Wang, and Chengcheng Li. 2020. "Human Activity and Hydrogeochemical Processes Relating to Groundwater Quality Degradation in the Yuncheng Basin, Northern China" International Journal of Environmental Research and Public Health 17, no. 3: 867. https://doi.org/10.3390/ijerph17030867
APA StyleGao, X., Li, X., Wang, W., & Li, C. (2020). Human Activity and Hydrogeochemical Processes Relating to Groundwater Quality Degradation in the Yuncheng Basin, Northern China. International Journal of Environmental Research and Public Health, 17(3), 867. https://doi.org/10.3390/ijerph17030867







